Running Head: Prevalence and Comorbidities of COPD
Funding support: There are no sources of funding or financial support for all authors
Date of acceptance: July 16, 2015
Abbreviations: chronic obstructive pulmonary disease, COPD; Area Development Districts, ADDs; Kentucky Behavioral Risk Factor Survey, KyBRFS; odds ratios, ORs; confidence interval, CI; coronary heart disease, CHD; chronic lower respiratory disease, CLRD; Centers for Disease Control and Prevention, CDC; Behavioral Risk Factor Surveillance System, BRFSS; cardiovascular heart disease, CVD
Citation: Kamour A, Mannino D, Kanotra S. Prevalence and comorbidities of chronic obstructive pulmonary disease among adults in Kentucky across gender and area development districts, 2011. Chronic Obstr Pulm Dis. 2015; 2(4): 296-312. doi: http://doi.org/10.15326/jcopdf.2.4.2015.0138
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most common chronic lower respiratory diseases (CLRD) and a major cause of morbidity and mortality throughout the world.1 CLRD is and was the third leading cause of death in the United States in 2011.2 The World Health Organization estimated that COPD will rank fifth in the global burden of disease by 2020.3 The National Health Interview Survey reported that emphysema is present in 18 persons per 1000 and chronic bronchitis in 34 persons per 1000.4 In 2012, the Centers for Disease Control and Prevention (CDC) reported that the prevalence of COPD in 2011 was 6.5% and increased with age, from 3.2% among those aged 18–44 years to more than 11.6% among those aged 65 years and older.5 The prevalence of COPD in Kentucky in that survey was 9.3%.5
Several studies have shown that COPD is associated with cardiovascular disease, depression, diabetes and hypertension.6-10 These comorbid diseases have a major impact on the prognosis and health-related quality of life of the patients.11-13
Cigarette smoking and older age are the most consistently established risk factors of COPD.1,14-18 Cigarette smoking can also cause an increased risk of COPD comorbidities because it is associated with an abnormal pulmonary and systemic inflammatory response. Cigarette smoking induces vascular oxidative stress, endothelial dysfunction and blood coagulation impairment.19-21 Regarding gender differences in COPD, the CDC reported that the prevalence of COPD in the United States was stable from 1998 through 2009 and has remained higher in women than in men.22
This study was done to determine the burden of COPD in Kentucky and to evaluate the link between COPD and the major comorbidities with regard to age, sex, cigarette smoking, education level, income and Area Development Districts (ADDs). Kentucky’s ADDs consist of 120 counties that share common geographical and economic development characteristics. These ADDs comprise a statewide network of multi-county planning and development organizations working along multi-jurisdictional lines to advance local, state, and regional priorities. We used the ADD as an analysis unit to overcome the insufficient Behavioral Risk Factor Surveillance System (BRFSS)respondents. The findings may help public health officials and clinicians to create effective programs to reduce the frequency and severity of COPD and its comorbidities.
Using the population-based BRFSS data, we estimated the prevalence of COPD and its comorbidities. The difference in the prevalence of both COPD and its comorbidities and the risk differences of these comorbidities across ADDs in Kentucky with regards to age, sex, smoking status, education and income among those diagnosed with COPD were also estimated.
We hypothesized that there would be variations in the prevalence of both COPD and its comorbidities and the risk of these comorbidties based upon gender and ADDs after adjusting for age, smoking status, education and income.
Methods
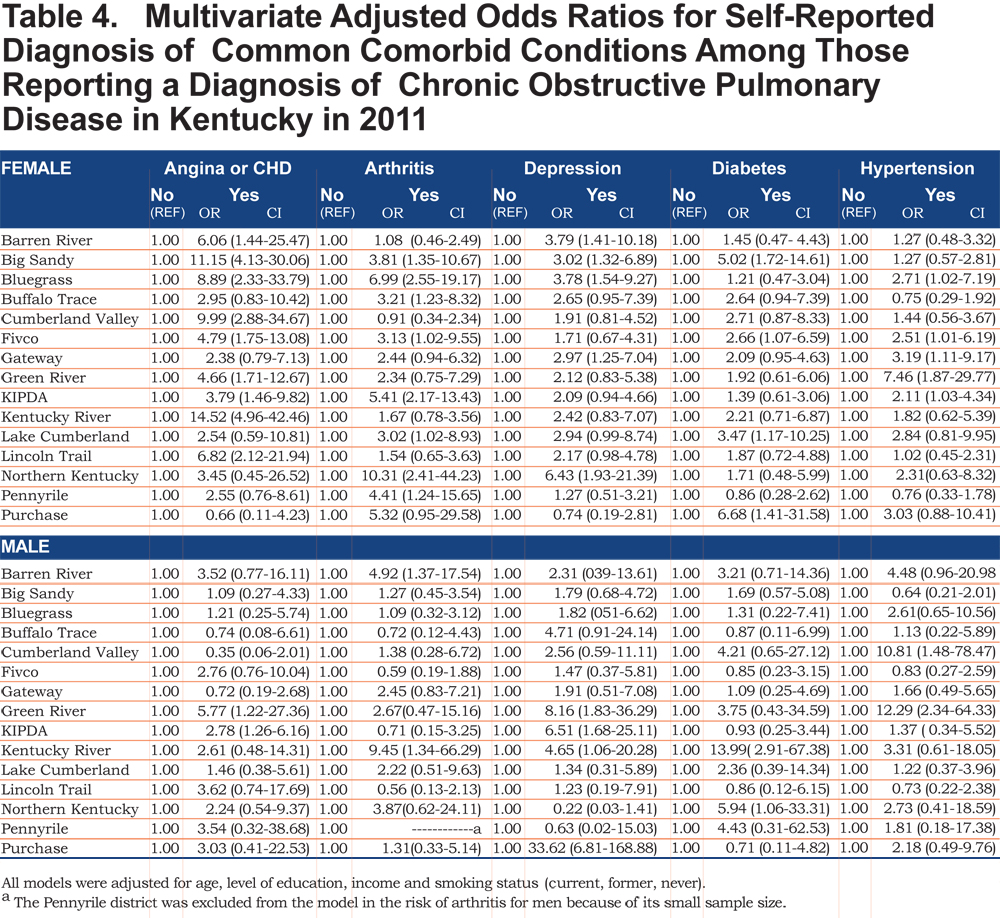
The data used in this study was from the 2011 Kentucky BRFSS. BRFSS is a state-based telephone surveillance system designed to collect data on individual risk behaviors, preventive health practices, and health-related conditions that are related to the leading causes of death and disability in the United States. It is supported by funds granted by the CDC and is conducted in all 50 states across the nation and some territories. This data was used to estimate the overall number of persons with COPD in the state among the estimated 3,344,426 adults living in Kentucky in 2011.1 The status of whether the participants have COPD was determined based on responses to the following BRFSS question, "(Were you ever told) you have (COPD) chronic obstructive pulmonary disease, emphysema or chronic bronchitis?” COPD comorbidities status in those with self-reported COPD was determined based on responses to the following questions: ” (Were you ever told) you had angina or coronary heart disease?", "(Were you ever told) you have some form of arthritis, rheumatoid arthritis, gout, lupus, or fibromyalgia?", "(Were you ever told) you that you have a depressive disorder, including depression, major depression, dysthymia, or minor depression?", "(Were you ever told) you have diabetes?" and "(Were you ever told) you have high blood pressure?" The sample of interest was all adult Kentucky residents 18 years and older (N = 10,794), of whom 1372 self-identified as having COPD.
The outcome variable for this research was self-reported COPD. The BRFSS had dichotomized COPD responses into "Yes or No”. Variables included in the analysis are age, educational level, income, smoking history, comorbidities and ADD. We categorized the data into the following variables’ classifications: sex (male and female), age-group (18-44, 45-54, 55-64 and 65+ years), education (less than high school, high school graduate and some college or college graduate), annual household income (less than $24,000, $25,000-$49,000 more than $50,000 and don’t know/not sure/refused), smoking status (never, former, and current), cardiovascular disease (CVD) (yes or no), arthritis (yes or no), high blood pressure (yes or no), diabetes (yes or no) and 1 of 15 ADDs (Barren River, Big Sandy, Bluegrass, Buffalo Trace, Cumberland Valley, Fivco, Gateway, Green River, Kentucky River, Kentuckiana Regional Planning & Development Agency [KIPDA], Lake Cumberland, Lincoln Trail, Northern Kentucky, Pennyrile and Purchase).
Statistical Analysis
Data was analyzed with the Statistical Analysis System version 9.3 (SAS Inc), WinEpi and Microsoft Excel.The following statistical analyses were conducted: First, a table for the descriptive analysis of the data was produced. Second, the age adjusted prevalence of self-reported COPD and its comorbidties in Kentucky, by area development districts were calculated. The prevalence estimates were calculated using raking weights provided by the CDC.23 All age-adjustments were calculated using the 2010 U.S. Census as the standard. Third, χ2 analysis was used to determine if there were differences in prevalence between men and women and across ADDs. A p-value less than 0.05 was considered statistically significant. Fourth,odds ratios (OR) of COPD, by sex and ADD were calculated by using multivariate logistic regression adjusted for age, educational level, income, and smoking history. Random effects meta-analysis models were conducted to calculate pooled prevalence estimates and odds ratios. Heterogeneity across gender and ADDs using I² measures were estimated.
Results
A total of 9585 participants from 15 ADDs were included in the study; of which 6283 (65.5 %) were females. Table 1 shows the demographic characteristics and important risk factors for COPD of all study participants across districts.
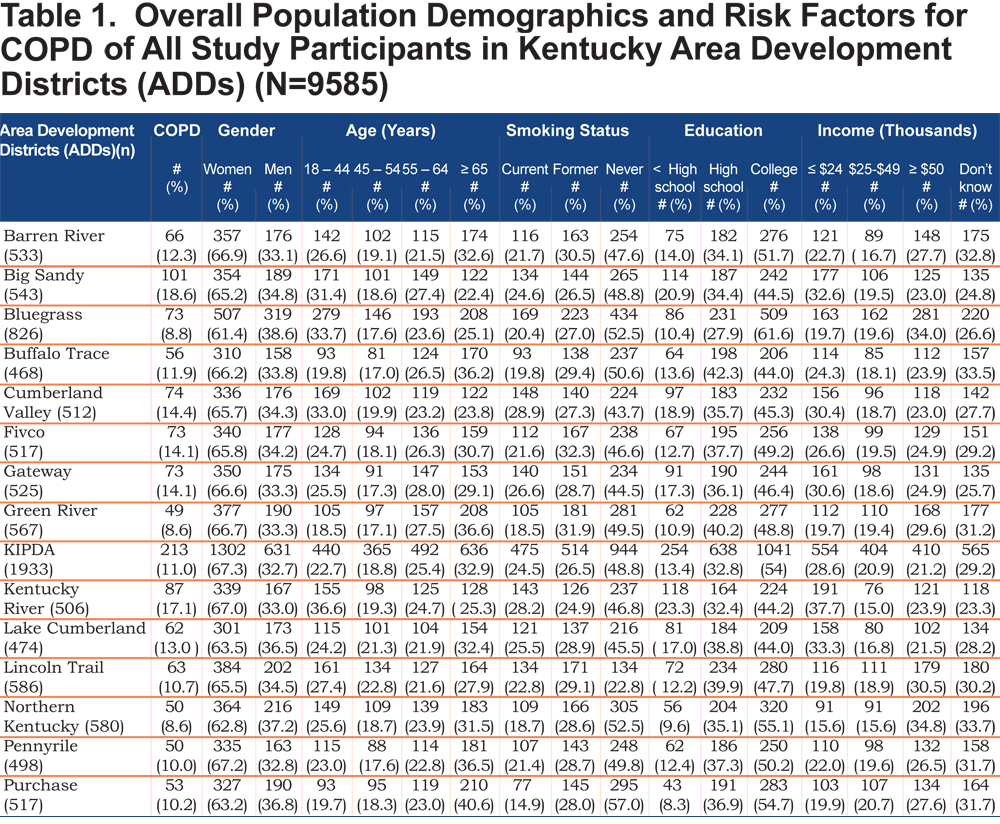
The mean age of females and males were 56.6 ±16. 4 years and 53.7 ±16. 4 years, respectively. The rate of COPD varies considerably among the ADDs. COPD was more frequent in the Big Sandy and Kentucky River districts (18.8% and 16.8%), respectively. Cumberland Valley and Kentucky River districts have the highest rate of current smoking, while Purchase and Green River districts have the lowest rate of current smoking. Prevalence of cigarette smoking varied widely across ADDs and between men and women. The level of education was low in these ADDs (Big Sandy and Kentucky River districts), which are located in the eastern part of the state. The highest level of education was recorded in the Bluegrass ADD. Participants from the Northen Kentucky ADD have the highest income rates compared with other districts.
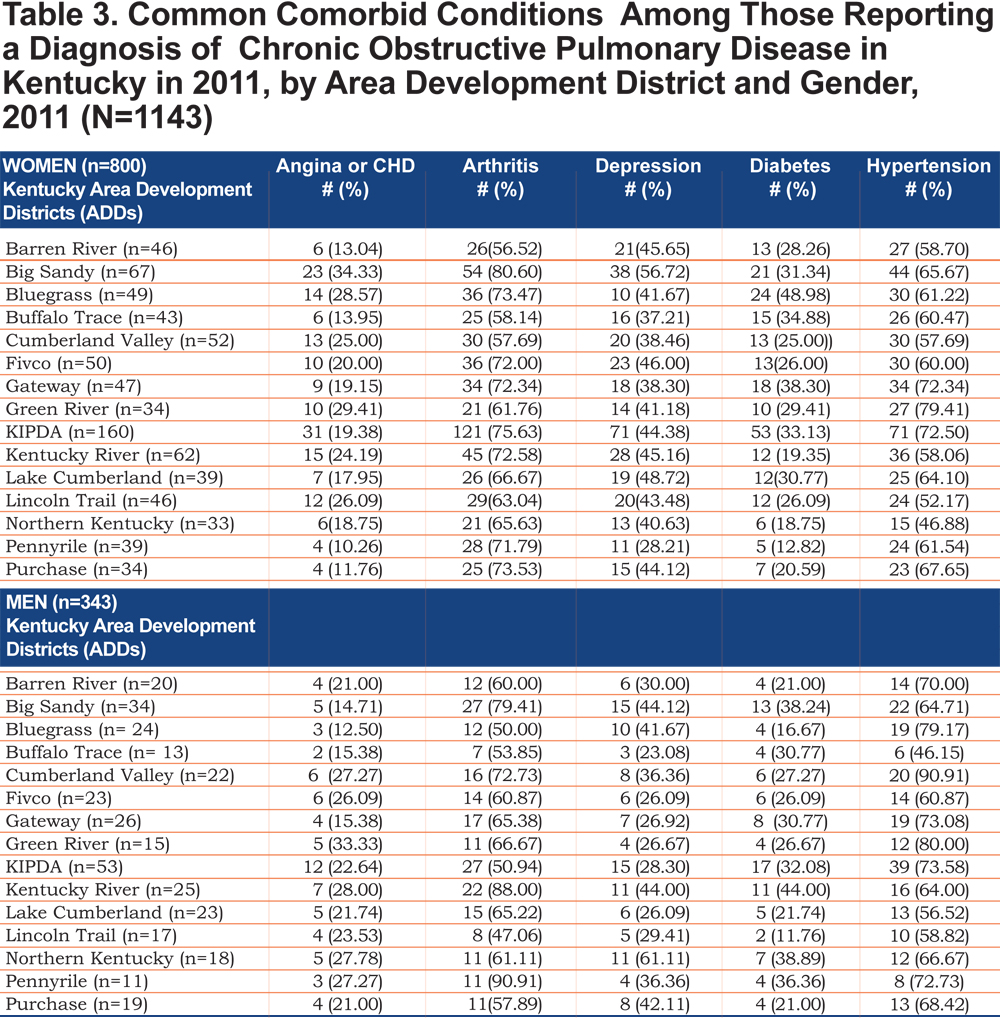
Tables 2 and 3 show the self- reported population characteristics and potential risk factors for COPD and its comorbidities of all participants recruited into the study with regard to sex and ADD.
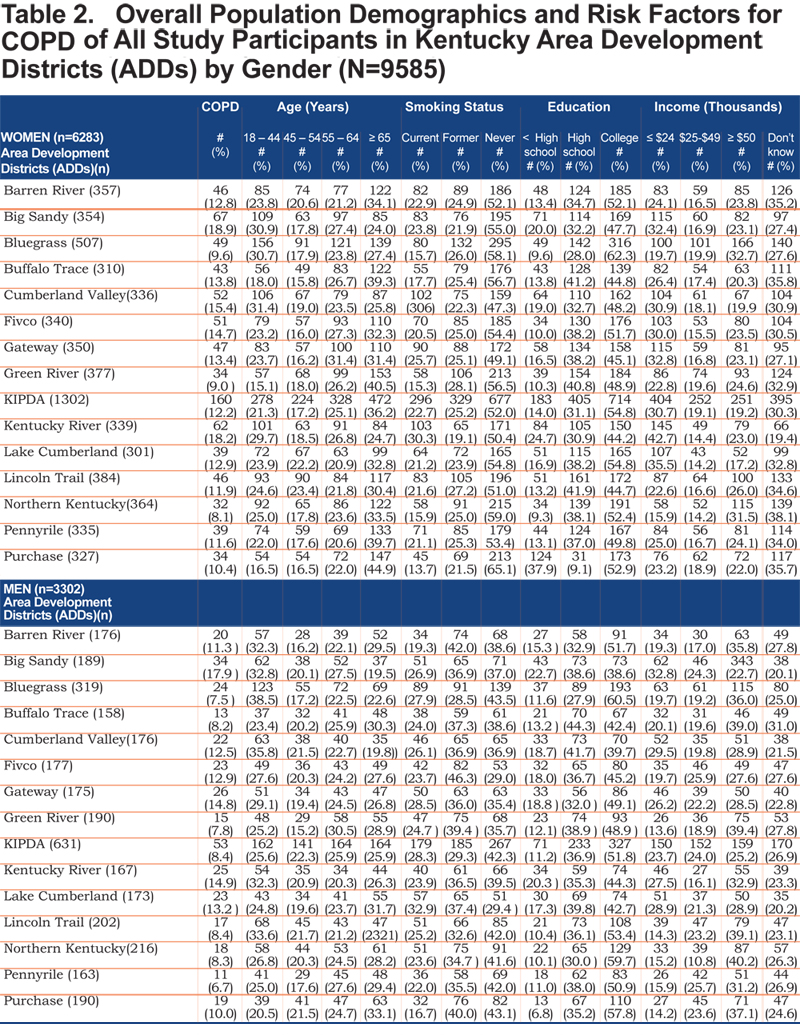
The overall prevalence of age adjusted self- reported COPD was 10.09% (95% confidence interval [CI] 9.99, 10.19), 8.85% for men (95% CI 8.76, 8.93), and 10.78% for women (95% CI 10.67, 10.88). Across all ADDs, self reported COPD was more common among females than males except in the Gateway area where the age adjusted prevalence in males was 12.63% (95% CI 12.75, 12.88) and 10.79% (95% CI 10.68, 10.89) in females. In regards to age-adjusted self reported comorbidities prevalence, they were most frequent in the Big Sandy district (arthritis 14.87% (95% CI 14.72, 15.01), hypertension 12.00% (95% CI 11.88, 12.12), depression 9.76% (95% CI 9.66, 9.85), diabetes 6.09% (95% CI 6.02, 6.15), angina 4.00% (95% CI 4.96, 4.04). Figure 1 shows the geographic distribution of age-adjusted self reported COPD prevalence and other comorbidities by ADD.
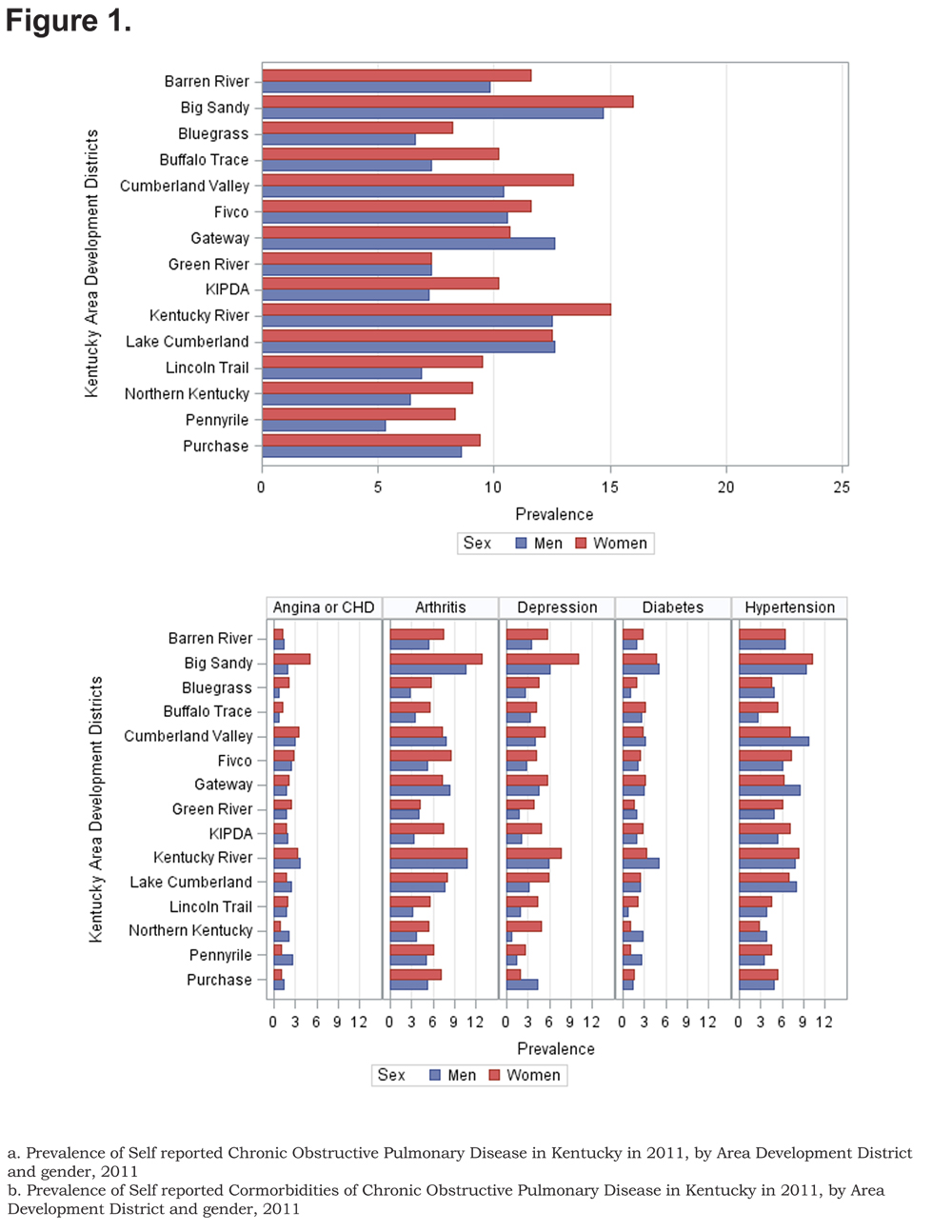
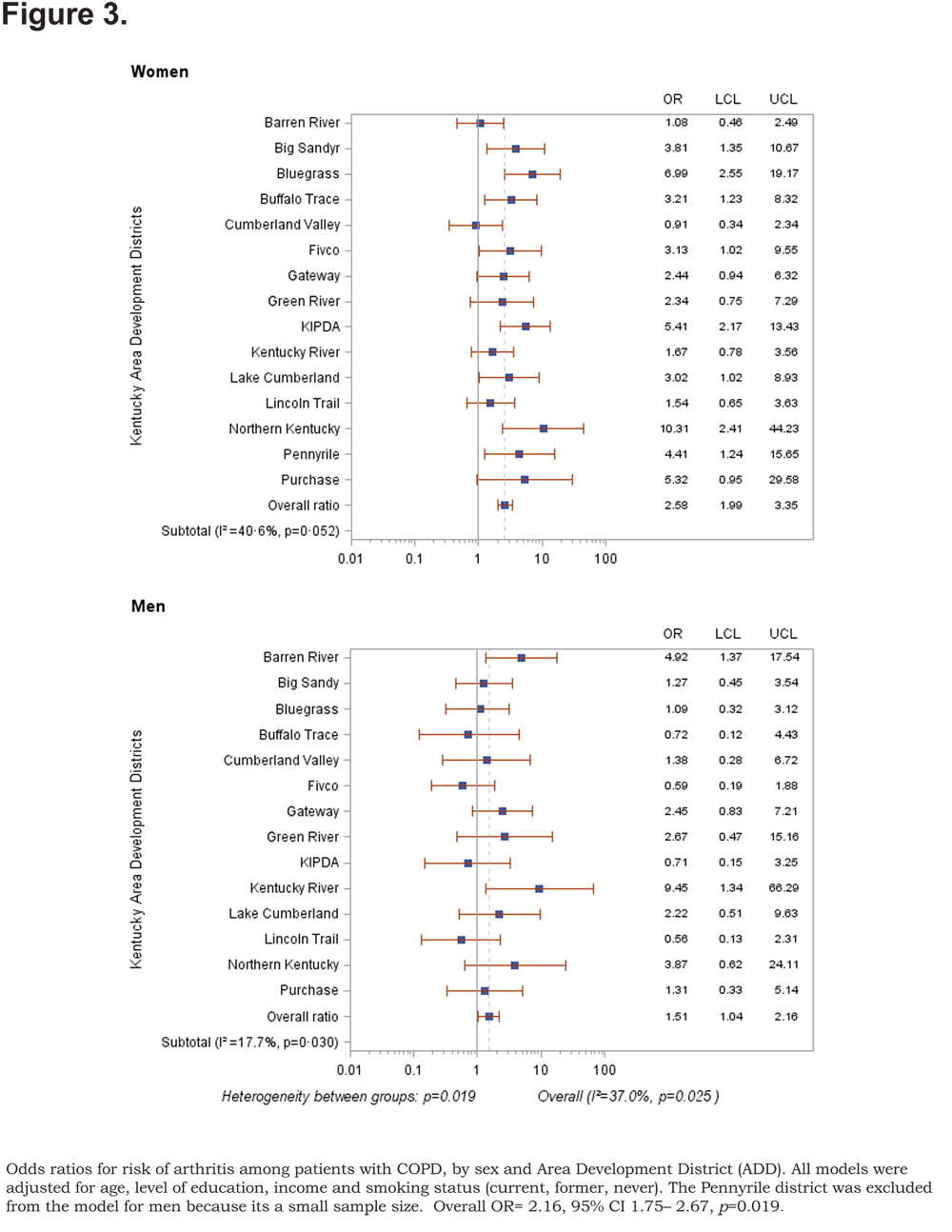
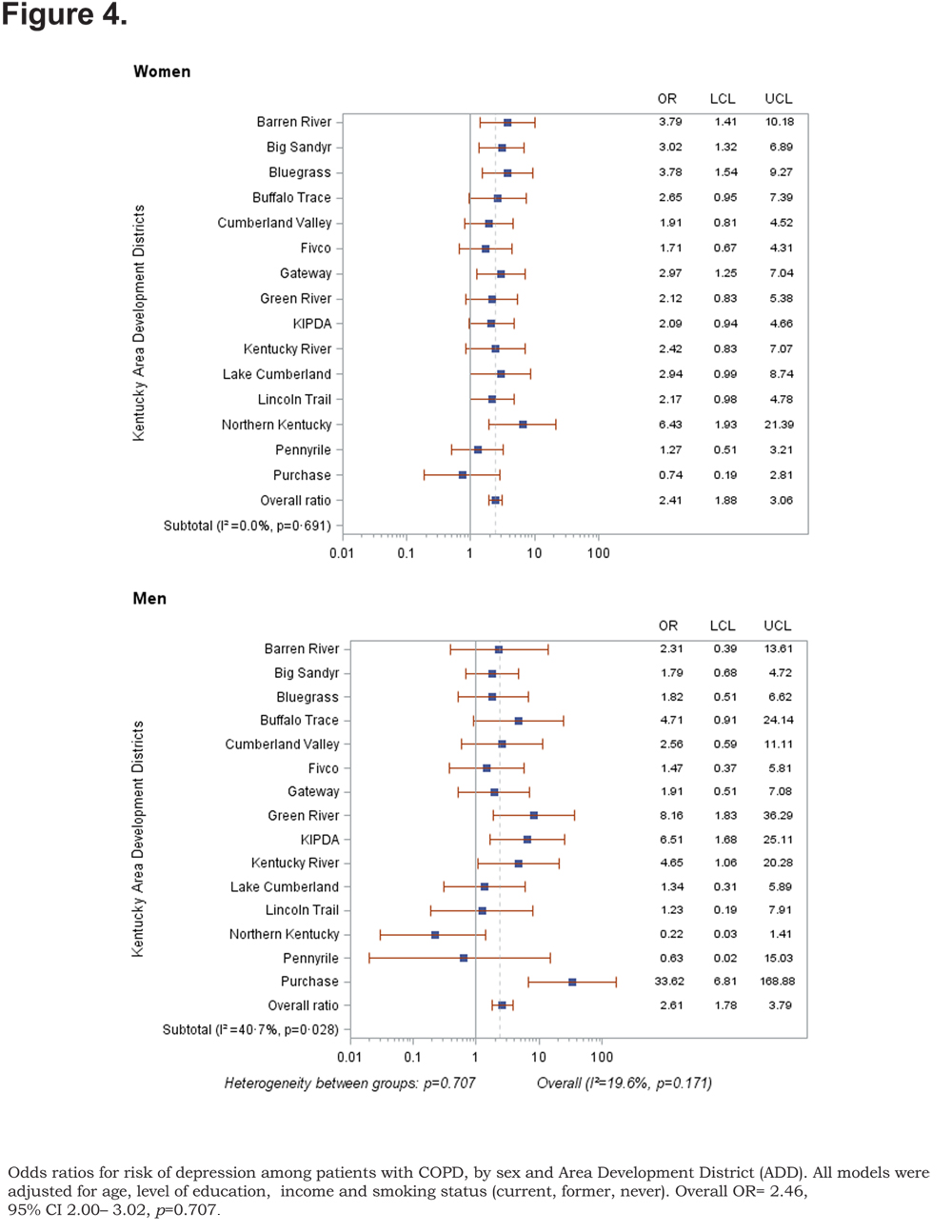
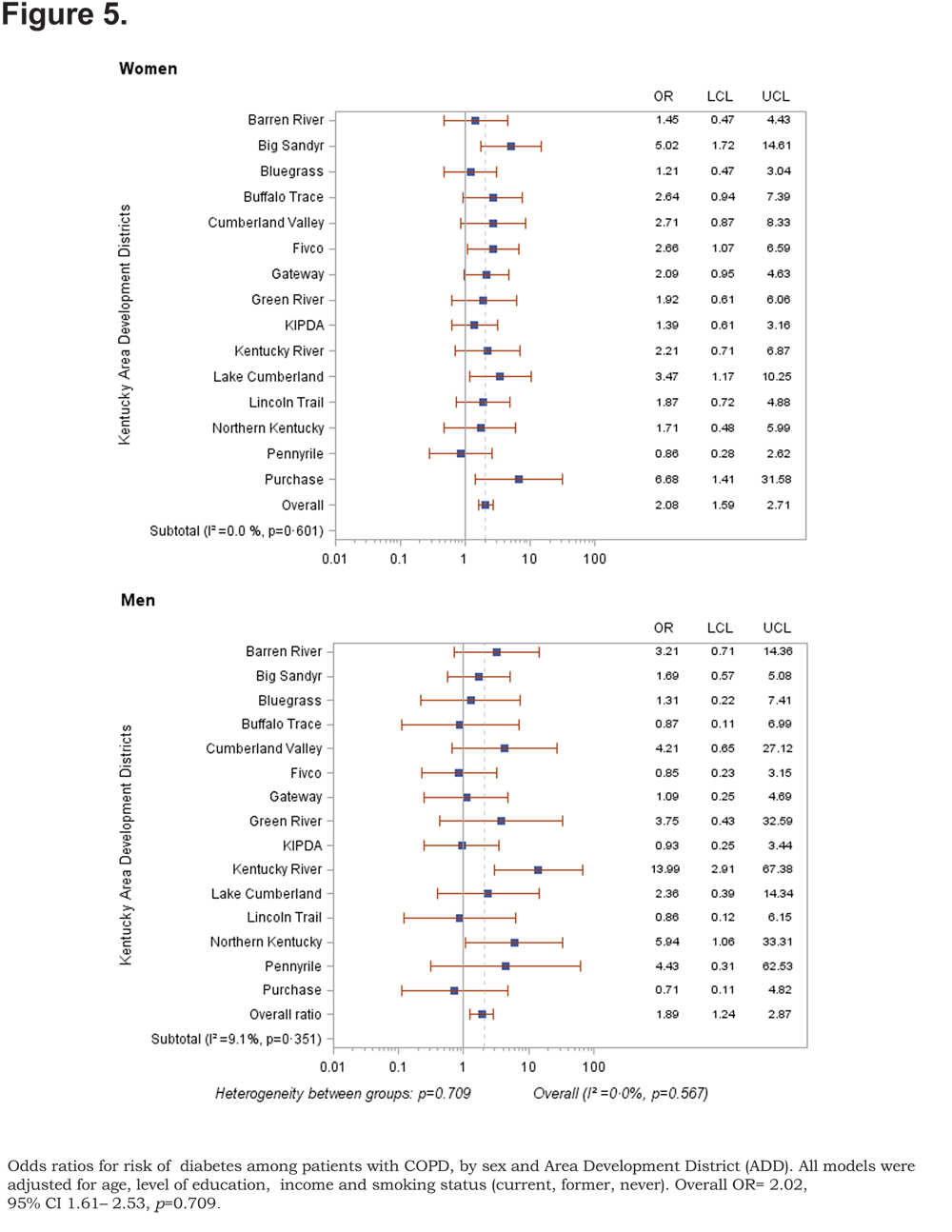
Out of 5 age-adjusted self reported comorbidities, as shown in Figure 1, the most common were arthritis 6.49 % (95% CI 6.42, 6.55), and hypertension 6.06% (95% CI 5.99, 6.12), followed by depression 4.32% (95% CI 4.27, 4.36), and diabetes 2.41%.(95% CI 2.38, 2.42). The prevalence of arthritis and depression were more common among women (for arthritis =7.22% [95% CI 7.14, 7.29], and for depression 5.08% [95% CI 5.03, 5.24]) than among men (for arthritis =5.19% [95% CI 5.13, 5.24], and for depression 2.97% [95% CI 2.49, 3.00]). In addition, differences in the prevalence of arthritis varied widely across gender (p=0.009). However, prevalence of arthritis showed borderline significant differences across ADDs (p=0.05). Table 4 and Figures 2-6 show ORs of COPD, by sex and site, associated with angina, arthritis, depression, diabetes and hypertension (in those with self-reported COPD only). Data was adjusted for age, level of education, income, and smoking status (current, former, never). Pooled ORs for these disorders among COPD individuals, compared to non-COPD individuals, revealed that people with COPD have a greater risk of angina, arthritis, depression, diabetes and hypertension than people without COPD (ORs for women=5.01 [95% CI 3.68–8.81], 2.58 [95% CI 1.99–3.35], 2.41 [95% CI 1.88–3.06] 2.08 [95% CI 1.59–2.71] and 1.96 [95% CI 1.32–2.17], and ORs for men=1.98 [95% CI 1.37–2.86], 1.51 [95% CI 1.04–2.16], 2.61 [95% CI 1.78–3.79], 1.89 [95% CI 1.24–2.87] and 1.68 [95% CI 1.16–2.42] respectively). There was significant heterogeneity among men in OR estimates for arthritis (p = 0·03), depression (p = 0·028) and hypertension (p=0·006) across the ADDs. Figures 2 and 3 show the tests for heterogeneity in the ORs between ADDs and sexes were statistically significant in angina (p= 0.0001) and arthritis (p= 0.0001). However, Figures 4, 5, and 6 show that the tests for heterogeneity between ADDs and between sexes were not statistically significant and the overall pooled OR estimate for depression, diabetes and hypertension were 2.46 (95% CI 2.00-3.02) and 1.68 (95% CI 1.37-2.07), respectively.


Discussion
The purpose of our study was to determine the prevalence of COPD, prevalence and risk of its comorbidities, and to investigate the pattern of their variation across ADDs and genders. In this population-based study, we observed that there was variation in the age-adjusted prevalence of both self-reported cases of COPD and its self-reported comorbid conditions between men and women across ADDs. Furthermore, after age, level of education, income, and smoking status adjustment, adults with COPD were more likely to report angina, arthritis, depression, diabetes and hypertension compared to adults without COPD. The study also reported that there were substantial differences in the risk of these comorbidties in adults with COPD across gender and ADDs.
Our study shows a higher prevalence of COPD among women versus men.24 These findings are consistent with previous results. Several studies suggest that the trends in COPD prevelance across gender have changed dramatically over the last two decades.22,25,26 For instance, a study based on clinical diagnosis of COPD conducted from 1980 to 1996 showed that the prevalence of COPD among women had increased while the prevalence among men remained stable.25 Moreover, the National Health Interview Survey Data from 1998 through 2009 reported that the prevalence of COPD among women aged 18-74 years was significantly greater than among men.22 The pattern for the prevalence of COPD among different sexes with controlling for smoking is still a controversial topic.27-30
The significant differences in the prevalence estimates of COPD among ADDs are more likely to be affected by prevalence of COPD risk factors (p<0.0001). Previous studies have also suggested that differences in the prevalence estimates of COPD may be influenced by prevalence of its risk factors.10,31-33 Cigarette smoking and other risk factors have a major impact on the variation of COPD across ADD.34 Geographically, the prevalence of COPD and other chronic conditions were more prominent in the eastern part of Kentucky. In our study, we found that prevalence rates were higher in the Big Sandy and Kentucky River districts. These districts are located in eastern Kentucky which has a long history of tobacco use, coal mine history and other risk factors.10,32,35-37
While susceptibility, clinical presentation, diagnosis, and treatment of COPD across gender have been assessed in current literature,38-41 data remains limited when it comes to the gender difference in the risk of COPD comorbidities. Although the ORs are adjusted for common risk factors such as age, sex, level of education, income, and smoking status (current, former, never), these OR estimates tended to be greater than 1, indicating that comorbidities were more common in persons who had COPD than in those who did not. Therefore, our study confirms a strong relationship between COPD and angina, CHD, arthritis, depression, diabetes and hypertension.6,42-44 It also suggests that the prevalence of arthritis and depression were more common among women than among men, while cardiovascular comorbidity and diabetes mellitus were more prevalent in men. The same observation has been reported by others 42,43 which lends support to the concept that women may be more susceptible to arthritis and depression then men.45-47 Di Marco et al reported that the high prevalence of depression in women is more likely due to exposure to poor quality of life caused by severe symptoms.46-48
The variation of these ORs across the ADD and sex after controlling for age, level of education, income and smoking status indicates that there are other determinants of the risk of comorbid diseases among both males and females. It has been reported that systemic inflammation and its mediators may play a major role in the mechanism of these comorbidities49,50 and genetic influence is also important to consider in the variation of the risk of these comorbid diseases across gender. However, the main mechanisms are still unknown. The ORs for risk of depression, diabetes and hypertension were not significant across ADDs nor for either women or men. One explanation of this result is the low number of partcipants with COPD who have these comorbid diseases or the participants may have a mild form of COPD.
COPD comorbidties have a negative impact on patient survival rate, financial burden of COPD, and risk of hospitalizations. 6,51-53 Although ADDs are heterogenous in burden of risk factors for COPD comorbidities, the findings of our study provide a reasonable approach for the evaluation of prevalence and risk differences of COPD comorbidities across ADDs. These significant differences in the state burden of COPD comorbidities and its risk across ADDs and between men and women draw attention to the need for public health officials and physicians to explore the knowledge of COPD comorbidites. They also need to do detailed, gender-specific analyses and intervention prior to the implementmentation of any population-based health services to improve the prognosis and health-related quality of life of individuals with COPD.
To our knowledge, this is the first study to use the population-based KyBRFS data in Kentucky to measure the types of chronic diseases associated with COPD and to investigate its risk variation across ADD by sex. The BRFSS is a unique population-based nationwide surveillance tool. In Kentucky, the surveillance, initiated in 1985, is a collaborative effort of the Kentucky Department for Public Health and the CDC. It is an annual statewide telephone survey of randomly-selected adult Kentuckains to collect data on lifestyle risk factors contributing to the leading causes of death and chronic diseases.54 We used age-standardized prevalence of both COPD and its comorbidities to overcome the effects of age differences on them since age itself is an important risk factor for this disease.
There are several limitations to our study. First, our analysis lacks laboratory testing to confirm the diagnosis of COPD. The diagnosis of COPD and its comorbidities are dependent on self reporting by respondents to KyBRFS. Therefore, the diagnosis of COPD needs to be confirmed by spirometry to validate the estimation of age adjusted COPD and other laboratory tests for diagnosis of associated comorbidities. Second, the small sample size of participants in each ADD limits our analysis in terms of sex-based comparisons. The Pennyrile district was excluded from the model for men because of its small sample size (Figure 3). Third, the study lacks the clinical evaluation of COPD through our survey. This may introduce misclassification bias because some comorbidities present only in participants with the most severe disease. In addition, young individuals (18–44 years) were included in the study to investigate the variability in the prevalence of COPD and risk for comorbidities at a young age. Severe alpha-1 antitrypsin deficiency is an inherited disorder that can lead to the development of severe emphysema at an early age. Misclassification bias was minimized by estimation of age adjusted COPD prevalence because the prevalence of COPD in individuals aged <45 years is low compared to those who aged >65 years. 55,56 Fourth, racial/ethnic characteristics were excluded from the analysis because of the small sample size of some racial/ethnic subgroups. Fifth, smoking statuswascategorized into:former, current or never smoker, which may introduce some degree of misclassification in smoking status, because the study lacks information on smoking duration or intensity.
Conclusion
This study suggests that COPD and its comorbidities are major public health issues in Kentucky. The study suggests that there is an association between angina, CHD, arthritis, depression, diabetes and hypertension and COPD. It also points to a significant impact of gender on the variation in prevalence of both COPD and its comorbidities, and the risk of these comorbidities across ADDs. The presence of these comorbid conditions is more likely to be associated with poor quality of life. Therefore, it important to determine risk factors that may underlie these gender differences in the risk of COPD comorbidities. In addition, designing gender-based programs is important to reduce the COPD comorbidities and to improve the prognosis and health-related quality of life of the patients with COPD.
Acknowledgment
Abdulbaset Kamour takes responsibility for the content of the manuscript, including the data and analysis. David Mannino and Sarojini Kanotra contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Declaration of Interest
Drs. Kamour and Kanotra have no conflicts of interest. Dr. Mannino has received honoraria/consulting fees and served on speaker bureaus for GlaxoSmithKline, Novartis Pharmaceuticals, Pfizer Inc., Boehringer-Ingelheim, AstraZeneca PLC, Forest Laboratories, Inc., Merck, Sunovion, and Amgen. Furthermore, he has received royalties from Up-to-Date and is on the Board of Directors of the COPD Foundation. He has also served as an expert witness on the health effects of tobacco use.