Running Head: Hiatal Hernia and COPD Exacerbation
Funding Support: National Institutes of Health funded COPDGene grants R01 HL089856 and R01 HL089897
Date of Acceptance: January 9, 2016
Abbreviations: gastroesophageal reflux disease, GERD; chronic obstructive pulmonary disease, COPD; hiatal hernia, HH; high-resolution computed tomography, HRCT; COPD Epidemiology study, COPDGene; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; modified Medical Research Council dyspnea scale, mMMC; body mass index, BMI; long-acting muscarinic antagonist, LAMA; long-acting beta-agonist, LABA; inhaled corticosteroids, ICS; short-acting beta-agonist, SABA; short-acting muscarinic antagonist, SAMA
Citation: Kim C, Ouyang W, Dass C, Zhao H, Criner GJ, COPDGene Investigators. Hiatal hernia on chest high-resolution computed tomography and exacerbation rates in COPD individuals. Chronic Obstr Pulm Dis. 2016; 3(2): 570-579. doi: http://doi.org/10.15326/jcopdf.3.2.2015.0158
Background
Patients with advanced chronic obstructive pulmonary disease (COPD) have a higher incidence of gastroesophageal reflux disease (GERD) that can often be asymptomatic and found only with esophageal pH monitoring.1-5 Patients with clinically significant GERD symptoms seem to have more COPD exacerbations and hospitalizations than individuals without these symptoms.6-8 In addition, poor control of GERD symptoms negatively impacts the quality of life in COPD.9 Given the significant morbidity and health care burden associated with acute exacerbations in COPD,10-12 finding a non-invasive marker for gastroesophageal disease could help identify patients prone to acute exacerbations.
The presence of a hiatal hernia (HH) has been identified as a risk factor for developing GERD symptoms through a complex range of mechanisms including anatomic disruption of the lower esophageal sphincter and delayed esophageal-emptying.13-15 HHs are easily identified on non-contrast computed tomography (CT) of the chest as a proximal displacement of the esophagogastric junction through the esophageal hiatus of the diaphragm into the mediastinum.16,17 Noth demonstrated the efficacy of using CT thorax images for identification of HH in patients with pulmonary fibrosis.18 We aim to investigate whether the presence of HHs on routine chest CT performed on COPD individuals can serve as a convenient marker for identification of individuals prone to frequent or severe exacerbations.
Methods
We performed a retrospective data analysis of prospectively collected data from the COPD Genetic Epidemiology (COPDGene) database, a multicenter prospective observational study involving 21 academic centers in the United State and 10,300 individuals. Both current and former cigarette smokers with and without COPD, self-identified as African-American and non-Hispanic white, were recruited. Individuals were 45 to 80 years of age with at least 10 pack years of smoking history. Exclusion criteria were pregnancy, history of other lung disease except asthma, prior lobectomy or lung volume reduction surgery, active cancer undergoing treatment, or known or suspected lung cancer.
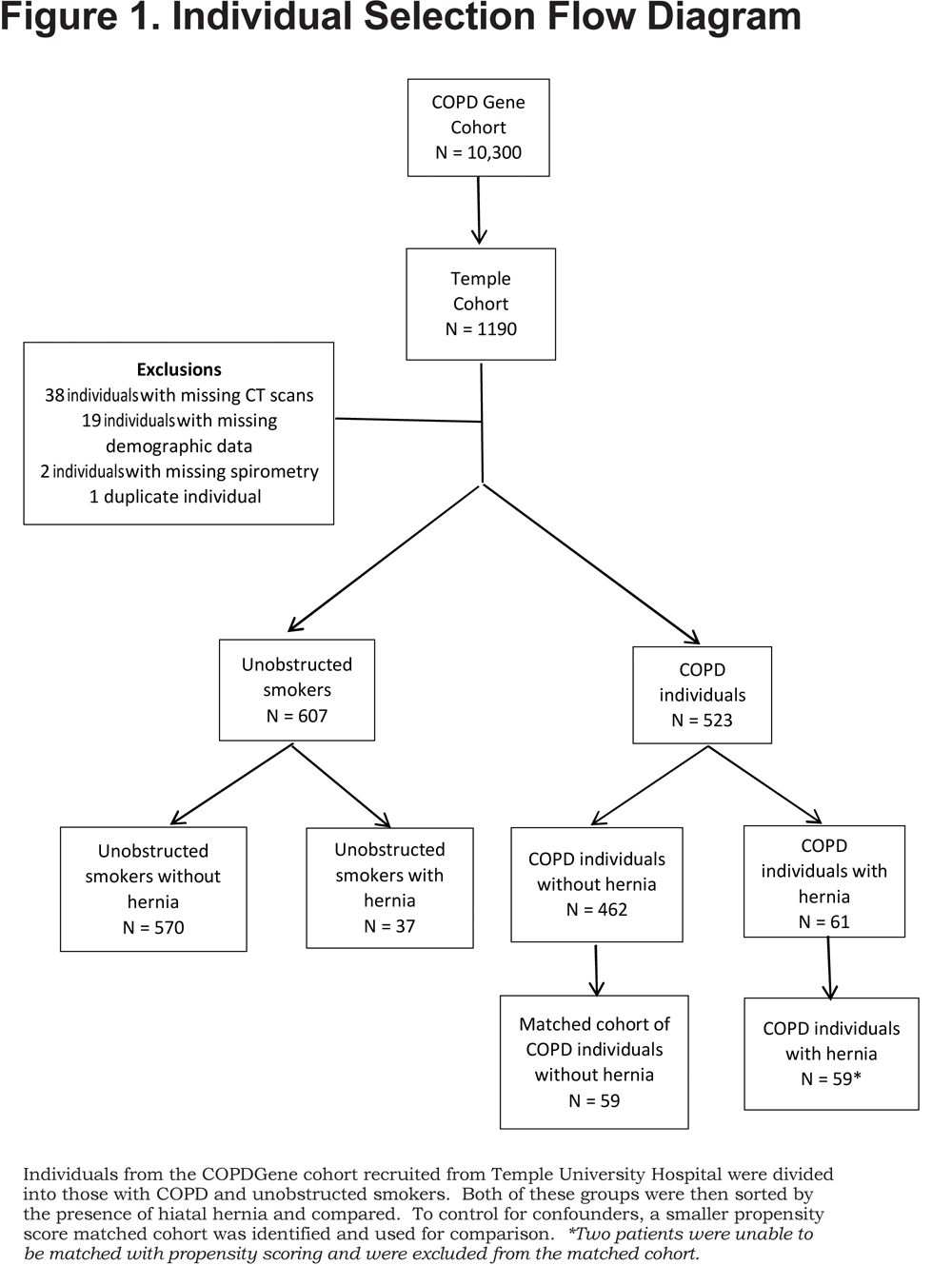
Approval was obtained from the governing body of the COPDGene database as well as the institutional review board of Temple University (protocol #22381). For our study, we used the 1190 individuals recruited from Temple University Hospital with available images for review. Those with incomplete data such as missing CT scans or database information were excluded leaving 1130 individuals who were then categorized by spirometry into those with COPD (forced expiratory volume in 1 second [FEV1] to forced vital capacity [FVC] ratio < 0.7) or smokers without obstruction (Figure 1).
Upon enrollment, individuals were surveyed regarding a number of clinical conditions. Those reporting a history of heartburn, acid reflux, or stomach ulcers were considered positive for GERD. Comorbidities such as angina, congestive heart failure, coronary artery disease, high blood pressure, and high cholesterol, as well as the presence and timing of symptoms such as cough and phlegm production were identified using a modified American Thoracic Society Respiratory Epidemiology questionnaire.19 Exacerbations were defined as any episode 1 year prior to enrollment during which the individual experienced shortness of breath or change in sputum production requiring an increase in medication. Frequent exacerbations were defined as having 2 or more exacerbations per year. Severe exacerbations were defined as those requiring hospitalization. Spirometry data and CT scans were performed prospectively at the time of enrollment. Dyspnea was assessed using the modified Medical Research Council (mMRC) dyspnea scale.
CT scans were acquired in spiral mode using multi-detector scanners (Siemens, Sensation-16 and Sensation-64, Malvern, PA, USA). Images were obtained with breath held on deep inspiration and at the end of normal expiration. The exposure factors were effective mAs of 200 for inspiration, 50 mAs for expiration, and 120 kVp for both. Tube rotation time of 0.5 seconds and pitch of 1.1 were used. Images were reconstructed in the axial plane at 0.75 mm slice thickness, with 0.5 mm interval, using both soft tissue (B31f) and high spatial frequency (B46f) algorithms. Monthly scanning, using a custom COPDGene phantom, provided monitored stability of CT measurements for each scanner. One-millimeter thick coronal and sagittal images were reconstructed.
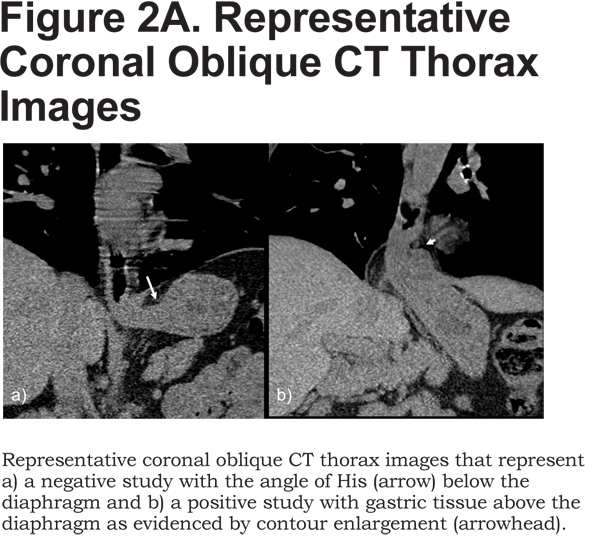
The presence of an HH was determined by visual examination of the inspiratory CT. Since mucosal detail is poorly visualized, extrinsic morphology was used to identify the level of the esophagogastric junction with respect to the esophageal hiatus of the diaphragm. The study was negative for HHs if the angle of His was identified below the diaphragm (Figure 2A).
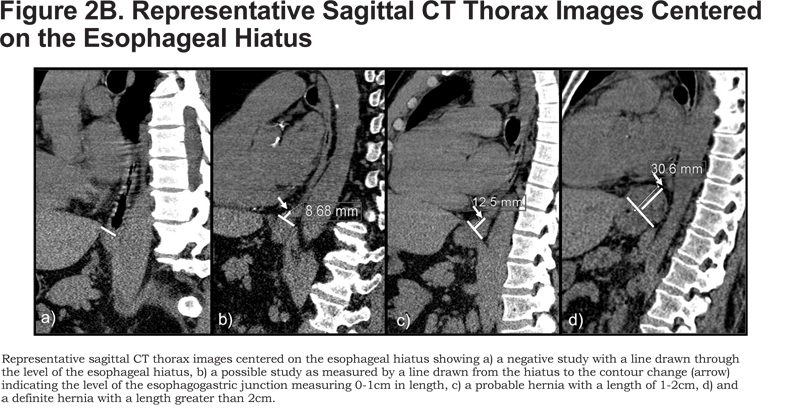
We used CT features classic for herniation of gastric tissue to identify herniation above the esophageal hiatus: abrupt concentric contour enlargement, rugal folds, and focal irregular lobulation. The hernias were then categorized as definite, probable, and possible based on length. First, an oblique line was drawn through the anterior and posterior aspects of the esophageal hiatus on a sagittal image centered on the hiatus. Next, the height was measured by drawing a perpendicular line up to the superior margin of the hernia (Figure 2B). Definite hernias were those longer than 2 cm, probable ones were those between 1 and 2 cm, and possible ones were those between 0 and 1 cm. Since by radiographic convention, an HH is defined as displacement of the esophagogastric junction by more than 1 cm above the hiatus,20 we considered possible hernias negative during data analysis. The maximum transverse diameters of the hernias were obtained on axial images.
Participant data was analyzed by JMP Pro software version 10.0.2d1. Groups with and without hernia (both COPD individuals and unobstructed smokers) were compared with t-test for normally distributed variables and by Wilcoxon rank sum test for all other variables. Unobstructed smokers without hernia were compared to those with hernia to examine if the presence of hiatal hernia alone caused respiratory symptoms in the absence of COPD. Further analysis was done to look at the effect of confounding variables on COPD individuals with an HH compared to those without an HH with respect to exacerbation frequency. Due to the size and distribution disparities between the COPD individuals with and without HHs, propensity scoring was used to create a matched cohort for comparison. The score was calculated using gender, race, age, body mass index (BMI), the presence of GERD symptoms and hyperlipidemia and then used to identify a matched cohort of COPD individuals without HHs comparable to the test population. Two COPD individuals with HHs did not have an identifiable propensity score matched control and were eliminated from that portion of the analysis (Figure 1). COPD individuals with HHs were then compared to the smaller propensity score-matched cohort to determine if any of the previously mentioned variables had an effect on exacerbation rates. Both sets of analyses are reported here. Data were considered significant with a p value < 0.05 and is presented in the form of either a percentage with the associated N value or as mean with standard deviation.
Results
Of the 1130 individuals investigated, 98 (8.6%) probable or definite HHs were identified. Of these, 54 (4.8%) were definite HHs and 45 (3.9%) were probable hernias. The maximum transverse diameter of the hernias ranged from 2.1cm to 10.1cm as measured on axial images. There was a significantly higher incidence of HHs among individuals with COPD as compared to unobstructed smokers (11.6% [61/523] versus 6.1% [37/607], p < 0.001). A total of 6.5% (N=34) of these individuals had a definite hernia.
Description and Exacerbation Rates of COPD Individuals
Of 1130 individuals, 523 had COPD as defined by spirometry with an FEV1/FVC < 0.7. The 61 COPD individuals with hernias (11.6%) were more likely to be female, white, older and have a higher BMI compared to COPD individuals without hernias (Table 1). COPD individuals with a hernia as compared with COPD individuals without a hernia had a similar rate of pack year cigarette use, level of obstruction on spirometry and the prevalence of long-term supplemental oxygen use. The majority of COPD individuals with and without HHs had Global initiative for chronic Obstructive Lung Disease21 (GOLD) Stage II or III disease (p = 0.68).
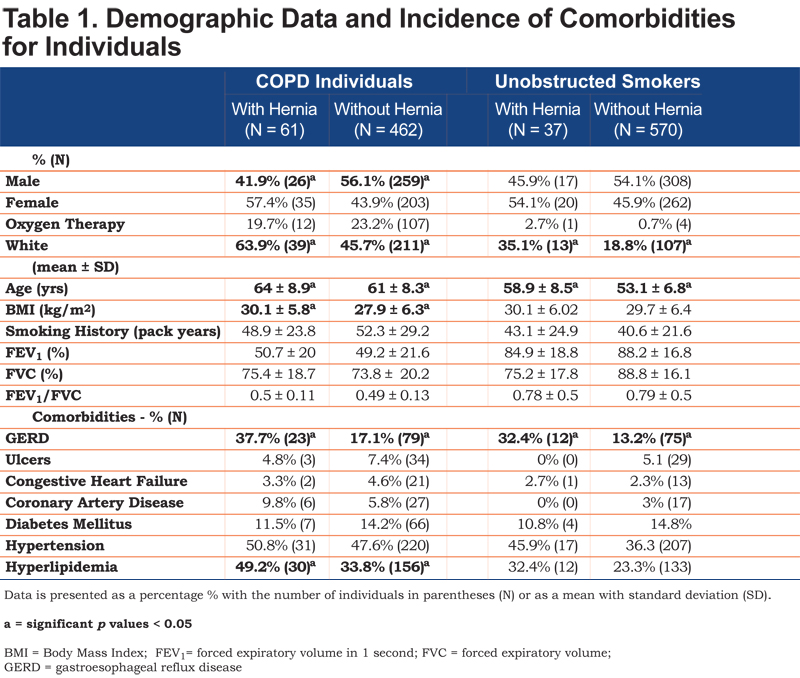
COPD individuals with an HH had significantly more GERD but similar rates of gastric ulcers. The incidence of comorbidities such as congestive heart failure, coronary artery disease, diabetes, and hypertension were equal between COPD individuals with and without HHs. Hyperlipidemia, however, was significantly increased in COPD individuals with an HH (Table 1). There were similar rates of cough, cough productive with phlegm and perception of dyspnea as defined by the mMRC.
There was no significant difference in self-reported exacerbation rates (0.67 ± 1.1 versus 0.83 ± 1.29, p = 0.37), or percentage of individuals experiencing severe exacerbations in the past year (23% versus 29.4%, p = 0.29). The percentage of individuals with frequent exacerbations was slightly lower in the group with hernia as compared with COPD individuals without HHs, but was not significantly different (11.5% versus 17.5%, p = 0.29).
Rates of rescue medications or controller medications use between COPD individuals with and without HHs were similar. COPD individuals with a hernia had an increased rate of long-acting muscarinic antagonists (LAMAs) use (59% versus 49.4%, p = 0.16) but the difference was not significant (Table 2).
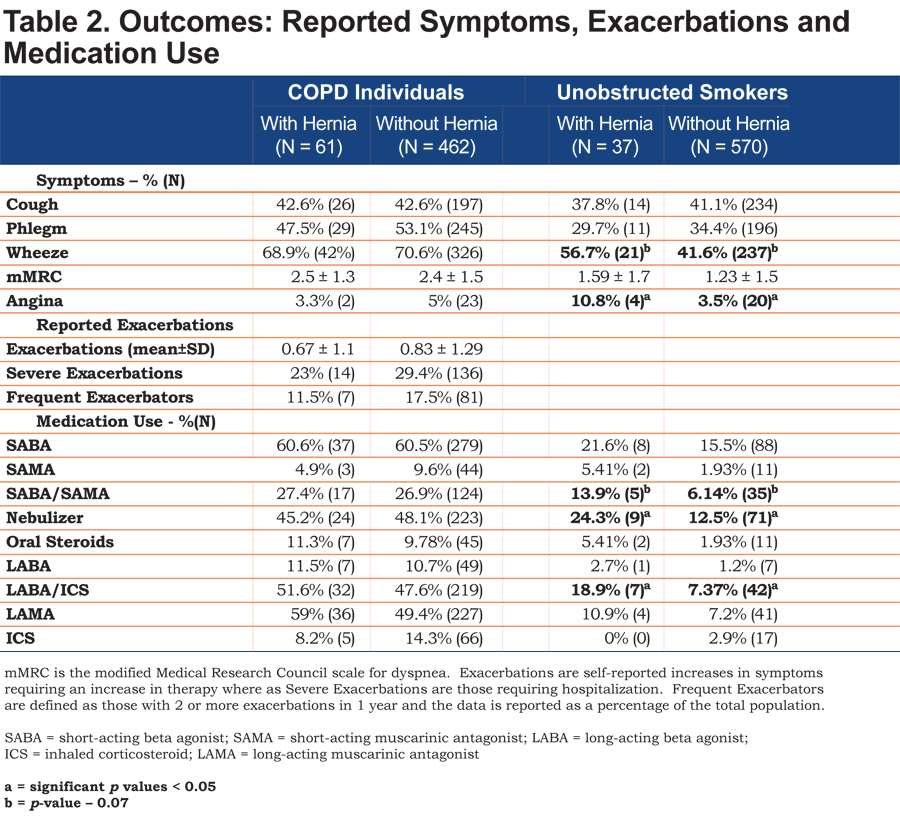
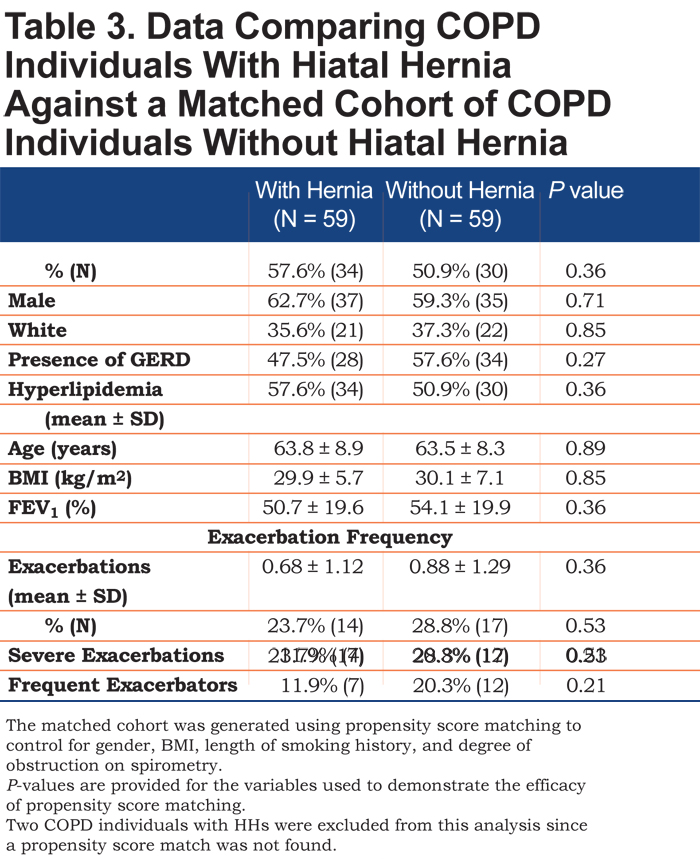
Long-term follow up data was available for 424 of the 523 individuals and showed no significant difference in exacerbations per year (0.8 ± 1.3 versus 0.7 ± 1.3, p = 0.47). There was also no difference in mortality between the 2 groups (8.3% versus 6.7%, p = 0.67). COPD individuals with hernias were matched to a propensity score matched cohort. The score was able to create a matched cohort of COPD individuals without HHs with similar gender, race, and incidence of acid reflux, hyperlipidemia, age, BMI and level of obstruction to that of the HH group to control for the effect of these variables. Repeat analysis between these 2 revealed similar mean exacerbation rates (0.68 ± 1.12 versus 0.88 ± 1.29, p = 0.36) and percentage of individuals with severe exacerbations (23.7% versus 28.8%, p = 0.53). (Table 3).
Description and Exacerbations Rates for Unobstructed Smoking Individuals
Statistically significant clinical differences were found within the 607 smoking individuals without obstruction (FEV1/FVC>0.7). As stated above, there were significantly fewer unobstructed smokers with hernias than COPD individuals with hernias. However, the 2 hernia positive groups shared characteristics of older age, white predominance and increased incidence of GERD. Unobstructed smokers with HHs were similar to unobstructed smokers without HHs in terms of gender, BMI, length of smoking history, and level of obstruction (Table 1).
Unobstructed smokers with hernias were more likely to complain of angina but had similar incidences of coronary artery disease, congestive heart failure, diabetes, hypertension, and hyperlipidemia. Frequency of cough, rates of phlegm production and mMRC were similar between the 2 groups. Increased wheeze in the hernia group trended toward significance. Those with hernia were significantly more likely to have a nebulizer at home and use a long-acting beta-agonist (LABA)/inhaled corticosteroids (ICS) combination. There was a trend towards more short-acting beta-agonists (SABAs)/short-acting muscarinic antagonists (SAMAs) use in the group with HHs that did not reach statistical significance (Table 2).
Discussion
In our study, we found that the presence of HHs on CT imaging does correlate with an older, more obese population with a higher incidence of GERD and that HHs occur significantly more frequently in individuals with COPD as compared with unobstructed smokers. Patients with HHs tend to be older, more obese, and female. This would suggest that though CT scan is not the traditional diagnostic method for hiatal hernias, it is can still be used to find clinically significant cases. However, this visual marker does not correlate with increased COPD exacerbation in our study cohort.
Acute exacerbations of COPD account for millions of inpatient visits and billions of dollars in health care costs each year. 10,22,23 These acute exacerbations are unequally distributed, with some patients exhibiting a frequent exacerbator phenotype, suffering from repetitive and severe exacerbations.24,25 These frequent exacerbators tend to have a greater impairment in health status, an elevated white-blood cell count, and a history of GERD.
Presence of an HH is a structural risk factor for GERD that can be easily visualized on CT imaging. American and European studies report an incidence of 14%-24%,26,27,28 higher than the 10% incidence found in our study population, which is likely related to patient selection and diagnostic method. While more women had HHs in our study, there is no clear gender association found in the literature.13 Traditionally, an HH is diagnosed with endoscopy or barium esophagram.29 However, it is commonly reported on CT scans of the thorax as an incidental finding.16 To our knowledge, there is no standard definition of size or parameters for measurement of HHs on CT scans. In addition to answering the clinical question posed, we devised a systematic method for defining hiatal hernia on CT. Since COPD patients routinely undergo CT scans of the thorax, our aim was to 1) formally define hiatal hernia seen on CT and 2) determine if these findings can be used as a visual marker to identify patients who may be frequent exacerbators, especially given the relationship between GERD and increased COPD exacerbations. We found no relationship between incidence of hiatal hernia and exacerbation rate.
Our understanding of the complex relationship between GERD and pulmonary disease is constantly evolving. Mechanisms involved are thought to include tracheal microaspiration of acid stimulating bronchospasm30 and increased airway hyper-reactivity associated with acid reflux into the lower esophagus.31,32 In our study, 19% of COPD individuals reported GERD symptoms as compared with 14% in unobstructed smokers, consistent with previously cited studies showing a higher incidence of GERD in COPD patients. As with other studies, we did find that GERD incidence increased with older age and obesity.33,34 To control for these risk factors, we used propensity scoring to create a control population that was matched for obesity and age among other factors and found no difference in exacerbation rates.
One interesting and unanticipated finding is the increased nebulizer and LABA/ICS use in unobstructed smokers with HHs that was independent of both dyspnea perception according to the mMRC and the presence of cough or phlegm, lending weight to the idea that acid reflux in the lower esophagus affects pulmonary function. Alternatively, this finding along with the increased rate of angina in the same group may be explained with symptom confusion on the part of the individuals, reflecting symptoms of acid reflux rather than true dyspnea or angina. The significantly increased rate of hyperlipidemia in the hernia group is most likely related to the increased incidence of obesity associated with HHs. Interestingly, other comorbidities such as hypertension and diabetes were not different between the 2 groups.
Weaknesses of our study include reliance on self-reported symptoms and diagnoses such as cough and acute exacerbations of COPD, the transient nature of HHs on imaging, and our relatively small sample size. Reports of acid reflux could not be confirmed with formal acid reflux questionnaires. However, the majority of validated GERD questionnaires use symptom-based questions similar to those in this study35 and guidelines by the American College of Gastroenterology36 support the clinical use of subjective symptoms and response to antacid therapy as a method of GERD diagnosis. Inconsistencies in self-reporting were observed with regards to exacerbation history, with some individuals reporting hospitalizations for shortness of breath in the previous year yet reporting having had no exacerbations. These individuals represented an extremely small minority, but highlight the drawbacks of subjective reporting. In addition, sliding hernias are by nature transient and our images only capture one moment in time. We chose to review inspiratory CT images to match the convention for CT thorax. However, very little is known regarding the behavior of HHs in relation to diaphragm movement during respiration. Further research comparing expiratory and inspiratory CT imaging for changes in the appearance of HHs would help clarify this question. Finally, there were fewer than anticipated HHs identified and as with any small sample size, there is an increased risk of type II error.
Strengths of the study include a large study population with extensively described symptom and medication history along with the availability of imaging for symptom correlation. We used a systematic method to standardize identification and characterization of HHs on HRCT imaging, adding weight to the use of this imaging modality as a diagnostic tool for HHs. In addition to examining the effect of respiration on HHs, future research in this unique cohort could also examine the effect of lung hyperinflation on HHs’ size and motion. Finally, our imaging evaluation of HHs focused on the presence and amount of gastric tissue that has herniated superiorly through the esophageal hiatus. It is possible that the size and integrity of the esophageal hiatus itself has more relevance to symptomatology, and therefore, serves as a better target for characterization with imaging.
In conclusion, the presence of an HH on an inspiratory HRCT scan did not predict increased symptoms or exacerbation rate in COPD individuals. Those with HHs were older, more obese, and more likely female compared to those without HHs. In addition, HRCTs of the chest may be used to identify and characterize HHs and may offer a convenient alternative diagnostic method.
Acknowledgements
The authors wish to thank the investigators of COPDGene for access to their considerable body of data and continued support of this research.
Declaration of Interest
None of the authors of this paper have any real or apparent conflicts of interest to disclose, nor have any financial or consulting relationships to report. The entire manuscript is the original work of the authors and there was no additional input from any agency or freelance writer.