Running Head: High Intensity Non-Invasive Ventilation for Hypercapnic COPD
Funding support: The study was funded by Philips Respironics, USA.
Date of acceptance: August 4, 2015
Abbreviations: high intensity non-invasive positive pressure ventilation, HI-NPPV; partial pressure of carbon dioxide, PaCO2; chronic obstructive pulmonary disease, COPD; forced expiratory volume in 1 second, FEV1; long term oxygen therapy, LTOT; body mass index, BMI; interquartile range, IQR; sleep stage 3, N3; rapid eye movement, REM; Chronic Respiratory Questionaire, SRQ; Calgary Sleep Apnea Quality of Life Index, SAQOL; Severe Respiratory Insufficiency, SRI; non-invasive positive pressure ventilation, NPPV; carbon dioxide, CO2; inspiratory positive airway pressure, IPAP; forced vital capacity, FVC; total lung capacity, TLC; apnea hypopnea index, AHI; arterial blood gas, ABG; maximal inspiratory pressure, PI max; polysomnography, PSG; Global initiative for chronic Obstructive Lung Disease, GOLD; expiratory positive airway pressure, EPAP; inspiratory to expiratory time ratio, I:E; residual volume, RV; bilevel positive airway pressure, BiPAP
Citation: Weir M, Marchetti N, Czysz A, et al. High intensity non-invasive positive pressure ventilation for stable hypercapnic chronic obstructive pulmonary disease patients. Chronic Obstr Pulm Dis. 2015; 2(4): 313-320. doi: http://doi.org/10.15326/jcopdf.2.4.2015.0145
Introduction
Once patients with chronic obstructive pulmonary disease (COPD) develop hypercapnic respiratory failure they are at increased risk for exacerbations, re-hospitalization and mortality.1-4 Evidence to support interventions for the treatment of chronic respiratory failure in COPD, except for the use of long-term oxygen therapy (LTOT), has been lacking.5 Non-invasive positive pressure ventilation (NPPV) is commonly instituted in COPD patients with hypercapnia during hospitalization for acute exacerbations and subsequently continued as an outpatient in a sporadic fashion. However, the evidence to support this intervention is poor with no consistent benefit in survival, need for re-hospitalization, dyspnea, quality of life, respiratory muscle strength, sleep efficiency or 6 minute walk distance.6 Several factors predispose severe COPD patients to chronic respiratory failure; severe airflow obstruction, hyperinflation, imbalances in the respiratory muscle length-tension relationship, malnutrition, chronic systemic steroid use and comorbid conditions.7 Some investigators have suggested that chronic nocturnal NPPV could provide respiratory muscle rest thus enhancing recovery from chronic respiratory muscle fatigue and thereby improving respiratory muscle strength, improving lung function and gas exchange.8 NPPV used nocturnally improves nighttime ventilation and can reduce PaCO2 both at night and during the day.9 An improvement in nocturnal hypoventilation is thought to reset the respiratory center sensitivity for carbon dioxide (CO2).10 It has been theorized that NPPV can improve ventilation/perfusion relationships through recruitment of poorly ventilated lung units although this has not been successfully demonstrated. Prior reports of chronic nocturnal NPPV‘s ability to improve outcomes in COPD patients with chronic respiratory failure have been inconsistent. This has been attributed to problems in prior study design - small numbers of participants, short duration of NPPV, a failure to select individuals with sufficient hypercapnia and inappropriate levels of NPPV that resulted in poor patient tolerance, limited adherence and insufficient levels of ventilation to improve gas exchange or provide respiratory muscle rest.11,12
Recently published studies have shown positive results with high-intensity non-invasive positive pressure ventilation (HI-NPPV),13-15 a different algorithm of NPPV utilizing higher inspiratory positive airway pressure (IPAP) and back up respiratory rates to provide greater ventilator support with an aim to provide more robust ventilation and respiratory muscle resting indicated by normalization of PaCO2. There is a growing body of evidence that HI-NPPV can improve blood gas,16 lung function,15 health-related quality of life17 and now survival.18 However, other groups have not been able to replicate these results.9 Concerns remain that the high inspiratory pressures of HI-NPPV will not be tolerated and could possibly induce worsening hyperinflation in severe COPD patients.
The purpose of this pilot study was to attempt to replicate the positive outcomes of HI-NPPV in stable hypercapnic COPD patients through assessment of daytime PaCO2 levels, tolerance, effect on quality of life measures and polysomnography.
Methods
Study Design
This was a multicenter non-randomized pilot study of stable hypercapnic COPD patients. Due to issues with patient recruitment at some clinical centers, all patients in this report came from Temple University Hospital. The HI-NPPV initiation and outpatient treatment protocol is based on prior work of Windisch et al.15
Patient Selection
Patients were recruited from Temple University outpatient pulmonary clinic if they had a prior diagnosis of COPD, age<80 years, FEV1<50% predicted, FEV1/forced vital capacity (FVC)<70%, total lung capacity (TLC)>90 % predicted, BMI<35 kg/m2 with a daytime PaCO2>50mmHg and symptoms of hypercapnia. Patients with acute hypercapnic respiratory failure were excluded but patients>14 days post hospital discharge were eligible. The patients were recruited over a 24-month period during 2011 and 2012.
Exclusion criteria included FEV1<15% predicted, a prior diagnosis of obstructive sleep apnea (apnea hypopnea index [AHI]>15 per hour), history of pneumothorax, diffuse lung disease other than emphysema, current users of NPPV, chronic corticosteroid use, LTOT>5L’s/min or a significant other medical comorbidity that would interfere with the study requirements.
The study received prior approval from Temple University Hospital institutional review board. After informed consent the participants underwent an initial screening including a medical history, physical exam, spirometry and daytime arterial blood gases (ABG) / SpO2. Participants that met all initial screening criteria underwent further tests including the Epworth Sleepiness Scale, baseline Quality of Life Questionnaires, pulmonary function tests (body plethysmography and diffusion capacity for lung carbon dioxide [DLCO]), maximal inspiratory pressure (PI, max) and a polysomnography study (PSG).
High-Intensity Non Invasive Positive Pressure Ventilation Initiation
All patients received conventional medical therapy for COPD and LTOT as per Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines.19 All patients were hospitalized to initiate HI-NPPV. On the day of admission, a resting arterial blood gas on room air was analyzed, if arterial PaCO2 was > 50 mmHg the patient was eligible for the study. The Respironics BiPAP Synchrony® (Philips-Respironics, USA) device used in the Spontaneous/Timed mode (pressure-limited, time-cycled). The IPAP setting was 16cm H2O with an expiratory positive airway pressure (EPAP) of 4cm H2O. The IPAP was then titrated to a minimum of 20 cm H2O with a goal to increase the IPAP sequentially to the maximum inspiratory pressure tolerated up to 30 cm H2O. In an attempt to establish controlled ventilation, the respiratory rate was gradually increased beyond the spontaneous rate with a targeted back up set respiratory rate of 18-22 breaths/minute. The inspiratory to expiratory time (I:E) ratio was set to individual patient tolerance but no less than 1:2. Arterial blood gas sampling was performed once daily after at least 4 hours of HI-NPPV to determine if hypercapnia was still present, if so the IPAP was further titrated with a goal of achieving optimal alveolar ventilation and normocapnia while maintaining patient comfort. Patients were discharged once normocapnia was achieved or the PaCO2 did not show further improvement after 3 days of HI-NPPV titration. Upon discharge the patients’ goal for adherence was to use the device for at least 6 hours in total during daytime naps and nocturnally.Adherence was assessed with daily phone calls for the first 4 days, then weekly for the first month. Adherence was defined as minimum of 4 hours use per night on 5 out of 7 nights per week. The patients underwent monthly visits to the research center after the first month to ascertain that they were using the device properly. The study staff reviewed the mask and ventilator settings for comfort and recorded any necessary adjustments.
Outcomes
The primary outcome of the study was the change in PaCO2 at 3 months compared to baseline levels. Secondary outcomes included patient tolerance and changes in lung function, maximal inspiratory pressures, 6-minute walk distance, health-related quality of life, sleep efficiency and quality by polysomnography without ventilator assistance at 3 months compared to baseline measures.All adverse events such as exacerbation, pneumothorax or aspiration pneumonia were recorded.
Statistical Analysis
Statistical analysis was performed using JMP PRo 10 and SAS. The data is presented as mean ± standard deviation for normally distributed variables and non-normally distributed data is presented as median and interquartile range (IQR). For the analysis of differences between pre and post treatment a student’s t-test was used and was checked with the Wilcoxon signed rank test. A p< 0.05 was considered statistically significant.
Results
Twenty patients were screened from Temple University Hospital, 9 did not meet inclusion criteria (Figure 1) and 2 dropped out of the study. The remainder of the results will concentrate on the 9 COPD individuals who completed the study.
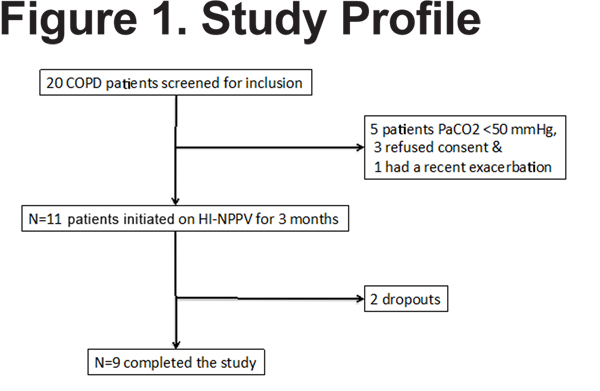
Demographics
The study included 2 males and 7 females. Mean age was 64.4 yrs. (SD ±6.6) all with moderate to severe COPD, mean FEV1 26 % of predicted (SD±6.73), median smoking was 55 pack years (IQR 45 – 90), 8 out of 9 used LTOT and the median BMI was 26.6 kg/m2 (IQR 25.5 – 32.5).
Outcomes
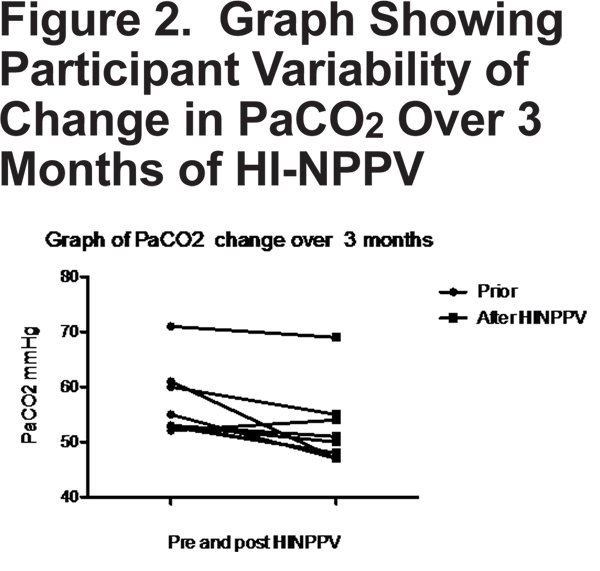
Daytime PaCO2 measured after 3 months of daily nocturnal HI-NPPV compared with baseline measurement showed a mean reduction of 4.66 mmHg (± 4.5 SD)(p=0.01). A graphical representation of individual variability in change in PaCO2 is displayed in Figure 2.
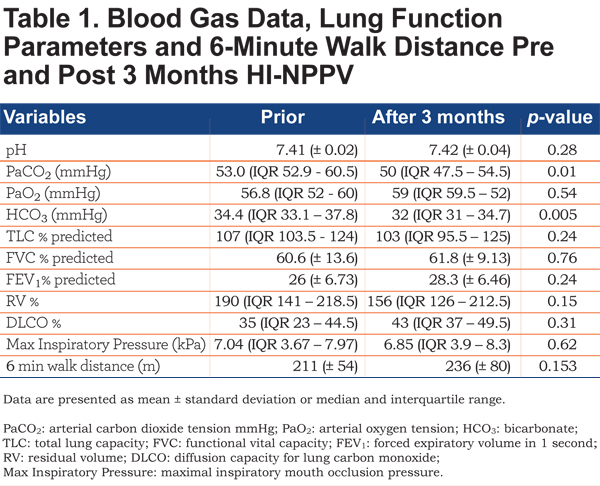
There was also a significant reduction in arterial blood gas bicarbonate level, mean reduction 2.16 mmHg (p=0.005). There were no statistically significant differences in lung function; maximal inspiratory pressures or 6 minute walk distance (Table 1). There was no statistically significant change in residual volume % or FEV1 % surrogate markers of compliance.
Sleep and Quality of Life
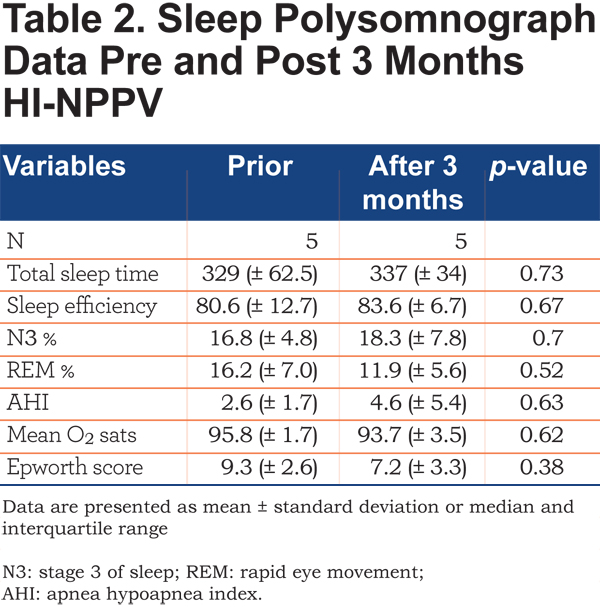
A baseline sleep study in the whole group (n=9) did not indicate the presence of significant sleep apnea, AHI median 2.4 (IQR 1.25 - 3.95). Not all patients completed the repeat polysomnography after treatment. In those that did (n=5) there was no statistically significant difference in terms of sleep duration, efficiency or quality as determined by percentage of N3 or REM (Table 2).
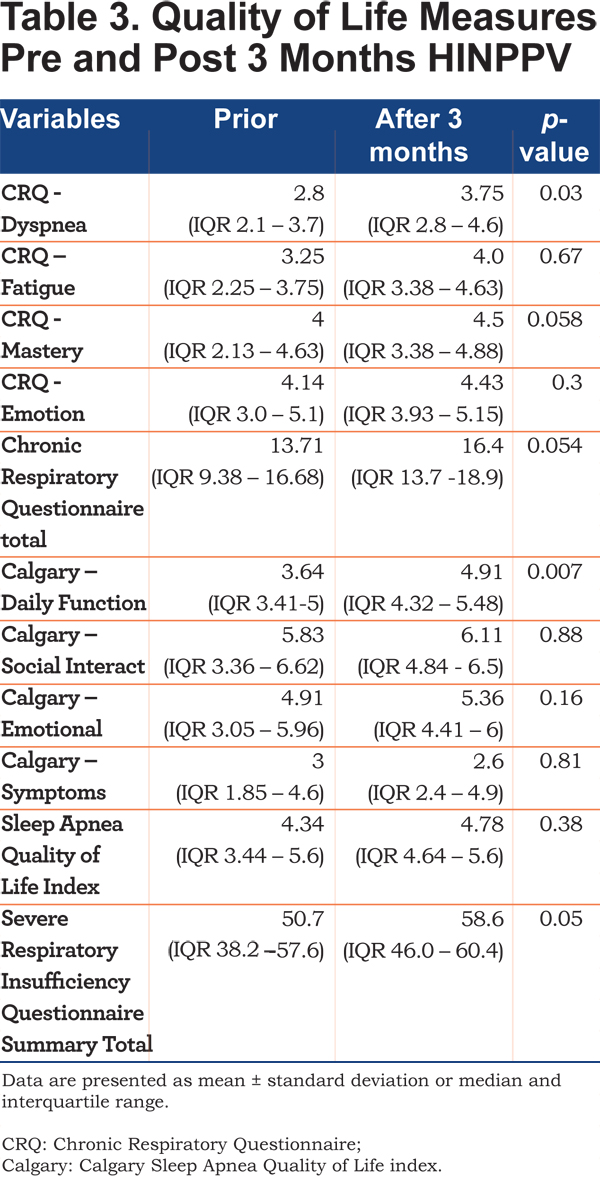
The Chronic Respiratory Questionnaire (CRQ)20 showed a trend to improvement overall with an increase of 2.69 (p=0.054), the dyspnea domain showed a statistically significant improvement (p=0.03). The Calcary SAQLI questionaire21 detected an improvement in domain A – daily functioning (p=0.007). The Severe Respiratory Insufficiency (SRI) Questionnaire22 showed an improvement overall (p=0.05) (Table 3).
HI-NPPV and Adverse Events
The ventilator settings were mean IPAP 27.3 (±4.7) cm H2O and EPAP 4 cm H2O and mean set rate 15.4 (±2.9) /min. Adherence with daily nocturnal HI-NPPV by patient reported use was good in 5/9 patients.6,17,18 Four patients had COPD exacerbations during the follow up period. There were no recorded events of pneumothorax, aspiration or pneumonia.
Discussion
This single center pilot study in stable severe hypercapnic COPD patients shows HI-NPPV has a positive effect on daytime PaCO2, a concomitant effect on HCO3 with improved health-related quality of life similar to previous studies.15,23,24 In patients who were adherent there was a consistent 5 mmHg improvement in PaCO2 compared with non-adherent individuals in whom there was no significant difference. Through a process of admitting the patients for initiation of HI-NPPV and tailoring the settings to the individual tolerance, we were able to achieve a comparable magnitude of improvement in daytime PaCO2 as reported by Dreher et al.24 They report a mean improvement of 6.2 mmHg in daytime PaCO2 with HI-NPPV compared to our 4.66 mmHg. Kohnlein et al18 suggest that it is necessary to reduce PaCO2 by 20% or to less than 48 mmHg (6.5 kPa) to get maximal benefit. We were unable to achieve these treatment goals despite aggressive pressures with mean IPAP 27.3 (±4.7) cm H2O and EPAP 4 cm H2O. We attempted to optimize patient ventilator synchrony by maximizing the I:E ratio; we did not increase EPAP as we had screened the group for sleep apnea and wanted to maximize pressure support. One difference between our study and other published studies is the female predominance in the study group. Overall, the small sample size, patient heterogeneity and adherence issues may explain the variable improvement in daytime PaCO2 and quality of life indices. It has been noted in past studies that with cohorts of COPD patients with severe hypercapnia and PaCO2 > 55 mmHg it is easier to demonstrate positive outcomes; our study had a median PaCO2 of 53 mmHg at recruitment.25 Kohnlein et al in a single study of HI-NPPV in stable hypercapnic COPD patients (PaCO2 > 51 mmHg) demonstrated a beneficial effect on survival at 1 year using similar inspiratory pressures and back up rates; they stressed the importance of targeting marked reduction in hypercapnia of 20% or to less than 48 mmHg. Other groups have been unable to replicate these results. The inability to replicate may reflect patient selection. Struik et al9 in a large multicenter prospective randomized controlled trial recruited patients after acute ventilatory failure episodes. They were unable to demonstrate a difference in time to readmission or mortality with the addition of NPPV. The majority of the control group in this study at 3 months had a PaCO2 < 51 mmHg and hence would not have qualified for the Kohnlein study. This may have provided a control group that would naturally improve without HI-NPPV support leading to a negative result. Our study was not designed to assess survival.
We noted an improvement in health-related quality of life using the SRI questionnaire and the Calgary SAQLI, 2 disease-specific measures of quality of life. The mechanism by which this is achieved is unclear but may reflect a reduction in residual volume. The small change in residual volume did not meet statistical significance but this may reflect population size. We were unable to demonstrate any improvement in lung function parameters or maximal inspiratory muscle pressures in contrast to the findings of Windisch et al.14,15 Additionally, repeat polysomnography at 3 months did not show any difference in sleep efficiency or quality. These polysomnographs were not performed with ventilator assistance, as such cannot be used as evidence that HI-NPPV has no detrimental effect on sleep architecture. Patient comfort with high pressures is often cited as reason not to adequately titrate NIV in COPD. Our patients tolerated the treatment well and 7 of the patients chose to continue the treatment after the study. COPD patients have been shown to tolerate chronic NPPV poorly in comparison to neuromuscular disease patients.26 Acclimatization to NIV in COPD may be slow and patients often increasingly use NIV as their disease progresses to ameliorate daytime symptoms. The mechanism by which PaCO2 is reduced would appear to be through HI-NPPV mediated augmentation of nocturnal ventilation. The beneficial effects of which are thought to include improved respiratory system efficiency, resting of chronically fatigued respiratory muscles and improved respiratory system compliance by reducing nocturnal atelectasis.11 Resetting of chemoreceptor sensitivity for hypercapnia has been shown to increase ventilatory responsiveness to hypercapnia after NIPPV.27
Adherence with HI-NPPV in our study was in line with previous studies.6,18 Two patients dropped out early due to poor tolerance with HI-NPPV, and of the 9 remaining participants, 5 (56%) were adherent with HI-NPPV. This is despite advances in ventilator design, usability and hospitalizing the patients for up to 3 nights to titrate and familiarize the patient with HI-NPPV. This calls into question the utility of HI-NPPV outside of the research setting.
The weaknesses of our study are its small sample size and lack of objective adherence data. The study was initially designed as a multicenter study but due to poor recruitment response at other clinical centers patients were enrolled only from Temple University Hospital. It was not felt to reflect protocol design or HI-NPPV. However, it was designed as a pilot study to assess feasibility and safety of HI-NPPV in our patient group and as result did not require a large sample size.
The strengths of the study were the inpatient titration of HI-NPPV to maximize tolerance and efficacy combined with the extensive characterization of the patient group. This is also the first time HI-NPPV has been studied in the United States and in a predominantly female population.
Conclusions
In our pilot study of HI-NPPV in stable severe COPD with moderately severe resting hypercapnia, use of HI-NPPV demonstrated a significant effect on reducing PaCO2 and improving quality of life measures. During the 3-month period of daily use, tolerance and adherence with HI-NPPV in our participants was similar to that reported in prior studies. Future studies are needed to delineate the optimal candidate for HI-NPPV, the most effective settings to provide optimal ventilation and enhance patient compliance with chronic nocturnal use.
Acknowledgements
The study was supported by Philips Respironics, USA but they were not involved in data collection, interpretation or manuscript preparation. All authors contributed to the study concept and design, the statistical analysis, interpretation, writing, and critical review of the manuscript. All authors read and approved the final manuscript. The authors thank anonymous reviewers for their comments and suggestions.
Declaration of Interest
M. Weir, N. Marchetti, A. Czysz and G. Criner have no conflicts of interest to disclose regarding the content of this article. N. Hill acts in an advisory capacity for ResMed, and Philips Respironics. F. Sciurba acts in an advisory capacity for ResMed. P. Strollo has received grants and consultant fees from Philips Respironics and ResMed.