Running Head: COPD Over/Underdiagnosis and Treatment
Abbreviations: chronic obstructive pulmonary disease, COPD; computed tomography, CT; National Lung Screening Trial, NLST; forced expiratory volume in 1 second, FEV1 ; forced vital capacity, FVC; forced expiratory flow between 25% and 75% of vital capacity, FEF25-75; University of Texas San Antonio, UTSA; electronic medical record, EMR; international classification of diseases, 9th edition, ICD-9; web-based interactive professional education, WipeCOPD™; quality improvement, QI; information technology, IT; plan do study act, PDSA; Global initiative for chronic Obstructive Lung Disease, GOLD; Body mass index-airflow Obstruction-Dyspnea-Exercise capacity, BODE index
Citation: Berry CE, Yawn BP. COPD overdiagnosis, underdiagnosis, and treatment. Chronic Obstr Pulm Dis. 2016; 3(1): 491-497. doi: http://doi.org/10.15326/jcopdf.3.1.2015.0172
Introduction
Spirometry is an essential tool in establishing the diagnosis of chronic obstructive pulmonary disease (COPD). In spite of its critical role in COPD diagnosis, its implementation in primary care practice has been limited. The role of spirometry in primary care clinics and factors influencing its implementation were discussed recently at the COPD9USA conference in Chicago, Illinois.
Spirometry Isn’t for Screening – So What Is?
Spirometry screening for chronic obstructive pulmonary disease (COPD) has been reviewed by both the United States Preventive Services Task Force and the United Kingdom National Screening Committee, and both organizations recommended against spirometry-based screening.1,2
In general, the benefits of screening studies are often dramatically overestimated by patients,3,4 which may be due in part to the strong messages that health care advocacy groups send about the importance of screening. But in fact, the benefits of smoking cessation far outweigh the benefits of screening for diseases caused by smoking. For example, in the National Lung Screening Trial (NLST), 3.2 lives were saved per 1000 screen computed tomography (CT) studies.5 This is in contrast to 60 lives saved for every 1000 patients treated for smoking cessation. Given that smoking cessation is 20 times more effective at saving lives than CT screening for lung cancer, health care providers should consider placing more emphasis on smoking cessation during their patient visits than screening for smoking-related malignancies.
It is also important to note that the first rule of screening is to do no harm, as all events that occur as a result of screening an asymptomatic individual are considered iatrogenic and preventable.6,7 Therefore, higher medical and ethical standards are necessary when we employ diagnostic tests for screening of asymptomatic individuals compared to their use in the evaluation of symptomatic patients. In the context of lung cancer screening, the potential for harm is relatively high, as 16 iatrogenic deaths occurred in the screening arm of NLST.5 When compared with the impact of smoking cessation, small reductions in smoking would completely offset any potential benefit of lung cancer screening. And of course, screening studies are performed on apparently healthy individuals who may even prefer to be left alone. Therefore, decision making about whether or not to perform screening should be shared between the patient and provider.
Although screening for COPD and other diseases is conceptually attractive, one must acknowledge the risks including physical harm to the patient, but perhaps even more importantly providers should understand the excess harms that may occur if they focus on screening rather than smoking cessation. The limited data that may support screening spirometry as part of a smoking cessation strategy suggests that performance of spirometry alone, regardless of the presence of airflow limitation, can positively influence quit rates.8
Screening for COPD in asymptomatic individuals is not recommended because it will not change practice. Providers should instead focus on risk factor modification, especially smoking cessation, and identifying patients with chronic respiratory symptoms.
Diagnosis of COPD in a Primary Care Midwest Practice
COPD is often described as a clinical diagnosis because there is no histopathologic or biochemical abnormality that can readily identify chronic airflow limitation, the defining hallmark of COPD. However, conceptualizing COPD as a clinical diagnosis can be misleading because post-bronchodilator spirometry is required to confirm the presence of chronic airflow limitation.9
Office spirometry is therefore routinely performed to assess individuals with respiratory symptoms and/or risk factors for COPD. Given the importance of spirometry in establishing the diagnosis of COPD, attention to factors influencing utilization, technical accuracy and reliability, and interpretation is essential. A large primary care Midwest practice recently completed a review of medical records including spirometry reports to evaluate their performance in this regard.
Across practice sites (n=12), a total of 2285 records were reviewed, and 827 cases of newly diagnosed COPD were identified between January 2010 and October 2014. Only 512 (62%) of these patients had spirometry performed to confirm the COPD diagnosis. There was evidence that spirometry utilization increased over time, with 54% of new cases being confirmed by spirometry between 2010-2012 compared to 73% of cases in 2013-2014 (p<0.01).
In order to examine the quality of spirometry and its interpretation, investigators reviewed a representative sample of 98 records of newly diagnosed COPD patients, including detailed review of the spirometry testing and interpretation. Review showed that 29 records (29.6%) included spirometry results demonstrating forced expiratory volume in 1 second (FEV1) / forced vital capacity (FVC) >0.7, with at least half of these reports including interpretations that lung function was normal. Seven reports (7.1%) demonstrated such poor quality spirometry that they were not interpretable, and none of these showed that the spirometry software automated quality metrics were utilized. For 31 records (31.6%), the automated interpretations were inaccurate, at times indicating restriction when there was actually evidence of obstruction and occasionally diagnosing obstruction based on forced expiratory flow between 25% and 75% of vital capacity (FEF25-75) rather than FEV1/FVC.
The investigators found that many providers were not using available features of the office spirometer, including automated quality metrics and data fields for smoking history and respiratory symptoms. There was evidence that providers relied heavily on automated interpretations, which were frequently inaccurate compared with established COPD guidelines for establishing the diagnosis. Recommendations for improving clinical practice based on these study findings include (1) perform spirometry to confirm all cases of COPD, (2) utilize automated quality indicators to assess spirometry adherence to American Thoracic Society standards for acceptability and reproducibility, and (3) turn off the automated interpretation provided by spirometry software which is frequently inaccurate and misleading.
What Happens in Primary Care Without Screening?
Spirometry is required for the diagnosis of COPD. However, many patients are treated for COPD in primary care clinics without ever undergoing spirometry to confirm the diagnosis. Recently a program was implemented at the University of Texas San Antonio (UTSA) ambulatory clinics to assess why spirometry was not being performed routinely and to determine if an interactive program that employs web-based provider education, staff training, and integration of best practices reminders into the electronic medical record (EMR) influences spirometry utilization rates.
At the start of this project, medical record review demonstrated that the prevalence of COPD among UTSA-based clinics was 3.8%, which is lower than reported estimates of both the statewide prevalence and national prevalence of COPD. Moreover, only 19% of patients who had an international classification of diseases, 9th edition (ICD-9) code diagnosis of COPD had spirometry performed within the past 16 years.
In response to these findings, the WipeCOPD™ (web-based interactive professional education) program was developed using input from all members of the ambulatory health care team, including patients, receptionists, nurses, providers, and other clinical staff members to serve as a continuing education resource for a larger quality improvement (QI) project. The overarching goal of this project was to apply interventions to change the behavior of health care professionals and their clinical care of COPD patients using continuing education and the plan, do, study, act (PDSA) approach. . Providers with both the highest and lowest spirometry utilization rates were interviewed in order to understand both what worked well and what were perceived barriers to spirometry use for the confirmation of COPD diagnosis, and their feedback was incorporated into the program.
The specific educational objectives for learners participating in the QI project included: (1) to improve confidence and knowledge in the diagnosis and management of patients with COPD, (2) to identify more patients with COPD in the primary care clinic, and (3) to demonstrate improved performance in ordering spirometry for patients with ICD-9 codes for COPD to confirm the diagnosis. Over 5 months, primary care providers completed WipeCOPD™ online education and participated in 5 monthly 30-minute live sessions with COPD content experts and local clinic champions who reinforced the online program and specifically addressed issues related to nihilism in the diagnosis of COPD. Clinic staff also completed WipeCOPD™ online modules and participated in weekly live spirometry performance training sessions. In addition, information technology (IT)-based reminders of COPD best practices were incorporated into the EMR. For example, providers caring for patients with an ICD-9 code for COPD who did not have a spirometry order received an electronic reminder. After spirometry was performed, Global initiative for chronic Obstructive Lung Disease (GOLD) grades were automatically assigned by the EMR, and best practices for the given GOLD grade were recommended to the providers. In the 5 months after initiation of this QI program, all clinic staff trained to perform spirometry achieved ≥80% competency based on a standardized checklist. The prevalence of COPD based on ICD-9 codes among UTSA-based clinic patients increased to 4.4%. Most importantly, among the patients diagnosed with COPD, spirometry was performed in 56% of cases to confirm the diagnosis, demonstrating dramatic change in provider practice compared to historical data. These findings suggest COPD screening programs are not necessary in primary care clinics when providers and staff are appropriately educated and EMR-based tools are employed to promote best practices for COPD care. Similar projects modeled after this successful UTSA program are now being implemented in other health care practices using QI tools, IT reminders, and interprofessional continuing education programs.
From Screening to Diagnosis to Management in a Busy Primary Care Practice
The average community-based primary care practitioner is quite busy on any given day, with provider time and attention divided by multiple face-to-face patient visits mixed with dozens of phone and email messages, prescription refill requests, laboratory results, diagnostic testing reports, and consultation letters.10 This is compounded by the fact that patient visits are often short (average 10-20 minutes) in spite of much to do, including addressing health behaviors, multi-morbidity, mental health issues, and care coordination. As a result, there is often little time left to consider the diagnosis or management of COPD,11 including the performance and interpretation of office spirometry.
Spirometry is essential to the diagnosis of COPD, as typical symptoms such as dyspnea commonly occur in other clinical conditions (e.g., heart failure, deconditioning, and obesity). Moreover, physical examination does not offer reliable indicators of COPD. And without proper diagnosis, practitioners may prescribe costly medications that offer no potential benefit in the absence of obstructive lung disease. Spirometry is therefore incredibly important and yet remains underutilized, which may be multifactorial resulting from lack of spirometer access, workflow constraints, competing priorities, absence of staff with proper spirometry training, lack of provider knowledge regarding COPD guidelines, and/or lack of provider confidence in spirometry interpretation.
Several studies have examined strategies to introduce spirometry or improve its utilization in primary care practice.12-14 These have included provision of open access mobile spirometry, visiting trained nurses, and on-site staff education and hands-on training. In general, spirometry utilization improved with these interventions, leading to more accurate COPD diagnoses and changes in medications. It should be noted that in some cases, spirometry findings were not concordant with the physician diagnosis, even after the results were available to the provider. In spite of initial success with these interventions, “dusty spirometers” were common among primary care practices and the improvements in spirometry utilization were not sustainable over time due to multiple factors such as staff turnover, lack of comfort in performing the procedure as a result of low volume, or concern about equipment maintenance.
To better understand why spirometry is underutilized, focus groups were conducted that consisted of internists from a single academic institution.15 In general, the participating physicians reported that they did not routinely obtain spirometry to confirm a diagnosis of COPD if a patient came to them with a prior diagnosis that was already being treated with respiratory medications. For those patients with a suspected diagnosis of COPD, the physicians indicated that they did not believe spirometry was always necessary to make the diagnosis or that spirometry-driven diagnosis would impact on patient care. They also described that prioritization of COPD during a patient visit was generally low unless the patient reported respiratory symptoms or needed medication refills, and the lack of a simple and routine point of care tool (e.g., blood pressure measurement or finger stick glucose) also affected this. The physicians did not feel that there were any patient or health system barriers to utilizing spirometry beyond those typical of other diagnostic tests. These focus group findings were then confirmed as being generalizable beyond the academic setting using a 75-question survey sent to a random sample of 1000 practicing community internists in Cook County, Illinois (response rate 30%).
Increases in spirometry utilization in primary care practices may occur with simple interventions but these have not proven to yield sustainable improvement over time. Studies of novel yet practical approaches to integrating spirometry and other tools for COPD diagnosis and management into busy primary care practices are currently underway. Nevertheless, the importance of continuing provider education emphasizing that spirometry is essential for COPD diagnosis should not be overlooked.
Practical Considerations of How Phenotype and Genotype Can Affect Management Decisions
Two cases are presented to demonstrate important points that warrant our attention and should promote our optimism with respect to COPD treatment.
Case #1:
Mr. C is a 69 year old male who worked as a roofer prior to his retirement. He has experienced exertional dyspnea over the past 7 years and is frustrated that this affects his ability to play with his grandchildren. He has hypertension, which is treated with hydrochlorothiazide and lisinopril, and he is a former smoker (1.5 packs per day x 40 years). He has never had a COPD exacerbation. He is currently using albuterol about 6-8 times per day for relief of dyspnea. His physical exam findings are notable for decreased breath sounds. Spirometry is performed, which demonstrates a post-bronchodilator FEV1/FVC ratio of 0.51 and FEV1 1.91 L (51% predicted) (GOLD grade B). He also has a 6-minute walk distance and modified Medical Research Council dyspnea score measured which establish his Body mass index-airflow Obstruction-Dyspnea-Exercise capacity (BODE) score as 2. He is prescribed tiotropium at the time of his visit. At his follow-up visit 1 year later, repeat spirometry is performed which shows his post-bronchodilator FEV1 is improved from 1.91 to 3.60 L. His FVC also improved dramatically from 3.71 to 4.92 L. His BODE score at the follow-up visit is 1.
This case demonstrates that treatment with a long-acting bronchodilator can result in dramatic improvement in lung function and symptoms in many patients, in contrast to the myths that COPD pharmacotherapy is relatively ineffective and COPD patients cannot significantly improve. For example, in the Understanding Potential Long-term Impacts on Function with Tiotropium-UPLIFT study, 53% of patients demonstrated 200-300 mL improvement after bronchodilator administration.16 (see Figure 1).
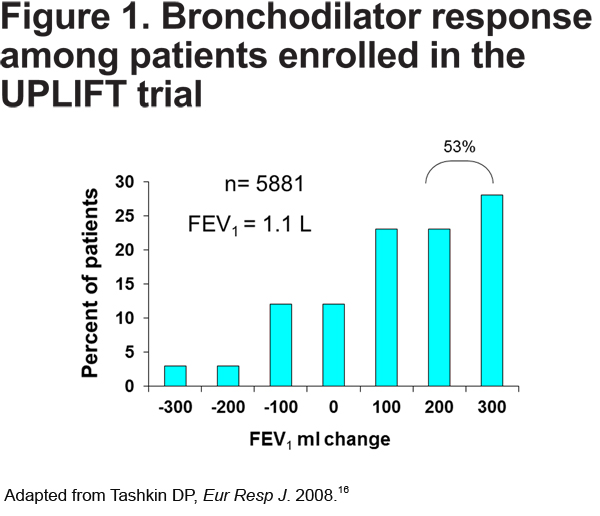
In clinical practice, serial spirometry may be underutilized to assess objective response to pharmacotherapy. Objective assessment is important in individuals with significant airflow limitation who may not experience dramatic change in symptoms in spite of improvement in lung function. It should also be noted that this patient experienced significant improvement in FVC as a result of decreased air trapping after long-term treatment with tiotropium. This observation is particularly common among those individuals with severe and very severe COPD who may not have substantial change in their FEV1 but may have more remarkable increases in their FVC in response to long-acting bronchodilator therapy.16 Thus, lack of improvement in FEV1 alone should not discourage providers from continuing long-acting bronchodilator therapy.
Case #2:
Mrs. K is a 44 year old woman who worked as a secretary until she became disabled after a COPD exacerbation 3 months ago. She is a mother of 2 children. Her alpha-1 antitrypsin level is 79 mg/dL and she was smoking cigarettes ½ pack per day until her most recent exacerbation. On presentation in the clinic, she is using accessory muscles to breathe and has high-pitched wheezes diffusely on auscultation of her chest. Post-bronchodilator spirometry demonstrates an FEV1 of 0.51 L (17% predicted). Her GOLD grade is D and her BODE score is 8. Upon review of her prior spirometry, it is clear she has experienced rapid decline in FEV1 over the past 4 years (FEV1 1.94 L in 2008, 1.01 L in 2010, and 0.51 L in 2012), but she did not receive aggressive treatment for her COPD until her most recent exacerbation. She is immediately referred for lung transplant evaluation.
This case describes an example of a COPD patient with rapid decline in FEV1. Until recent years, rapid decline in lung function was thought to be a universal feature of COPD pathogenesis. However, we now know that many patients with COPD do not demonstrate decline in FEV1 that is any different from that of the general population with aging, suggesting rapid decliners may represent a distinct COPD phenotype. Several studies have now shown that rapid decline is not an essential characteristic of COPD17-19 suggesting it is important to distinguish among individuals who have early COPD versus mild COPD. Patients with early COPD that is rapidly progressive may be challenging to identify unless serial spirometry is performed, as symptoms may not be a reliable indicator of disease progression given COPD patients will commonly modify their activities and reduce exertion to minimize dyspnea. This is in contrast to patients with mild COPD who may demonstrate little decline in FEV1 over time. Early recognition of COPD that is rapidly progressive allows for appropriate intervention including aggressive smoking cessation counseling and therapy, pulmonary rehabilitation referral, and prescription of pharmacotherapy to minimize symptoms, increase exercise tolerance, and maximize preservation of exercise capacity and physical activity as much as possible to prevent deconditioning.
Overall, these clinical cases demonstrate that COPD is indeed treatable and that spirometry is important in assessing objective response to therapy as well as progression of disease over time. COPD phenotypes such as rapid lung function decline are important to recognize in order to optimize care, and serial spirometry permits identification of these patients early in their disease course.
Summary
Screening spirometry is not currently recommended to identify COPD, but this should not be translated to undermine the importance of spirometry to confirm the diagnosis of COPD in suspected cases. In general, office spirometry is underutilized in primary care, but interventions focused on provider and staff education and training along with automated EMR reminders of best practices have demonstrated success in improving spirometry utilization. Although physicians encounter many barriers to spirometry use in their daily practice, it is important to recognize that spirometry is a useful tool for purposes beyond confirming COPD diagnosis. Spirometry also allows for objective assessment of response to pharmacotherapy and permits identification of individuals with early COPD who have progressive disease warranting aggressive intervention.
Declaration of Interest
Dr. Au has received funding from the National Heart Lung Blood Institute, Department of Veterans Affairs, and the Centers for Disease Control and Prevention. He has received travel funds and honoraria from Boehringer Ingelheim. He also reports associations with the Society of Hospital Medicine and the American Board of Internal Medicine examination writing committee. Dr. B Yawn has received funding for COPD screening studies from National Heart Lung and Blood Institute and Boehringer Ingelheim. She serves on COPD advisory boards for Aztra Zeneca, Novartis, GlaxoSmithKline, Boehringer Ingelheim, Pfizer, Merck and Pulmone. Dr. Adams has received funding from the National Institutes of Health, Department of Veterans Affairs, the University of Texas System, Chest Foundation, Astra Zeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis. She is also the president of the WipeDiseases Foundation. Dr. Joo sits on the GRIFOLS Primary Care Advisory Board for Alpha-1 Antitrypsin Deficiency. Dr. Celli has received grants from Astra Zeneca, GlaxoSmithKline, Boehringer Ingelheim, Almirall, Novartis, Forest, and Aeris. He also sits on the advisory boards for GlaxoSmithKline, Boehringer Ingelheim, Dey, Altana, Astra Zeneca, Almirall, Sepracor, Pfizer, Rox, and Novartis.
Click here to take the post-test for the activity. Please note that when you click here, you will be redirected to the website of our accredited provider, University of Nebraska Medical Center, Center for Continuing Education (UNMC CCE). On the UNMC CCE website, you will be required to register so that you may receive your CME certificate. Your registration information will be shared with the COPD Foundation and you will also receive free quarterly subscriptions to our publications which you can unsubscribe from at any time.
Thank you.