Running Head: Patient-Reported Consequences of CAP in COPD Patients
Funding Support: This study was sponsored by Pfizer.
Date of Acceptance: September 18, 2018
Abbreviations: community-acquired pneumonia, CAP; chronic obstructive pulmonary disease, COPD; community-acquired pneumonia burden of illness questionnaire, CAP-BIQ; COPD Patient-Powered Research Network, COPD PPRN; Patient-Centered Outcomes Research Institute, PCORI; COPD Assessment Test, CAT; standard deviation, SD; Behavioral Risk Factor Surveillance System, BRFSS; Centers for Disease Control and Prevention, CDC
Citation: Pasquale CB, Vietri J, Choate R, et al. Patient-reported consequences of community-acquired pneumonia in patients with chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2019; 6(2): 132-144. doi: http://doi.org/10.15326/jcopdf.6.2.2018.0144
Online Supplemental Material: Read Online Supplemental Material (526KB)
Introduction
Community-acquired pneumonia (CAP) is a significant cause of morbidity and mortality affecting more than 5 million U.S. adults and resulting in $8 billion in hospitalization costs annually.1-6 The 18-25 million U.S. adults with chronic obstructive pulmonary disease ([COPD]—including chronic bronchitis and emphysema)7 have a higher risk of developing CAP with incidence rates 6 to 8 times that of healthy individuals in the same age group.2-4,8-10
CAP carries an increased risk of morbidity, mortality and economic burden for individuals with COPD compared to the general population.11-14 In addition, CAP greatly increases the likelihood of a COPD exacerbation15 which results in major disruptions to patients’ and families’ lives with 587,000 hospitalizations and 1.86 million emergency department visits each year.6 An exacerbation may begin a downward spiral from which the individual may only slowly or never fully recover.16-18
To date, much of the CAP literature focuses on the burden and recovery from CAP in the general population, with only a few publications addressing the patient-reported burden and impact of CAP.5,19-22 Even less data is available on the impact on individuals with COPD.
In addition to respiratory symptoms, CAP is associated with generalized symptoms such as fatigue, weakness, balance problems, confusion or trouble thinking and sleep disturbances.19,20,22 Many of these symptoms are more common and may take longer to resolve in older adults and those with chronic disease comorbidities such as COPD.13,23-25 Such data are rarely available from health records, therefore, a full understanding of the burden of CAP requires use of direct patient reporting among those recently diagnosed with CAP. Patient-reported outcome data may provide the insights needed to develop policies, guidelines and prevention strategies for COPD patients who are at increased risk of developing CAP. The objectives of this study were to: (1) assess the symptom burden of CAP; (2) report time to symptom recovery; and (3) assess the impact of CAP on daily activities from the COPD patient perspective.
Methods
Study Design
This survey study included 2 surveys. A baseline survey was completed within 120 days of the onset of self-reported CAP which was defined as being told by a health care provider (e.g., doctor, nurse, etc.) they had pneumonia. The follow-up survey was requested 30 days later. Both surveys queried about CAP-related symptoms and impact of CAP on work and daily life. Participants were a convenience sample of adults 18 years of age and older who reported they had been diagnosed with COPD, emphysema or chronic bronchitis. The study was approved by a central institutional review board, and all participants provided informed consent prior to their participation. Multiple resources of the COPD Foundation were used to recruit potential participants (See Supplemental Table 1 in the online supplement). These included Facebook postings, posts on the COPD Foundation’s COPD360Social online network, and outreach to existing research participants in the COPD Patient-Powered Research Network (COPD PPRN), a secure online interactive patient registry maintained by the COPD Foundation and funded by the Patient-Centered Outcomes Research Institute (PCORI). Individuals interested in participating were directed to the study portal within the COPD PPRN platform and invited to complete a screening questionnaire to self-report diagnosed COPD and recent episodes of pneumonia. (See Supplemental Table 2 in the online supplement). If eligible (reported having COPD [chronic obstructive pulmonary disease, emphysema or chronic bronchitis] and being diagnosed with pneumonia within the previous 120 days), they were asked to provide eConsent. Consented participants were then directed to a baseline survey to complete at enrollment, with the follow-up survey available 30 days after completion of the baseline data. Study participants were reimbursed $10 after completing each of the 2 surveys.
Measures
The baseline survey collected basic demographic information including age, gender, highest education attained, income, employment, insurance status, and living arrangements. Self-reported presence of other chronic diseases including diabetes, hypertension, asthma and heart disease was also queried. All were asked to complete the COPD Assessment Test (CAT) which provides information on the respiratory and general symptom burden associated with COPD.26 Information on the burden of CAP was collected using a modified version of the CAP-Burden of Illness Questionnaire (CAP-BIQ), which is a content-validated patient-reported outcome measure.19,20 The CAP-BIQ consists of questions developed through a qualitative process including concept elicitation interviews and cognitive debriefing, and includes items on symptoms, comorbid conditions, as well as other impacts of CAP on daily life and activities. Participants were also asked if they had received a chest radiograph or computerized tomography to diagnose or confirm their CAP. The follow-up survey focused on CAP symptoms that were reported to be unresolved at the time of the baseline survey completion. A COPD patient stakeholder reviewed all recruitment and study materials to ensure their readability, clarity, and to assess participant burden and relevance to the COPD community.
Data Collection & Follow-up
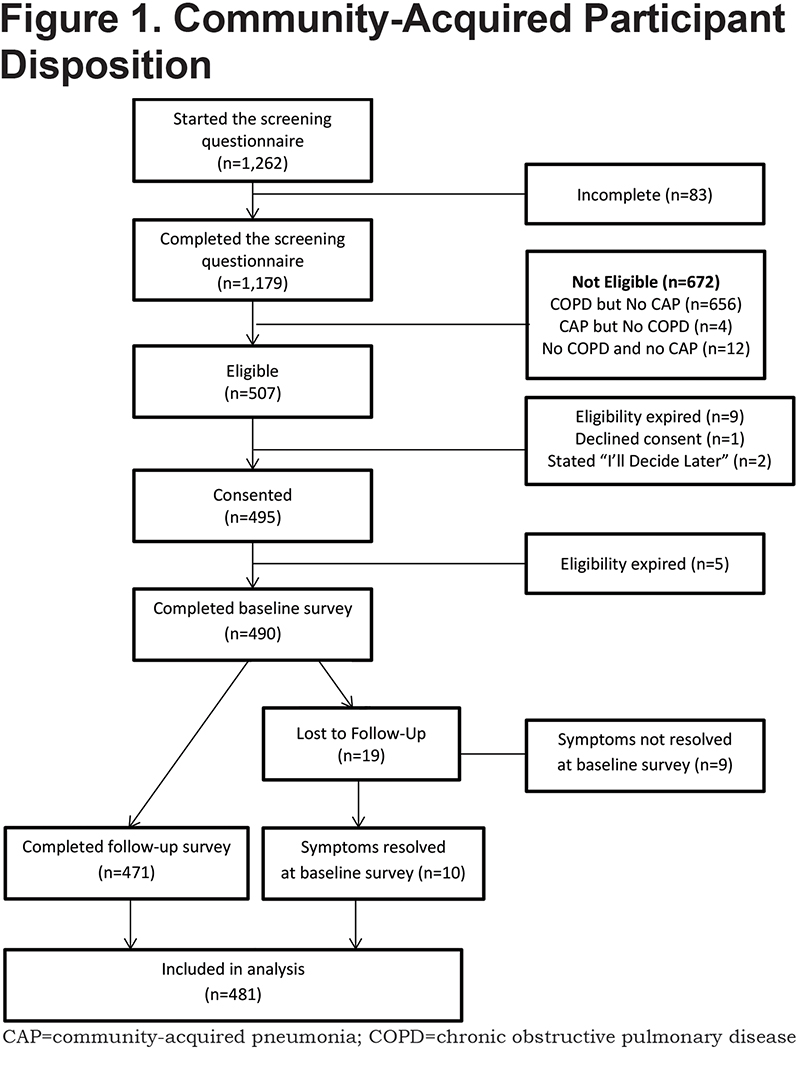
All information was collected online through the secure COPD PPRN dashboard assigned to the participant at the time of providing eConsent. If a consented participant did not complete the baseline survey immediately, they received email reminders on days 1, 2, and 7 after providing consent. On or shortly after day 8, participants who had not responded but provided a phone number were called by a representative of the C.O.P.D. Information Line, a service of the COPD Foundation which provides peer-to-peer information and referral for COPD patients and caregivers. No surveys were administered by telephone. If the participant had not completed the survey on the 30th day after providing consent, a final reminder email to complete the baseline survey was sent. Participants were notified of the availability of the follow-up survey 30 days after the completion of the baseline, and when necessary were reminded via email on days 31 and 37. For those who had still not responded, they were telephoned on the 8th day the follow-up survey was available, and a final email reminder was sent 60 days after completion of the baseline survey. Participant’s eligibility to complete the baseline survey was terminated at 120 days from the reported date of CAP diagnosis. (See Figure 1.)
Statistical Analysis
Analyses were completed using data from all participants who either submitted both the baseline and the follow-up surveys or reported that their CAP symptoms had resolved at the time of the baseline survey.
Descriptive statistics were computed for the participants’ baseline characteristics, including demographics, baseline CAT score and prevalence of CAP symptoms at diagnosis. Mean and standard deviation (SD) as well as median and interquartile range (Q1-Q3) were calculated for continuous variables, and categorical variables were summarized by frequencies and proportions. For those who reported at least 1 day with any CAP symptom on the CAP-BIQ, time to symptom resolution was calculated and reported as mean (SD), median and (Q1-Q3). Time to return to work, to usual level of work productivity or for those not working, usual daily activities, as well as the number of days of missed work or missed daily activities, were calculated based on the time of the survey completion and the date of pneumonia diagnosis provided by the participant. The frequency of patient-reported impact of CAP symptoms on family and daily life such as trouble with self-care, inability to get out of the home or drive and need for caregiver assistance, were also computed. As exploratory analyses, results were analyzed stratified by hospitalization for CAP (yes/no) and age group (<50, 50 to 64 or ≥65 years of age). Values between the strata were compared using t-test or analysis of variance for continuous variables, and Chi-squared test for categorical variables. The significance level was set at 0.05. All statistical analyses were performed using SAS 9.4.
Results
A total of 1262 individuals initiated the screening questionnaire. Of these, 1179 (93.4%) completed the questionnaire and 507 were eligible for enrollment. (Figure 1). There were 481 participants with sufficient data to be included in the analysis either due to the complete resolution of symptoms at the baseline survey (n=10) or by completion of both the baseline and follow-up surveys (n=471). Although CAP diagnoses were self-reported without medical record verification, 96.5% of participants stated they did have a chest radiograph or computerized tomography to diagnose or confirm their CAP.
The participants’ mean age was 48.9 years (SD 11.1 years), and 64% were men. Most were white and approximately half had completed a college or postgraduate education, 52% were currently employed and 49% were current smokers. Additional baseline characteristics of respondents including the CAT results (mean 24.9 which is high and the mean was not significantly different across age groups) are depicted in Table 1. Self-reported chronic conditions included asthma (23.5%), hypertension (23.5%), diabetes (15.4%), and heart disease (8.5%). Of the 481 participants included in the data analysis, 91.1% (n=438) reported being hospitalized for their CAP. The mean time between the diagnosis of CAP and completion of the baseline survey was 70.4 days (SD 26.7 days, median 73 days). Average time to completion of the follow-up survey was 32.8 days (SD 26.6 days, median 35 days) after completion of the baseline survey.
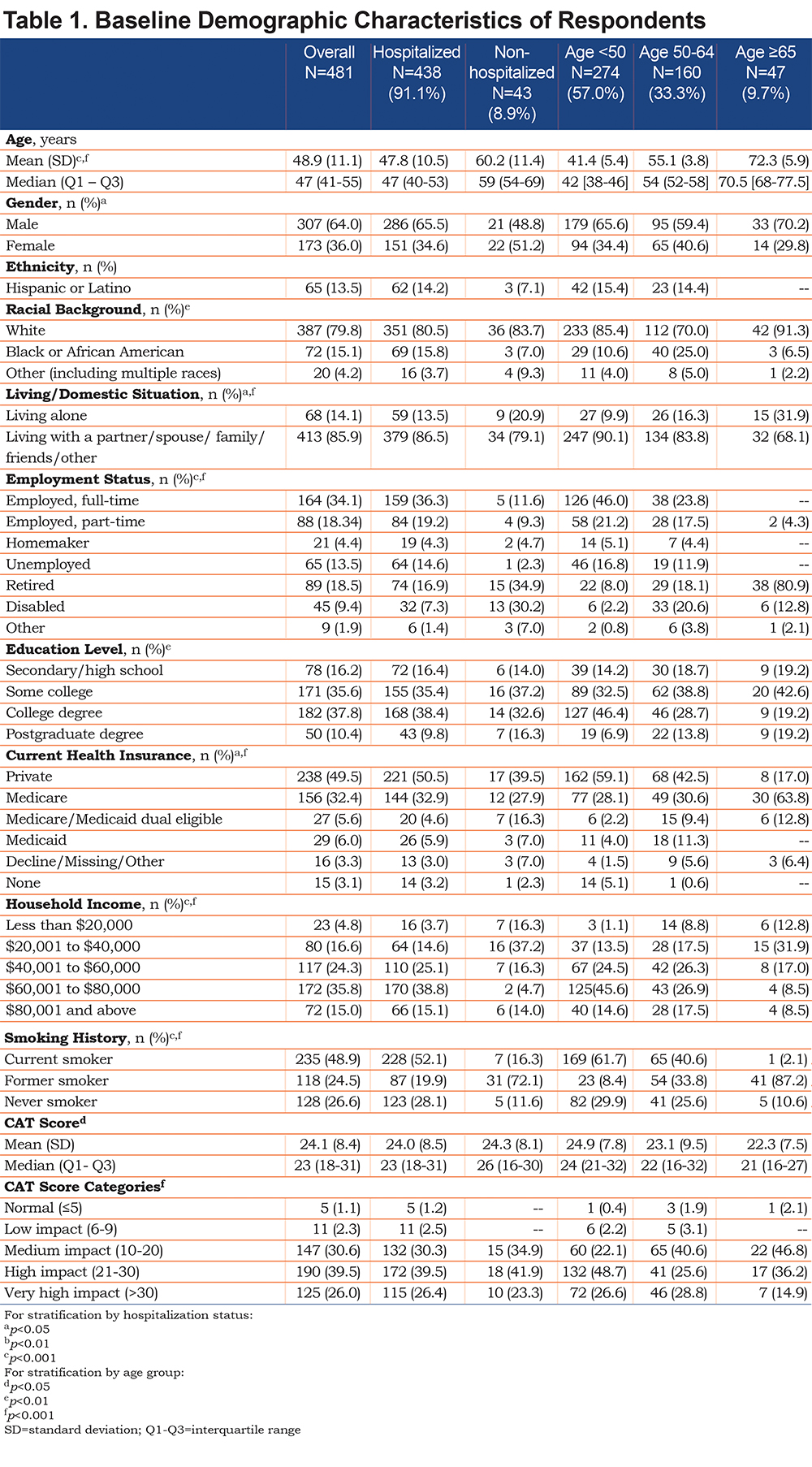
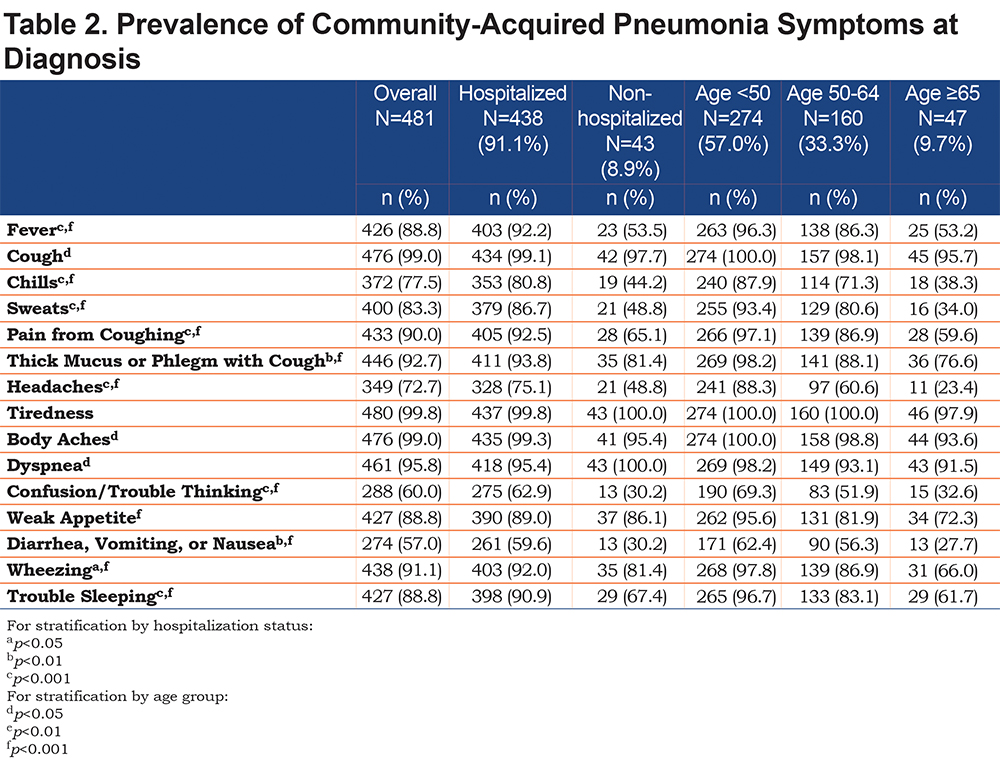
Over 90% of all participants reported the presence of respiratory-related symptoms including dyspnea, cough, thick mucus or phlegm, wheezing, pain from cough, as well as fever, and sweats at the time of CAP diagnosis. Generalized symptoms of weak appetite and trouble sleeping were reported by more than 60% while headaches, confusion or trouble thinking were reported by about half of the participants as being present at the time of CAP diagnosis. (Table 2).
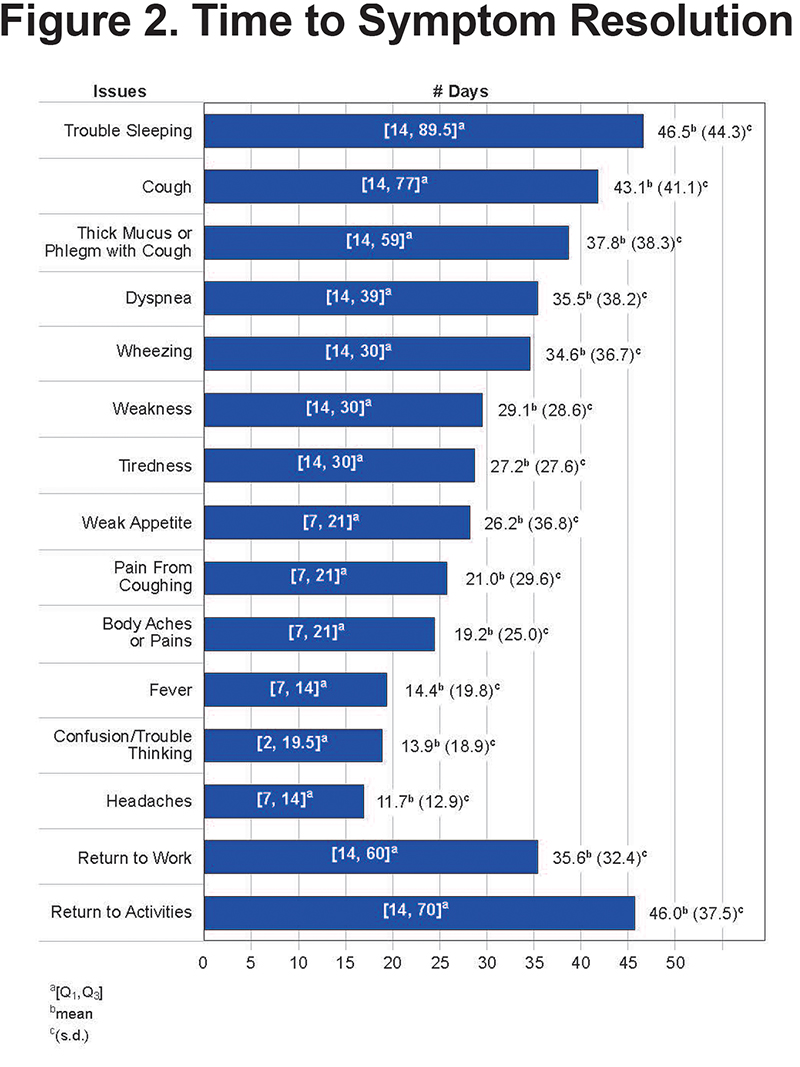
On average, most respiratory-related symptoms required 3 weeks or longer for resolution. Some of the more generalized symptoms such as headaches, gastrointestinal symptoms, chills and fever required less time to return to normal. Figure 2 displays the mean time to symptom resolution.
CAP Impact on Daily Activities
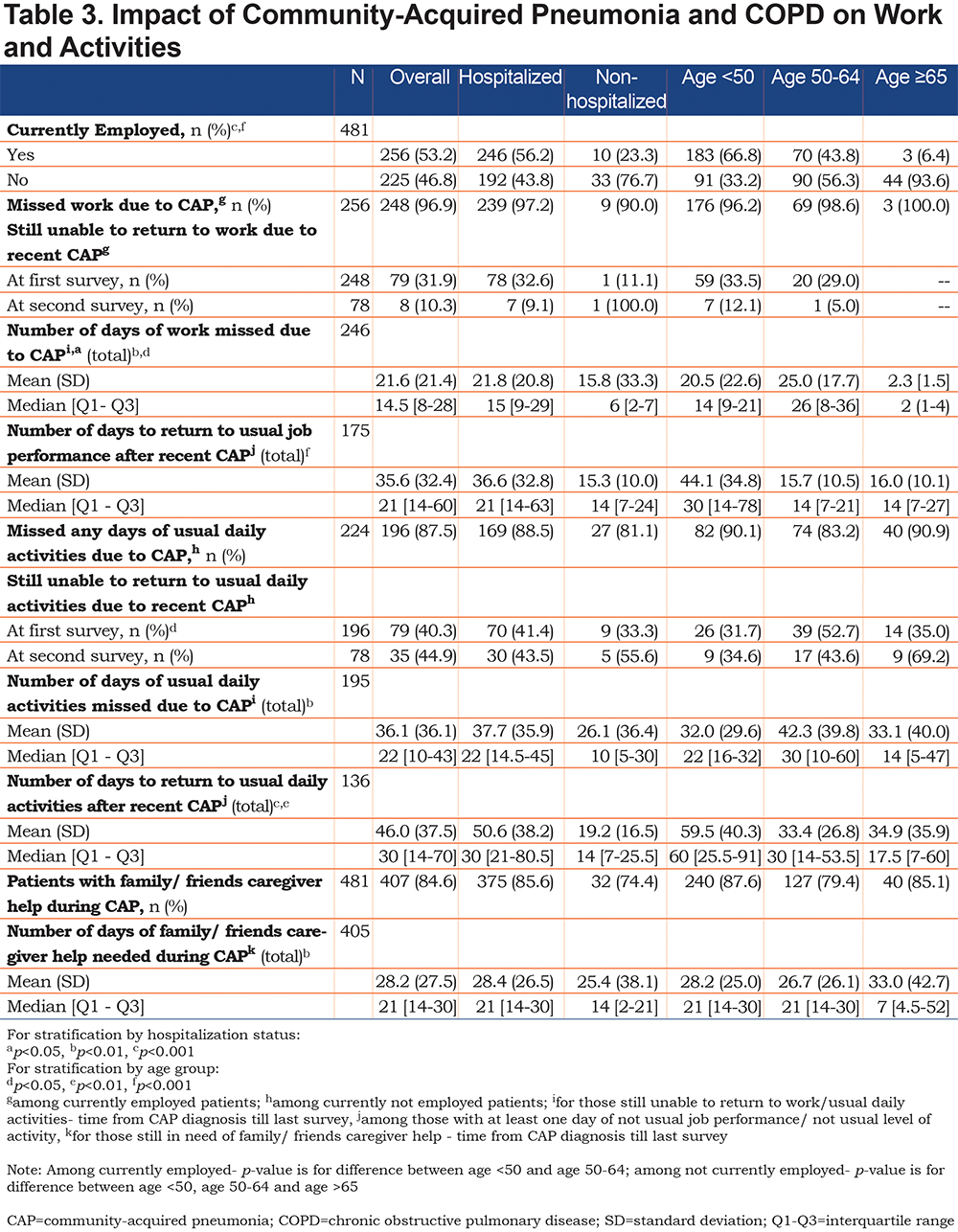
CAP had significant impact on both work and usual activities. (Table 3). Of those employed part or full time, 96.9% reported missing work with an average of 21.6 days of missed work and another 14 days required to return to “usual” job performance. For those not working, 87.5% reported missing 1 or more days of usual activities with an average of 36.1 days missed, and an additional 10 days on average to return to their usual activity performance. Overall, 84.6% of the sample reported that they required help from family, friends or caregivers during their CAP recovery, with an average of 28.2 days of help needed. (Table 4).
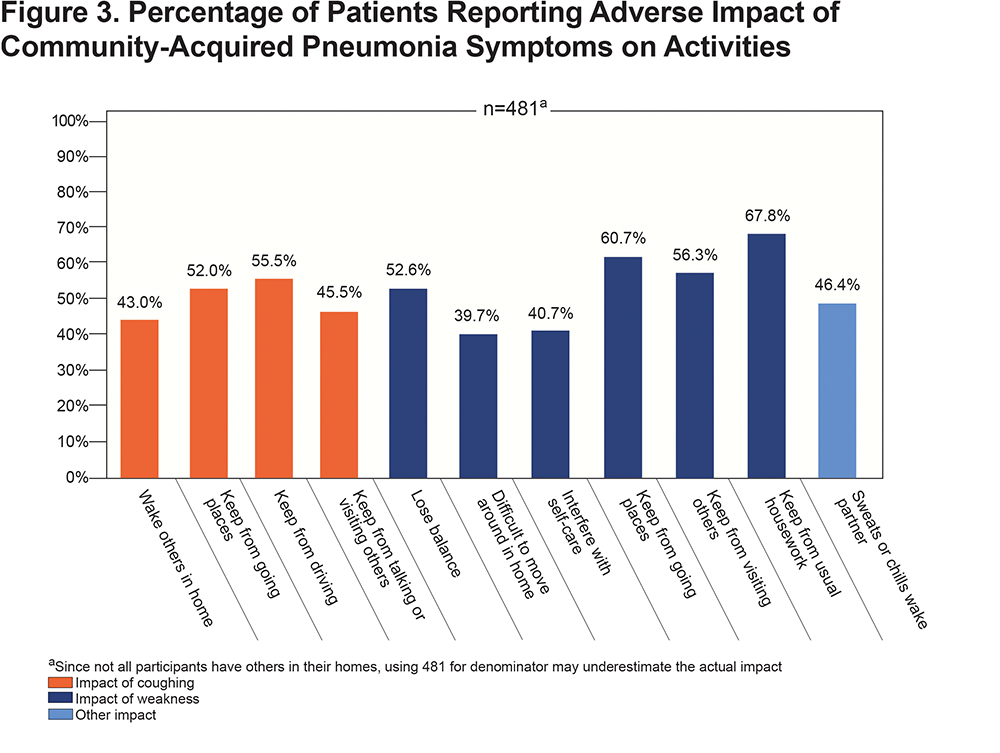
CAP-related cough and weakness were reported to have broad impacts on the participants’ lives that are seldom recorded in medical encounters. Figure 3 highlights that the majority of patients reported a negative impact of symptoms on others in their household as well their ability to go outside their home or visit or talk with others. Participants also used the opportunity to provide comments in an open text field to report problems with incontinence due to cough (n=6). One participant summarized the impact of CAP by saying “My ‘normal’ has been adjusted downward, again” and another likened their experience with CAP added onto their COPD as “in an invisible prison”.
Exploratory Impact of Hospitalization and Age
Most participants reported being hospitalized due to CAP. Among those hospitalized, almost all reported symptoms were more frequent compared to those treated as outpatients with the exception of dyspnea and weak appetite which were equally common in both groups. (Table 2). Time required for resolution of symptoms was similar in hospitalized and non-hospitalized participants, except for cough, phlegm, fever and trouble sleeping, which on average lasted longer in those hospitalized. Those hospitalized due to CAP experienced more missed days of work and activities as well as a longer period to return to usual job performance and usual activities. (Table 3).
The prevalence of all reported symptoms differed according to age group, with lower prevalence in the older age groups, though it is important to note that less than 10% of the participants were 65 years of age or older. Age did not appear to lengthen the time to recovery for most symptoms with the exception of coughing, pain from coughing, thick phlegm and fever in which longer resolution time was associated with older age. (See Supplemental Table 3 in the online supplement.)
Discussion
In this group of individuals with COPD and CAP, numerous respiratory symptoms were reported that required an average of 3 or more weeks to resolve. In addition to respiratory symptoms, participants experienced fatigue, weakness, poor appetite, confusion or trouble thinking, body aches, and pain associated with their coughs. The burden of CAP resulted in missed work and usual activities for almost all individuals with an average of 21 missed days of work for the employed and 36.5 days of missed activities for those who were not employed. Returning to usual job performance or usual level of activities required an even longer period of time. The impact of the CAP was also felt by the individuals’ families and care givers with almost 85% of participants reporting they required help with daily chores and self-care. Almost half reported they woke others in their home with their coughing or had sweats or chills that woke their sleeping partner. While pneumonia is always a potentially serious diagnosis, the burden was extensive among this group of people with COPD.
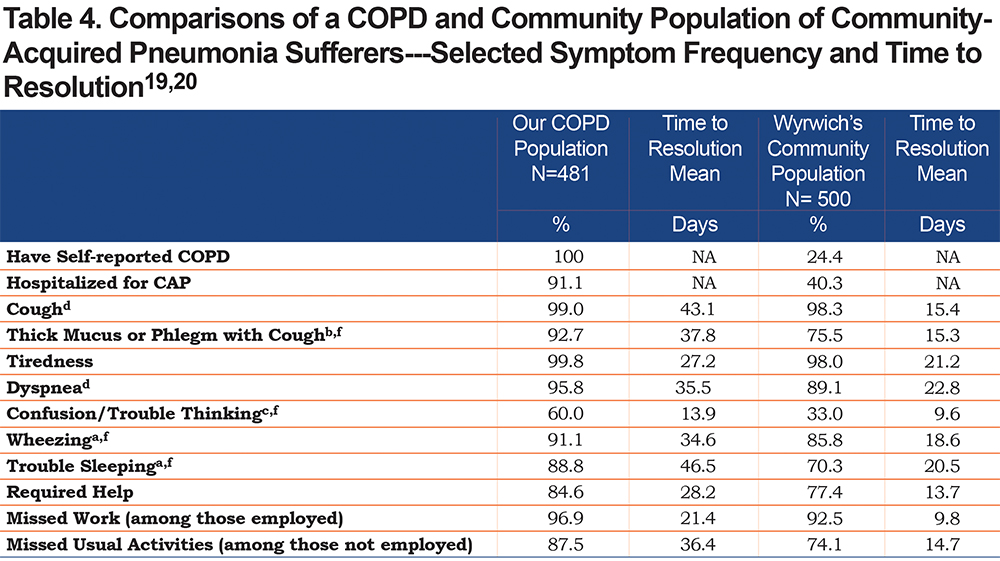
In a similarly designed survey study of U.S. adults with CAP by Wyrwich et al, the same CAP-BIQ instrument was used to identify symptom frequency, duration and impact from the patients’ perspective.19 That study included 500 individuals in the baseline survey whom, as in the present study, had self-reported CAP within the past 120 days. However, there are noteworthy differences between the 2 studies: first, 100% of our patient population has self-reported COPD versus COPD self-reported in 24.4% of Wyrwich et al’s community-based population and secondly, the mean age of our population was 48.9 years versus 62.4 years for the Wyrwich et al group. The comparison of selected common symptom frequency and time to resolution in the 2 populations are shown in Table 4.
The higher prevalence of most of the common symptoms, the longer time to symptom recovery, greater numbers of missed work and activity days, as well as the greater percentage of individuals requiring assistance and for a longer period of time, highlights the even greater burden CAP places on people with COPD compared to a general community population reported in the Wyrwich et al studies.19,20 While Wyrwich et al did not report the specific data for their 24.4% subsample with COPD, they did report that the individuals with COPD/emphysema had the longest time to recovery compared to those without COPD and that the younger COPD/emphysema patients had the longest recovery period.20 Insufficient data was available to do statistical comparisons.
Other studies of CAP impact included a study of patients in a Canadian trial of a treatment pathway for CAP.22,23 In the Canadian study patients were on average older (mean of 61.6 years) and only 26% had COPD. Symptom frequency was lower for all symptoms except cough with less than 80% reporting dyspnea (shortness of breath), less than 70% reporting sputum production and less than 40% reporting fever compared to 95.8%, 93.8% and 88.8%, respectively, in our study participants. Although the patients in the Canadian study were older on average, our group with COPD were more symptomatic, and compared to other groups of patients, also required longer to have symptoms resolve and allow for the return to work.
Other earlier studies of symptom burden in CAP provide additional context to our study results.27,28 Among “low risk” patients studied, e.g., those without major chronic comorbidities, median time to symptom “cure” was 21 days with fatigue and cough being the slowest to resolve. While the time to symptom recovery is longer among our participants, the long duration of cough and tiredness or fatigue are similar with 51% of the patients in that study reporting continued fatigue at 90 days post pneumonia diagnosis.27 In studies of patients hospitalized with bacteremic pneumococcal pneumonia, mean time to return to work was 16 days 29 while in the PORT study of patients treated in an ambulatory setting, pneumonia was associated with a much shorter time to return to work, with an average of 7 days. 30
While we did not formally collect any qualitative data, an opportunity to offer comments was provided. Some of those comments illustrate powerful images of the impact of CAP on people already living with COPD. The image of a “prison” limiting all activity and interactions and the need to again downgrade the anticipated “norm” for activities are both disheartening and revealing.
Many cases of CAP are preventable with smoking cessation and completion of recommended pneumococcal vaccines and yearly influenza vaccination.31-36 Almost half of this study’s participants reported themselves to be current smokers providing a significant prevention opportunity. In addition, the 2016 Behavioral Risk Factor Surveillance System (BRFSS) data reports 64.3% of individuals with COPD self-report having received a pneumococcal vaccine.37 The Centers for Disease Control and Prevention (CDC) reports that in the 2016-2017 influenza season, 43.3% of adults ≥18 years of age received a flu vaccine.38 The most recent CDC survey data from 2016 indicates that 66.9% of adults ≥65 years of age received a pneumococcal vaccine.39 These data are well below the Healthy People 2020 goals of 70% and 90% for influenza and pneumococcal vaccination in these populations, respectively.40 These data highlight an opportunity for health care providers to improve influenza and pneumococcal immunization rates among their COPD patient population. In our study population, nearly half of this study population reported themselves to be current smokers, providing another prevention opportunity. Educational opportunities to underscore the importance of immunizations and smoking cessation among COPD patients and health care providers should be utilized.
This study has several strengths as well as limitations that may affect the generalizability of the results. It is a strength that we collected information directly from patients. Patients are the best source of information related to symptom prevalence, duration and impact on their daily lives, though reliance on self-report may be less reliable for reports of medical diagnoses. However, self-report of CAP and COPD may have introduced some error into these measurements, including the presence and timing of CAP and the validity of the COPD diagnoses. The timing of the self-report may also be a limitation as this study required individuals to recall CAP symptoms that on average occurred 70 days prior to their report on the baseline survey. This is particularly true for those individuals whose symptoms resolved fairly quickly, as the recall period for those who had longer-lasting symptoms would be shorter for the same time elapsed since diagnosis; presumably there would not be recall bias for those whose symptoms persisted to the time of the survey. Likewise, as the follow-up focused on symptoms still present at the time of the baseline survey, recall from the follow-up survey may be of less concern since it required only 30 days of recall. The study was conducted online, potentially limiting participants to those with higher technological literacy or only those who have access to the internet. In addition, the sampling frame from which participants were recruited may not fully represent COPD patients with CAP as a whole, as participants were recruited through the resources of a patient advocacy organization. Finally, it is possible that self-selection resulted in a sample with more severe COPD who have higher risk of exacerbations and CAP. However, this is an important population since many health care professionals and policy makers may forget that the population with COPD and CAP includes many individuals who experience not only disruption to their usual activities but also extended absence from jobs.
Conclusions and Practice and Patient-Related Implications
In individuals with COPD, CAP results in a significant and lengthy burden affecting families and caregivers as well as the patient. Efforts to improve the provision of CAP prevention strategies including supporting smoking cessation and increasing pneumococcal and annual influenza immunizations are needed.
Acknowledgements
The authors thank David Mannino, MD, for his substantial work on and contributions to the CAP Study. The authors also wish to sincerely thank the late Edna Shattuck for her contributions to the study team and for her tireless work for the COPD community.
The research reported in this publication was conducted using PCORnet®, the National Patient-Centered Clinical Research Network, an initiative funded by the PCORI. The statements presented in this publication are solely the responsibility of the author(s) and do not necessarily represent the views of organizations participating in or collaborating with PCORnet® or of PCORI.
Author Contributions: Ms. Pasquale made substantial contributions to the conception and design of the study, oversaw data acquisition and writing the article and revised the paper in response to co-author comments. Dr. Vietri contributed to the design of the study, data interpretation, and reviewing the article. Ms. Choate designed and conducted data analysis, significantly contributed to data interpretation, and writing and reviewing the article. Dr. McDaniel contributed to conception and design of the study and revising the article. Dr. Sato contributed to the conception and design of the study, data interpretation, and reviewing the article. Dr. Ford contributed to the conception and design of the study, data interpretation, and reviewing the article. Ms. Malanga made substantial contributions to the conception and design of the study, data acquisition, and writing and reviewing the paper. Dr. Yawn oversaw data analysis and made substantial contributions to data interpretation, writing the article and revising the paper in response to co-author comments. Dr. Yawn developed the original draft of the manuscript and all authors reviewed and revised it for important intellectual content.
Declaration of Interest
J. Vietri, A. McDaniel, R. Sato and K.D. Ford are employees and stock holders of Pfizer, Inc. B.P. Yawn reports serving on advisory boards for GlaxoSmithKline, AstraZeneca and Boehringer Ingelheim related to COPD. All other authors have nothing to declare.