Running Head: Home Monitoring in COPD
Funding Support: This work was funded by Midmark Corporation.
Date of Acceptance: August 3, 2023 | Publication Online Date: August 7, 2013
Abbreviations: AECOPDs=acute exacerbations of COPD; ATS=American Thoracic Society; CAT=COPD Assessment Test; CI=confidence interval; COPD=chronic obstructive pulmonary disease; COVID-19=coronavirus disease 2019; ERS=European Respiratory Society; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; IC=inspiratory capacity; ICS=inhaled corticosteroid; IQR=interquartile range; LABA=long-acting beta2-agonist; LAMA=long-acting muscarinic antagonist; mMRC=modified Medical Research Council; RPM=remote patient monitoring
Citation: Rydberg M, Burkett P, Johnson E, Drummond MB. Home telemonitoring program in individuals with COPD during the coronavirus disease 2019 pandemic: a pilot study. Chronic Obstr Pulm Dis. 2023; 10(4): 437-443. doi: http://doi.org/10.15326/jcopdf.2023.0431
A portion of this manuscript was presented at the 2021 American Thoracic Society International Conference as a poster presentation.
Introduction
There has been significant interest in innovative ways to improve the care of chronic obstructive pulmonary disease (COPD) patients. Remote patient monitoring (RPM), also called telemonitoring, is a method of health care delivery that gathers patient data outside of traditional health care settings. The coronavirus disease 2019 (COVID-19) pandemic has moved telemedicine to the forefront of care1,2 accelerating the need to study remote monitoring in COPD patients. RPM tools including home spirometry, pulse oximetry, and daily questionnaires have been shown to have the potential to detect acute exacerbations of COPD (AECOPDs) earlier and improve patient-reported outcomes in COPD.3-7 Given the older age and numerous comorbid conditions of many COPD patients, it remains unclear if RPM interventions are feasible and acceptable by this patient population.8-12 To address this, we conducted a 12-week pilot study of a novel in-home telemonitoring system, consisting of 3components: a home spirometer, a Bluetooth®-enabled home pulse oximeter, and a tablet-based data collection system with avatar-assisted technology with the goal of determining impact on the COPD Assessment Test (CAT)13 score and adherence to device measurements. The study was designed to be conducted entirely remotely given the COVID-19 pandemic.
Methods
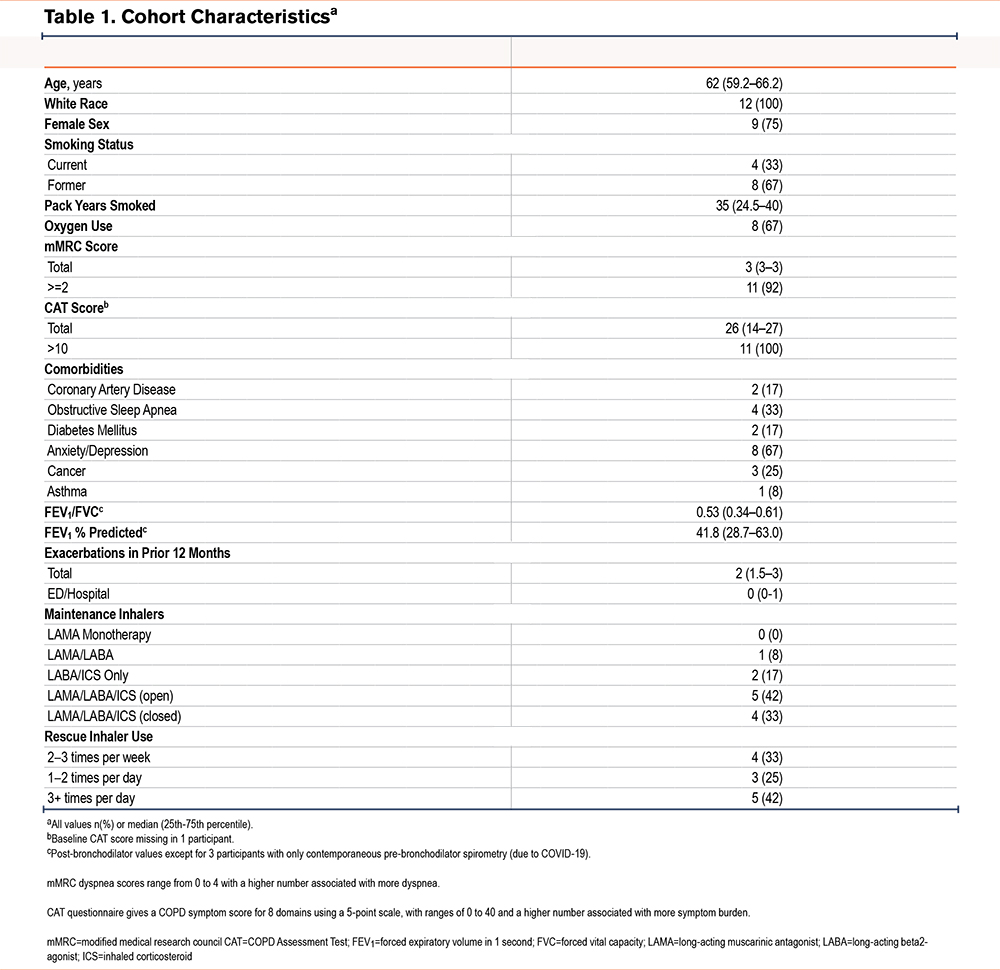
Twelve participants were enrolled from a single site (the University of North Carolina at Chapel Hill) from July 23, 2020 through February 19, 2021. Eligible participants were male or female, 40–80 years old, English speaking, with spirometry-confirmed COPD and forced expiratory volume in 1 second (FEV1) <80% predicted within 24 months prior to screening, and an increased exacerbation risk (1 admission for AECOPD or 2 outpatient AECOPDs requiring steroids and/or antibiotics in the prior 12 months). Eligible participants were approached in a sequential manner. The study was approved by the University of North Carolina at Chapel Hill Institutional Review Board (Study #20-0107) and registered with clinicaltrials.gov (NCT04369885). Participants were contacted via phone and underwent an IRB-approved phone screening and consent.
The remote monitoring technology consisted of a home spirometer (GoSpiro®, Monitored Therapeutics, Dublin, Ohio), a pulse oximeter (NoninConnectTM Nonin Medical, Plymouth, Minnesota), and a data collection platform (GoHomeTM Personal Health Monitor, Monitored Therapeutics, Dublin, Ohio) (Figure 1). The GoSpiro spirometer meets the 2019 American Thoracic Society/European Respiratory Society (ATS/ERS) criteria for accuracy and reproducibility. 14 The GoHome data-collection platform includes avatar-assisted technology to coach the participants through each measurement, assessing acceptability against ATS/ERS standards in real-time with coaching feedback. The program uses a statistical process control procedure shown to reduce fatigue and improve adherence in COPD patients.15 Participants were prompted to perform forced spirometry on Tuesdays and Thursdays and slow spirometry the other 5 days. Participants were also prompted to complete daily oxygen saturation measurements and a daily questionnaire assessing symptoms of shortness of breath, cough, and mucus as well as difficulty in leaving the house. The highest individual score from the domains of breathing difficulty, cough, and mucus was used to determine a daily automated COPD action plan response to be delivered to the participant. Adherence to the intervention was defined as the percentage of participants achieving >50% completion of all planned device measurements (spirometry, pulse oximetry, and questionnaires) over the 12-week study period. This threshold was selected based on prior publications of anticipated adherence to multi-component in-home monitoring of COPD patients.16,17 Participants completed the CAT questionnaire at enrollment and again at the end of weeks 4, 8, and 12. Participants were enrolled for 12 weeks of data collection, with data uploaded to a web portal immediately after collection. An automated e-mail report was sent to the study principal investigator daily along with summarized action plan scores and flags indicating any physiologic change (decrease in FEV1 or forced vital capacity [FVC], or inspiratory capacity [IC] >10% from baseline or oxygen saturation <88%) or lack of data transfer. Actions based on the flags were at the discretion of the principal investigator. Participants were advised that the study team physician reviewed results daily and might call the participant to further investigate flags.

Results
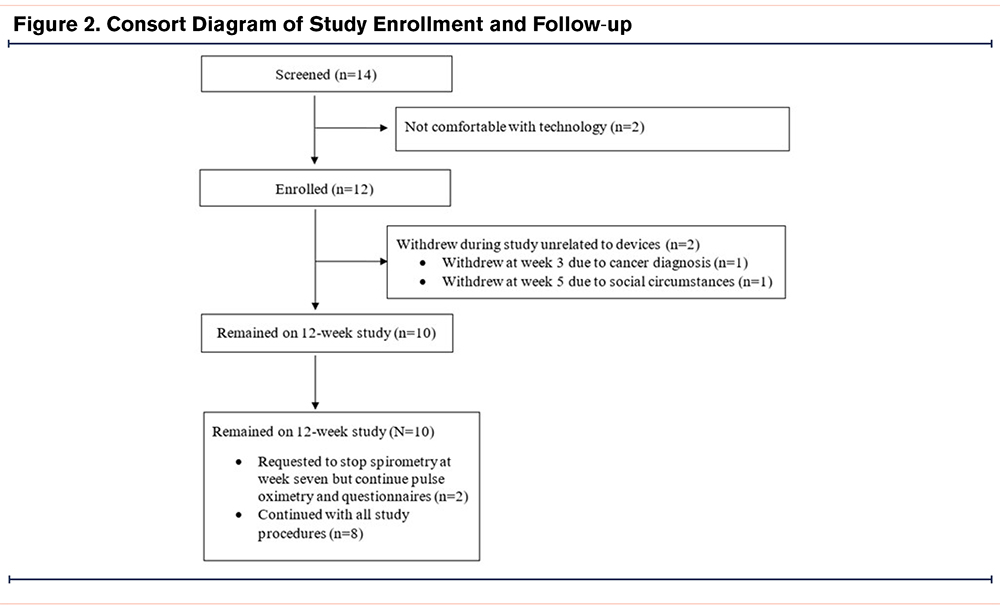
Figure 2 outlines participant enrollment and retention in the study. A total of 14 participants were screened to enroll 12 participants in the study, with 2 participants withdrawing from the study for reasons unrelated to the study conduct (e.g., new diagnosis of cancer, social situation). Enrollment demographic and clinical characteristics are shown in Table 1. On days when forced spirometry was completed, the best spirometry of the day met ATS/ERS standards 96% of the time, with no observed difference among those with FEV1 <50% predicted (95.5%) compared to participants with FEV1≥50% predicted (94.7%).
Of the 12 participants enrolled, 7 participants had initial and final CAT scores to permit an assessment of change from baseline in total CAT scores. Four of 7 participants, reflecting 57% of eligible participants, achieved a ≥2-point decrease in their CAT score from baseline to 3 months (95% confidence interval [CI] 18% to 90%). The median change in CAT score at 12 weeks was -2.5 points (interquartile range [IQR] -5 to 0; range -9 to 8).
Of the 12 participants initially enrolled, 10 participants contributed 3 months of data and were analyzed for adherence data. Five of 10 participants (50%) demonstrated >50% adherence to all planned device measurements. Although not statistically different due to the small sample size, adherent individuals were qualitatively older (67 versus 60 years), more commonly female (80% versus 60%), more likely former smokers (80% versus 40%), reported oxygen use (80% versus 60%), and had lower lung function (FEV1% predicted 38% versus 51%). Diabetes, anxiety/depression, and a history of cancer were more common in adherent individuals. When examining adherence to the individual device measurements among participants who completed all 12 weeks of study follow-up, 60% of participants achieved >50% adherence to forced vital capacity and 80% achieved >50% adherence to slow vital capacity maneuvers. A total of 90% were >50% adherent to home pulse oximetry measurement and 90% were >50% adherent to home questionnaires.
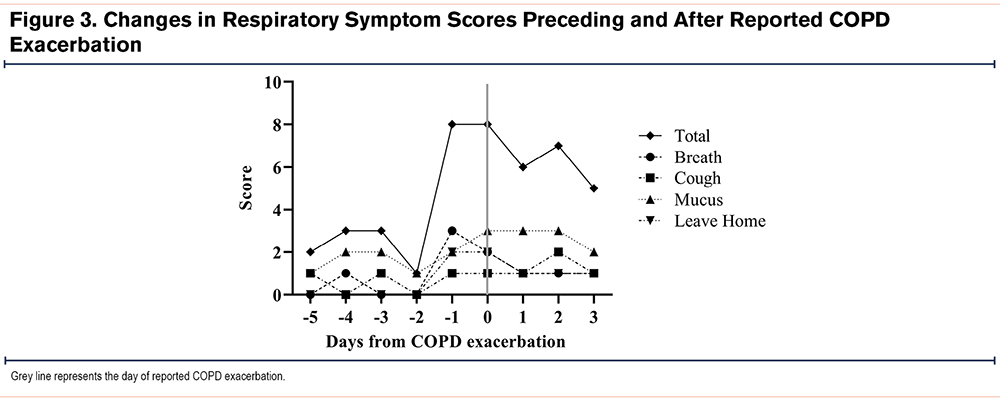
A total of 5 exacerbations occurred among 4 participants (2 requiring hospitalization). Of the 5 exacerbations, one participant triggered alarms for a >10% decline in FEV1 and FVC as well as oxygen saturation (Sp02)<88%, 48 hours prior to reporting the COPD exacerbation. This individual also had an abrupt increase in symptom scores 24 hours prior to reporting the COPD exacerbation (Figure 3). The other 4 exacerbation events were not associated with physiologic alarms or clear increases in reported respiratory symptoms preceding the exacerbation event.
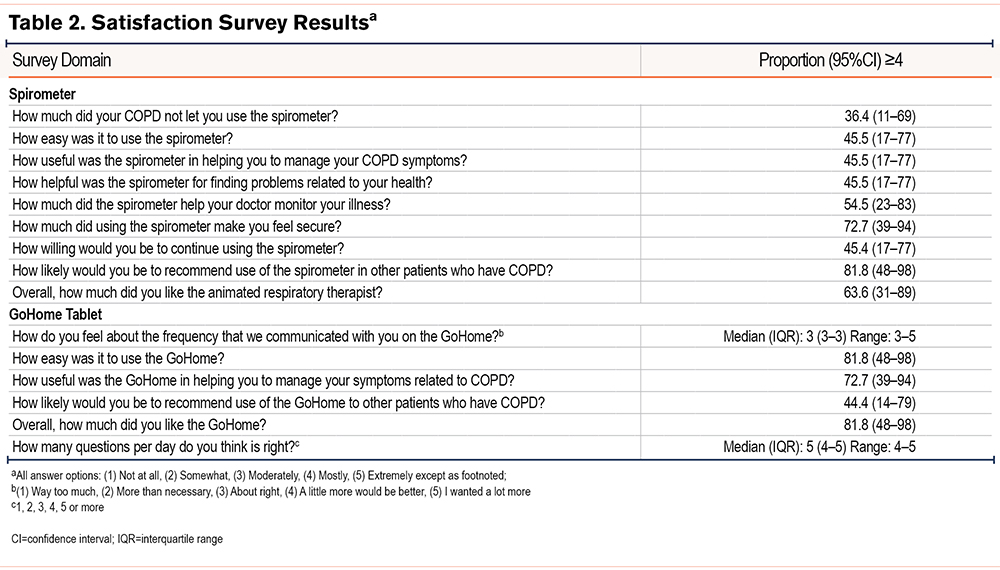
Participant satisfaction survey scores were measured at the conclusion of the study using a 15-question investigator-developed survey (Table 2). Of the 12 participants initially enrolled, 11 completed the survey. When assessing the home spirometer, the highest satisfaction scores were reported for “likelihood of recommending the spirometer to others with COPD” (82%), and if “using the spirometer made participants feel secure” (73%). Nearly two-thirds (64%) liked the animated respiratory therapist that guided the performance of spirometry. A total of 55% of participants felt that the spirometer helped their doctor monitor their illness. Regarding the GoHome tablet, 82% found it “easy to use” and a similar proportion “liked”using it. A total of 73% felt it was “useful in helping manage COPD-related symptoms.”
Discussion
This study is the first to evaluate a telemonitoring system consisting of the GoSpiro spirometer, NoninConnect pulse oximeter, and GoHome data collection system with avatar-assisted technology in the COPD population. Importantly, this study involved no in-person visits. We found that half of the participants were able to complete >50% of scheduled home study assessments. Among participants with adherence to device questionnaire schedules, nearly 60% achieved a qualitative improvement in COPD symptoms as assessed by the CAT score, informing the potential impact of this in-home telemonitoring system on COPD-related patient-reported outcomes. Participants felt that the home devices increased their feeling of security and physician monitoring. The majority of participants would recommend the use of the GoSpiro spirometer to other patients with COPD.
There has been concern among the medical community that technology-based interventions could not be successfully deployed in COPD patients. We found that a telemonitoring system could be deployed without any in-person interaction with COPD patients. More than 50% adherence to individual component measurements ranged from 60%-90% despite a rigorous daily schedule. Daily data collection was chosen to help patients establish a routine that could maximize adherence. It is possible that daily measurements were too intensive, and that a less frequent schedule may result in improved patient adherence with unchanged clinical benefits. Moreover, individuals with lower adherence tended to have less COPD disease burden and fewer comorbidities. These findings suggest that telehealth interventions can be accepted by COPD patients and may have implications for broader access to care. The devices in this intervention could transmit data without internet access, an important factor when considering the lack of widescale broadband internet availability. This intervention may result in improved access among rural and underserved patients who find it difficult to seek in-person care.
Only one exacerbation was preceded by an increased symptom burden. This may suggest that the algorithm deployed in this study is not sensitive enough to detect exacerbations. Alternative thresholds to define alerts may have improved the sensitivity to detect impending exacerbations. This study was not powered to draw conclusions about the sensitivity of the algorithm to detect COPD exacerbations. The majority of participants (73%) reported that the device was helpful in managing COPD-related symptoms. While limited by the small sample size, findings suggest that this intervention has the potential to reduce COPD-specific symptoms. A total of 73% of patients stated that using the spirometer in our intervention led to them feeling more secure; it is possible that awareness of the study physician’s monitoring or increased disease knowledge and confidence by participants may contribute to a sense of security, decreased CAT scores, and improved perceptions of their disease.
This study has limitations. This is a small pilot study intended to demonstrate the preliminary feasibility of a remote monitoring system. The study cohort, largely White, female COPD participants engaged in clinical care at a quaternary clinical center, is not reflective of the general COPD population. Acceptance and patient adherence may vary with a different composition of study participants. The study relied on self-reporting of exacerbation events and inhaler use, which may predispose to recall bias. We were not able to collect specific reasons for the lack of adherence to study procedures.
This prospective pilot study demonstrated that a telehealth intervention consisting of daily spirometry, pulse oximetry, and questionnaires can be adopted by COPD patients with a reasonable degree of adherence without any in-person visits required. The interventions were associated with a potential qualitative improvement in COPD symptoms and a feeling of increased security by participants. These findings support the conduct of larger clinical trials of this intervention to determine the potential benefit in a more generalized COPD population.
Acknowledgements
Author contributions: PB, EJ, and MBD substantially contributed to the conception and design of the study. PB and MBD substantially contributed to data acquisition. MR, PB, and MBD substantially contributed to data analysis. MR and MBD drafted the article, with all coauthors providing substantial contributions in revisions. All authors approved the final version. MBD takes sole responsibility for data analysis.
Data Sharing: Primary results, protocol, and the statistical plan are available on clinicaltrials.gov per clinical trial reporting requirements. The data are not publicly available due to the small study size and single-center enrollment that could compromise the privacy of research participants. Data requests should be sent to the corresponding author, MBD. Restrictions may apply to the availability of these data which were generated under an agreement with the funder. Data requests will need to align with university and funder policies prior to release, and only with the permission of the funder.
Declaration of Interests
Study devices were provided by Monitored Therapeutics, Inc. MR has no declarations of interest. PB is an employee of Monitored Therapeutics. EJ is an employee of Midmark Corporation. MBD has served as a paid consultant for Midmark and received support through a grant from Midmark for this work.