Running Head: KF4 Anti-CELA1 Antibody in AAT-Deficient Emphysema
Funding Support: Funding for this work was supported by the National Institutes of Health/ National Heart, Lung, and Blood Institute (R01HL141229-Varisco), the Alpha-1 Foundation (498262-Varisco), and the National Institutes of Health/National Institute of General Medical Sciences (R01GM115973- Zingarelli).
Date of Acceptance: November 11, 2024 | Publication Online Date: November 26, 2024
Abbreviations: AAT=alpha-1 antitrypsin; ANOVA=analysis of variance; BUN=blood urea nitrogen; CELA1=chymotrypsin-like elastase 1; CLP=cecal ligation and puncture; IgG=immunoglobin G; MLI=mean linear intercept; mRNA=messenger RNA; PBS=phosphate buffered saline; PPE=porcine pancreatic elastase; pro-Sftpb=pro-Surfactant protein C
Citation: Devine AJ, Smith NJ, Joshi R, et al. KF4 anti-chymotrypsin-like elastase 1 antibody and purified alpha-1 antitrypsin have similar but not additive efficacy in preventing emphysema in murine alpha-1 antitrypsin deficiency. Chronic Obstr Pulm Dis. 2025; 12(1): 12-22. doi: http://doi.org/10.15326/jcopdf.2024.0535
Online Supplemental Material: Read Online Supplemental Material (5132KB)
Introduction
Alpha-1 antitrypsin (AAT) deficiency is the most common genetic factor predisposing people to emphysema.1 Patients typically present in the fourth or fifth decade of life, and despite meta-analyses suggesting only marginal benefit,2 replacement therapy with purified, human AAT is the standard of care.3
AAT is an antiprotease that neutralizes4,5 neutrophil elastase, cathepsin g, and proteinase-3. Our group has shown that AAT also neutralizes an alveolar type 2 cell-secreted serine protease called chymotrypsin-like elastase 1 (CELA1).6-8 CELA1 plays a physiologic role in the postnatal lung by reducing lung elastase,6 and at baseline Cela1-/- mice have slightly smaller alveoli than wild-type mice. In antisense oligonucleotide6 and genetic7 models of AAT-deficiency, Cela1-/- AAT-deficient mice are protected from emphysema. CELA1-AAT protein complexes can be found in the human lung indicating that AAT likely neutralized CELA1 by covalent binding.8
The KF4 anti-CELA1 monoclonal antibody is a mouse immunoglobin G (IgG)1 that covalently binds to a N-terminal region of the CELA1 molecule.8 KF4 protects wild-type mice from cigarette smoke-induced emphysema and from emphysema progression following tracheal administration of porcine pancreatic elastase (PPE). Based upon the premise that AAT neutralization of CELA1 plays a key role in the pathogenesis of emphysema in AAT-deficiency, we sought to test whether Cela1-neutralization using KF4 antibody could protect AAT-deficient mice from emphysema and compare its efficacy with AAT replacement therapy. To do so we used previously reported mice that carry deletions of the Serpina1a, Serpina1b, Serpina1c, Serpina1d, and Serpina1e genes.9
Methods
Animal Use
Animal use was approved by the Cincinnati Children’s Hospital Institutional Review Board under authorization 2020–0054 (BMV). In addition, we utilized previously collected images from mice utilized under authorization IACUC2021–0087 (BZ). Results from these studies were previously published.10,11
Tracheal PPE Administration
C57BL6/JSerpina1em3Chmu male and female mice aged 8 to 10 weeks (called AAT-/-hereafter) were anesthetized with 2% isoflurane and suspended by the incisors on a mouse intubating board. The tongue was withdrawn using forceps, and 0.2 units of PPE (Sigma Aldrich, E1250), in 100µL of phosphate buffered saline (PBS) was instilled into the oropharyngeal cavity. The nose was occluded with a finger and the mouse remained suspended until it began recovering and aspirated the solution. The mouse recovered in a cage and was observed until ambulation resumed. The day of PPE administration was considered day 1.
Lung Tissue Elastase Assay
Lung elastase activity of AAT-/- mice treated with different doses of intraperitoneal KF4 was quantified using N-Succinyl-Ala-Ala-Ala-pNitroanilide, substrate for human leucocyte elastase and PPE (#NS945, Elastin Products Company; Owensville, Missouri). Lung tissue was homogenized in PBS and 10mg was incubated in the assay for 4 hours per manufacturer instructions with KF4 or IgG antibody. A signal at 4 hours was used as a measure of elastase activity.
KF4 and AAT Treatment
Based on inhibition of lung elastase activity, KF4 1mg/kg (10 mice), purified human recombinant AAT 60mg/kg (6 mice), combined KF4 and AAT (11 mice), or IgG 1mg/kg (12 mice) was administered intraperitoneally every 7 days starting on day 1 (i.e., the same day as PPE administration) to AAT-/- mice with equal or nearly-equal numbers of male and female mice in each group.
Mouse Cecal Ligation and Puncture
As described previously,10 mice were anesthetized with isoflurane and the cecum visualized through a midline incision. The cecum was ligated with 3-0 silk and a single through-and-through puncture made with a 22-gauge needle. Cecal contents were expressed, and the abdomen was closed with a prolene suture. For sham surgeries, the intestines were manipulated without ligation or puncture. The mice were treated with buprenorphine and recovered. At 6 and 18 hours, mice were sacrificed and vital organs collected.
Tissue Processing
All tissues were fixed in 4% paraformaldehyde in PBS overnight, brought into 100% ethanol by serial dehydration, and paraffinized. For liver specimens, only the left lobe was placed in the cassette for embedding, and only the left kidney was embedded. Whole hearts were embedded, but only left ventricles were assessed. For lung lobes, the 4 right lung lobes were arranged in a cassette with random orientation and embedded. Five micron sections of each specimen were mounted on a slide and stained using hematoxylin and eosin.
Imaging
Using a Nikon Ti2 microscope, for liver, kidney, and heart tissues, five 10X and five 40X images per slide were obtained. For lung images, five 10X images from each lobe were obtained and a total of five 40X images from each lung lobe were obtained.
Mean Linear Intercept (MLI) Analysis
Using the methods of Dunnill et al12 after de-identification, MLI values were determined for each 10X lung photomicrograph by a reviewer (AD).
Toxicity Assessment
Procedure: 8-week-old wild-type female C57BL6 mice were administered 5, 12.5, or 25mg/kg KF4, or 25mg/kg mouse anti-Human IgG (711-005-152, Jackson Laboratories; Bar Harbor, Maine), peritoneally. For 5, 12.5, and 25mg/kg doses, a maximum of 5mg/kg was administered at any given time and antibody was administered 1, 3, and 5 times per week respectively. At 6 weeks, mice were anesthetized and exsanguinated and major organs were collected for processing as above.
Tissue Injury Scoring
Our 3 blinded scorers (JD, BBP, and ANV) were instructed on how to perform injury scoring of photomicrographs of lung, liver, kidney, and heart tissue using the rubrics in the online data supplement. Tissues from KF4 or IgG treated wild-type mice were duplicated and de-identified and a score for each image determined. Unpublished cecal ligation and puncture (CLP) images were used as positive controls. The average image scores were analyzed by treatment and reviewer agreement assessed.
Immunohistochemistry
For immunohistochemistry, the ABC Vectastain kit was used with a previously validated anti-CELA1 guinea pig polyclonal antibody.5 Images were obtained using a Nikon TiE microscope.
Immunofluorescence
Mouse bone marrow aspirates and lung sections were immunostained with the same guinea pig antibody and Cd45 (Millipore Cat#05-1410) or pro-Surfactant Protein C (Seven Hills Bioreagents Cat#WRAB-9337) antibodies with appropriate secondary stains.
Statistical Analysis
Ordinal and nonparametric data was analyzed using the Kruskal-Wallis test with Dunn’s post hoc comparison. Parametric MLI data was compared by one-way analysis of variance with the Holm-Sidak post hoc test. All tests were 2-sided and a p-value of less than 0.05 was considered statistically significant. Parametric data is presented as line and whisker plots with lines representing mean and whiskers standard deviation. Nonparametric and ordinal data is presented as box plots.
Interrater reliability measurements are presented as Kirppendorff’s alpha values defining excellent agreement as >0.8, good 0.67 to 0.8, moderate 0.5 to 0.67, and poor <0.5. The R statistical computing platform, rstatix, irr, and ggpubr packages were used for analysis and figure generation.13-16
Results
Expression of Cela1 in Murine Tissues
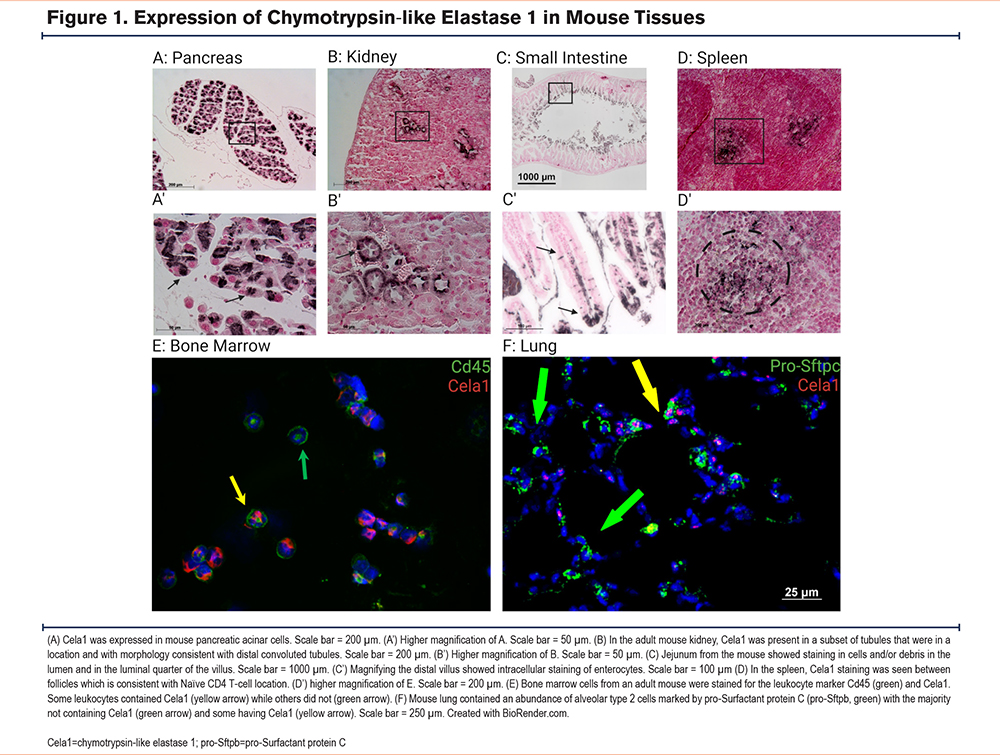
To understand the potential toxicities of CELA1 inhibition, we first evaluated normalized messenger RNA (mRNA) counts in the mouse ENCODE transcriptome library using the National Center for Biotechnology Information Gene. Duodenum, small intestine, colon, and spleen samples had the highest count values (Supplemental Figure 1A in the online supplement). Notably, pancreas was not in the list of evaluated tissues. We then performed immunohistochemistry on selected mouse tissues. As expected, expression was strong throughout the pancreas (Figure 1A). In the kidney, a subset of cells that appeared to be distal convoluted tubules had Cela1 protein (Figure 1B). In the small intestine, Cela1 protein was present in enterocytes in the luminal 1/3 of the villus. This staining was intracellular, indicating with that and mRNA expression that this was not from pancreas-secreted Cela1 (Figure 1C). To further identify cell types potentially expressing Cela1 in the spleen and in consideration of our previous finding that there were more numerous leukocytes in the lungs of Cela1-/- mice,8 we evaluated what leukocytes were reported as having CELA1 mRNA in the Human Protein Atlas.17 The 2 cell types with the highest mRNA levels were basophils and Naïve CD4 T-cells (Supplemental Figure 1B in the online supplement). Consistent with Cela1 expression in Naïve CD4 T-cells, we observed Cela1 staining in the red pulp of the spleen between follicles (Figure 1D) and in CD45-expressing bone marrow cells (Figure 1E). In the mouse lung, Cela1 was expressed in sparse, contained cells (Figure 1F) consistent with its expression in alveolar type 2 cells. These data indicated that Cela1 inhibition could result in digestive, renal, or immunological toxicities.
Toxicity Assessment of KF4 Anti-CELA1 Antibody
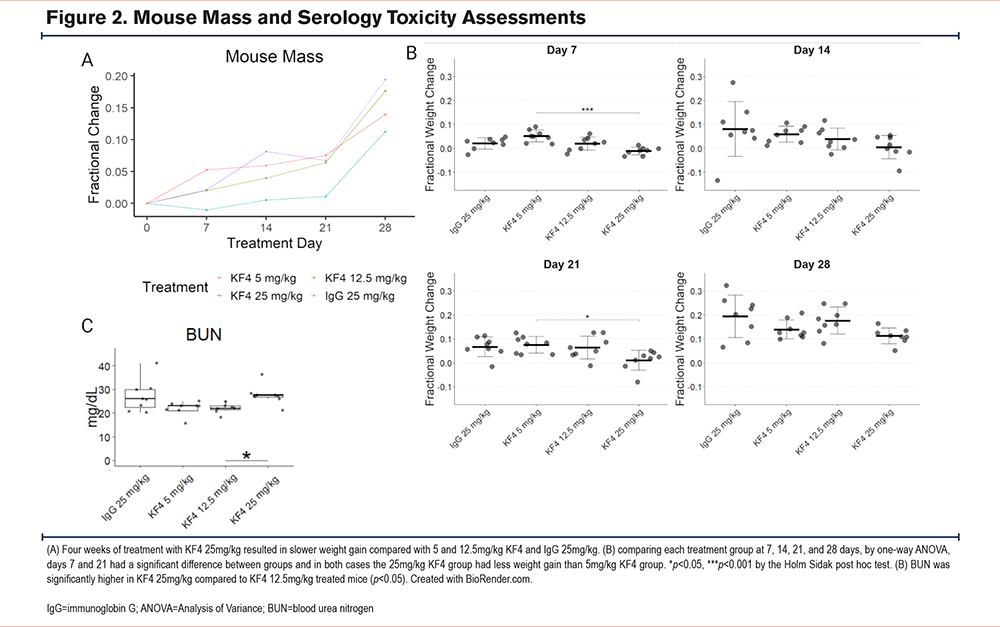
To test for toxic effects of KF4, we assessed administered intraperitoneal injections totaling 5mg/kg KF4, 12.5mg/kg KF4, 25mg/kg KF4, or 25mg/kg KF4 weekly for 4 weeks. We recorded mouse health assessments 3 times weekly, mouse mass weekly, and at 4 weeks collected blood for serology and performed blinded tissue injury scoring of mice. Throughout the 4 weeks, no mouse showed evidence of distress (i.e., scored less than 3 on a 0–3 scale of mouse health). Mice treated with 25mg/kg KF4 per week had less weight gain than lower dose KF4 or IgG treatment (Figure 2A-B). Serology of these mice after 4 weeks of treatment showed no differences in liver function tests, kidney function tests, myocardial or other muscle injury, or lipid profile except for a slightly higher blood urea nitrogen (BUN) level in KF4 25mg/kg treated mice (Figure 2C, Supplemental Figure 2 in the online supplement). These data suggest mild toxicity in mice treated with KF4 25mg/kg weekly.
We performed a 3-reviewer blinded histological scoring of lung, liver, kidney, and heart tissues from these mice. As a positive control, we used tissues from mice subjected to CLP—a mouse sepsis model in which these scoring systems have been validated. Sham-treated mice in these experiments were also evaluated. For antibody-treated and control images, 3 blinded reviewers scored each image on 4 criteria using the rubrics in the online supplement.
In histologic evaluation of lung injury, both CLP and sham animals had more evidence of lung injury than antibody-treated animals (Figure 3A). Reviewer 1 consistently scored animals as having a higher level of lung injury resulting in a Krippendorff’s alpha coefficient of 0.35, but this difference was similar across groups. In otherwise healthy mice, escalating doses of KF4 do not cause any greater degree of lung injury than that of nonspecific immunoglobulin and levels similar to that of an animal undergoing a sham abdominal surgery.
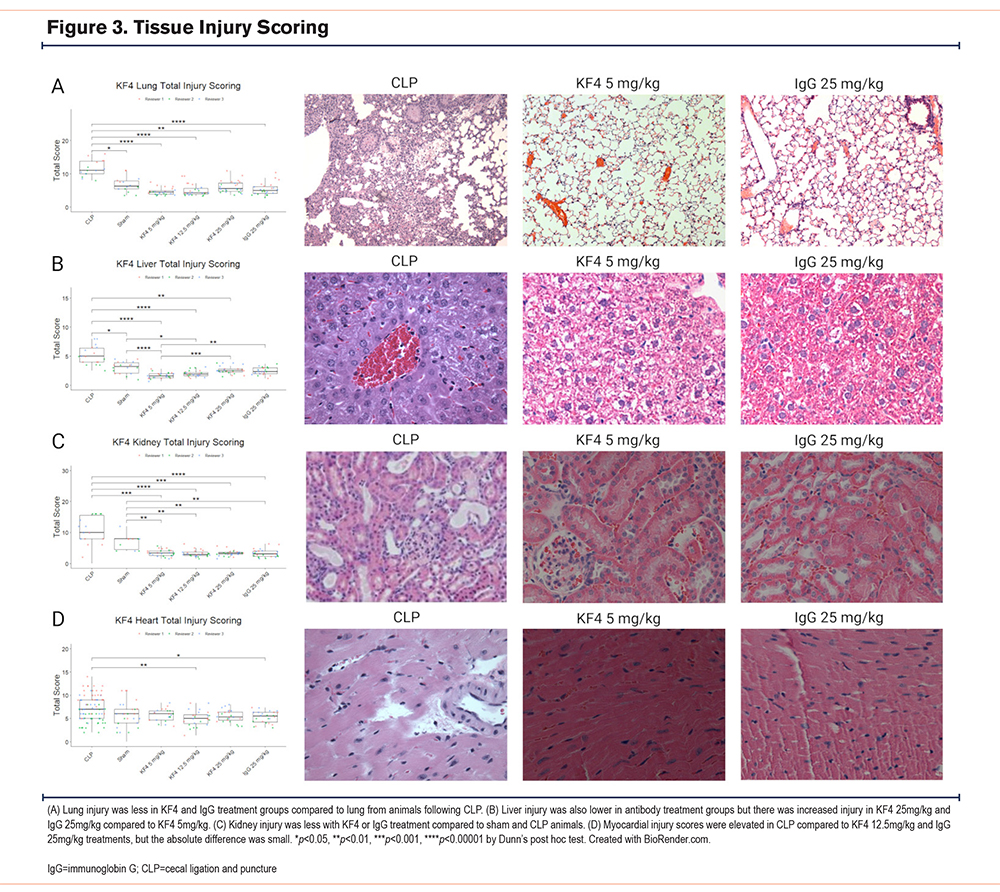
A similar pattern was observed in the liver. The injury levels of both CLP-treated animals had higher injury levels than all other groups, and sham-treated animals had greater injury than KF4 5mg/kg and 12.5mg/kg doses. A small but significant increase in liver injury was noted comparing both IgG and KF4 25mg/kg doses compared to KF4 5mg/kg (Figure 3B). Interreviewer agreement was moderate with a Krippendorff’s alpha of 0.54.
For kidney analysis, CLP and sham-operated animals had higher levels of injury than any of the antibody-treated groups (Figure 3C). There was substantial interrater variability, and agreement was poor with an alpha value of 0.32.
For myocardial injury scoring, only mild injury was noted in the CLP group which was greater than the injury observed in KF4 12.5mg/kg and IgG 25mg/kg administration (Figure 3D). Interrater agreement was again poor with an alpha of 0.279.
Taken together these data show evidence of mild liver injury with administration of KF4 or IgG 25mg/kg that did not result in liver function test abnormalities.
Efficacy of KF4 in Preventing Emphysema
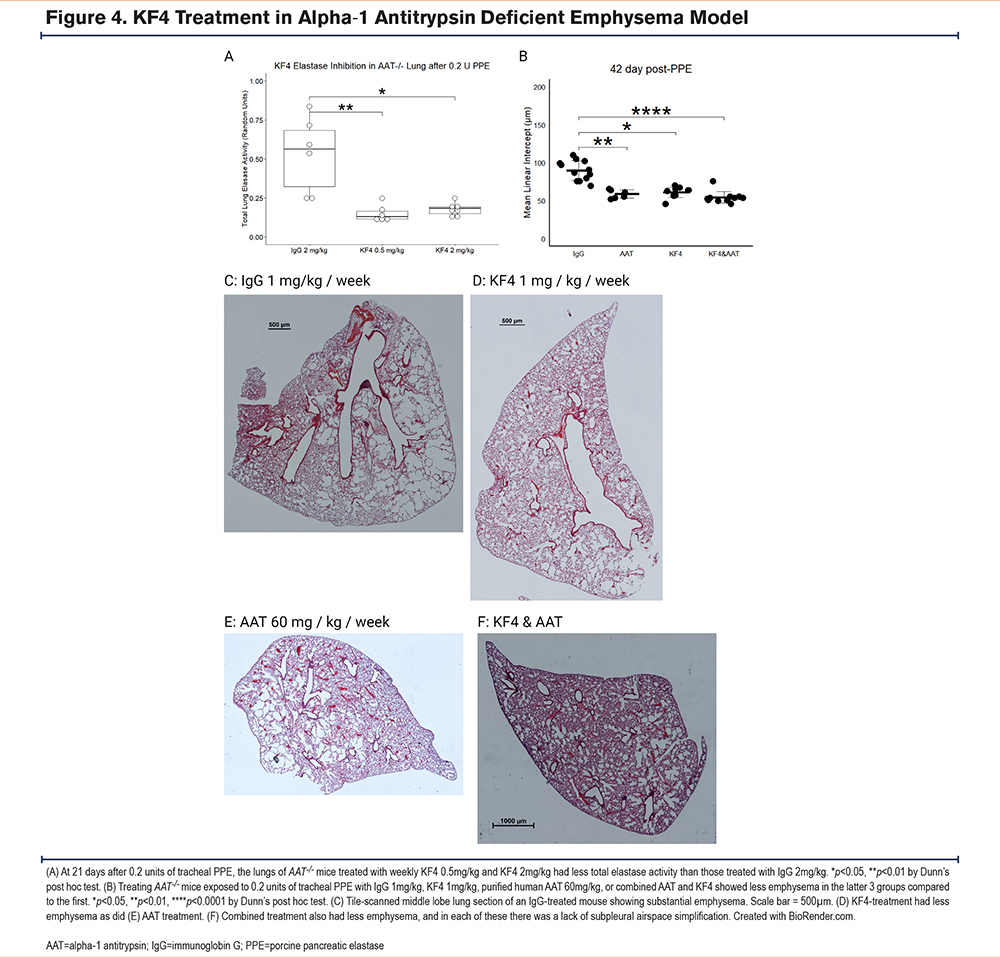
Using the previously described low-dose PPE model of AAT-deficient emphysema,7 we performed a dose titration experiment using lung homogenate from AAT-/- mice administered 0.2 units of tracheal PPE and treated with IgG 2mg/kg, KF4 2mg/kg, or KF4 0.5mg/kg in a colorimetric elastase activity assay. Both KF4 doses significantly reduced lung elastase activity (Figure 4A). We then performed an efficacy assay by administering the same tracheal PPE dose to AAT-/- mice and treating them with IgG 1mg/kg/week, KF4 1mg/kg/week, purified human AAT 60mg/kg/week, or combined KF4 and AAT. These last 3 treatment groups had less emphysema at 42 days than IgG treatment (Figure 4B). In evaluating tile-scanned lung sections, it appeared that this protection was largely due to a reduction in subpleural emphysema (Figure 4C-F).
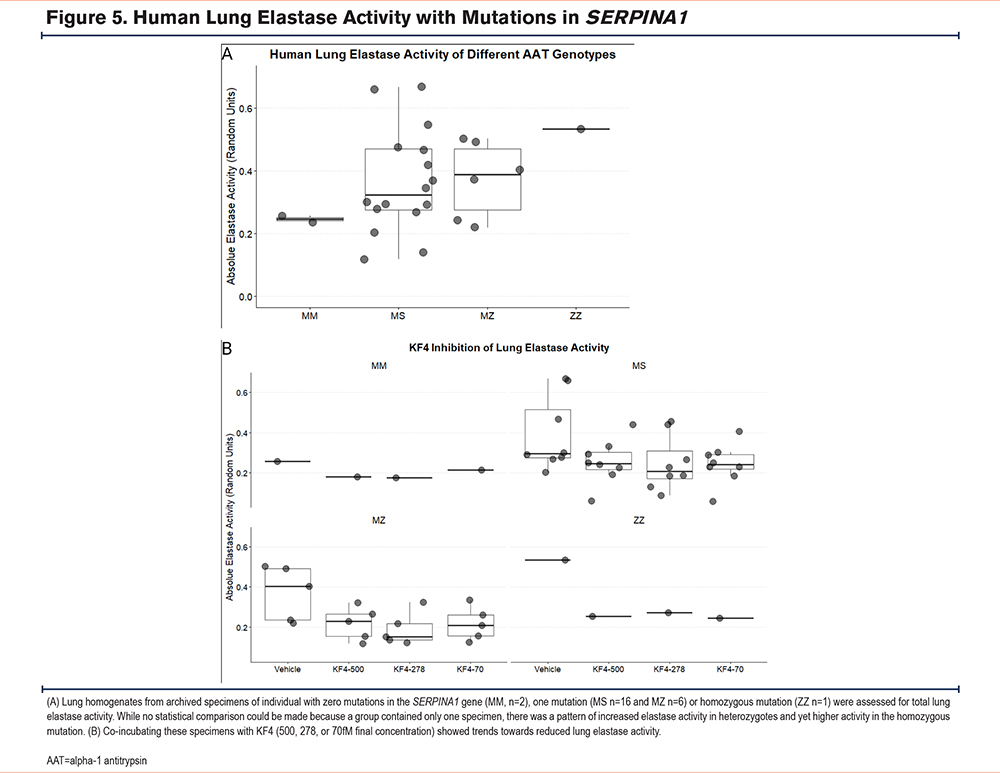
Ability of KF4 to Reduce Human Lung Elastase Activity in AAT-Deficient Emphysema
We obtained archived frozen lung specimens of individuals with mutations in the SERPINA1 gene and quantified homogenate elastase activity. The set included 2, 16, 6, and 1 MM, MS, MZ, and ZZ genotypes, respectively. While there was substantial variability, the elastase activity of MS and MZ specimens was about 40% higher than MM, and ZZ was a little more than double (Figure 5A). Co-incubating specimens (1, 8, 5, and 1 of MM, MS, MZ, and ZZ, respectively) with 500pM, 178pM, and 70pM KF4 demonstrated that KF4 reduced elastase activity in all of the genotypes although none reached statistical significance (Figure 5B). These data suggest that KF4 can reduce the lung elastase activity of individuals with genetic mutations in the SERPINA1 gene.
Discussion
In this study, we showed that the KF4 anti-CELA1 antibody and purified human AAT were similarly effective at preventing emphysema in a mouse model of AAT-deficient emphysema and that KF4 has the potential for renal and hepatic toxicity. Combining AAT and KF4 treatment did not result in less emphysema than either treatment alone. These data are supportive of a central role of CELA1 in AAT-deficient emphysema and suggest that selective CELA1 neutralization could be an alternative for purified AAT replacement therapy in AAT-deficient emphysema.
Our murine toxicity studies suggest that KF4 has a therapeutic index of at least 12.5 and that the 2 toxicities of greatest concern are renal and digestive. Renal toxicity was evidenced by an elevation of BUN, and evidence of histologic liver injury was observed in the highest administered KF4 dose. Although we did not see any elevation of liver injury markers, these may have become elevated with a longer duration experiment. It is likely that reduced mouse weight with KF4 25mg/kg treatment compared to lower doses and IgG is due to intestinal malabsorption. Since IgG is secreted into the intestinal lumen,18 it is likely that both pancreatic and brush border-synthesized Cela1 is neutralized and at 25mg/kg, malabsorption is induced. Stool studies are needed to confirm this. Due to the asynchronous nature of our experiment, we were unable to perform any hematologic assessments which is a limitation of our study. Interestingly, in humans CELA1 is not expressed in pancreatic acinar cells19,20 due to a mutation in Pancreatic Transcription Factor 1. This coupled with the fact that CELA1 but not CELA2A, CELA2B, CELA3A, or CELA3B is highly conserved in placental mammals6 and loss of function mutations are more rare in CELA1 than expected8 suggest an important, nondigestive role for CELA1. In our short-term murine toxicity studies and inhibition studies in emphysema models, at least one of these roles appears to be postnatal lung remodeling with potential intestinal and immune roles that need to be better defined.
We previously reported that Cela1-/- mice gain weight at rates comparable to wild-type mice.6 The finding of slower weight gain in mice treated with KF4 25mg/kg compared to IgG 25mg/kg suggests the possibility that other digestive enzymes are upregulated with Cela1 is absent throughout development; although, we cannot rule out some other developmental effect. The feces of mice treated with KF4 25mg/kg were indistinguishable from those in other groups, but a biochemical assessment of those feces might reveal important differences.
We chose to employ the low-dose PPE model of AAT-deficient emphysema based on previous work showing that Cela1-ablation protected AAT-deficient mice in this model.7 The model is relatively quick (42 days) and has a high signal-to-noise ratio, but it does not accurately capture many important clinical elements of AAT-deficient emphysema. AAT<-/- mice will develop spontaneous emphysema with age which is consistent with the clinical scenario of diagnosing patients with AAT-deficiency after emphysema is discovered in the fourth and fifth decades of life.21 A more rigorous assessment of KF4 in AAT-deficient emphysema needs to test whether it can prevent this age-related emphysema. Another concern with the PPE model is that since KF4 neutralizes one of the proteases that comprises PPE, it may be that the difference between KF4 and IgG is due to modifying the insult rather than intrinsic lung protective effects. Arguing against this is that Cela1 ablation in AAT-/- mice demonstrated similar levels of protection,7 in wild-type mice, delaying KF4 initiation by one week also protected mice against PPE-induced emphysema.8
Our findings are most consistent with our previously described model that CELA1 acts in the progression but not the initiation of emphysema.8 This would explain seemingly contradictory findings that Cela1-/- and KF4-treated mice develop less emphysema in response to 6 months of cigarette smoke exposure compared to wild-type or IgG-treated mice,8 but cigarette smoke-exposed AAT-/- and Cela1-/- mice have slightly but significantly greater emphysema than AAT-/- mice.7 Daily cigarette smoke exposure causes persistent inflammation and the activity of leukocyte-associated proteases like neutrophil elastase, cathepsin G, and matrix metalloproteinases are likely greater in this model than in the PPE model used in this study. Since AAT-/- mice lack the canonical anti-protease to counter these proteases, they are perhaps more important than Cela1-mediated remodeling in the context of continued inflammation. In wild-type mice, endogenous AAT mitigates the activity of these proteases, and the Cela1 effect is more prominent. Exactly how the genetic absence of Cela1 potentiates the effect of these other proteases, whether and how Cela1 changes the behavior of Naïve CD4 T-cells, and whether these findings also occur with Cela1 inhibition need further study. Such studies are needed because increased inflammation is present in the lungs of AAT-deficient individuals with normal lung function,22 T-cells are activated and bronchus-associated lymphoid tissue larger in AAT-deficiency,23 AAT modulates oxidative activity in neutrophils,24 and AAT seems to play a role in alveolar macrophage function.25
To the best of our knowledge, this study is the first to use ex vivo lung tissue to test whether AAT or any other therapeutic can reduce overall lung elastolytic activity. In the 1970s before electrophoretic shift and molecular diagnosis approaches were available, serum elastase inhibitory capacity was used for diagnosis of AAT deficiency.26 In a small study, the anti-elastase capacity of alveolar lining fluid was shown to be elevated after treatment with human purified AAT.27 Many studies including the RAPID trial use serum anti-elastase capacity as a measure of efficacy.28 The approach of using lung tissue from genotyped individuals is obviously limited by specimen availability, but it could be a valuable last preclinical test of novel compounds and biologics aimed at reducing lung remodeling in AAT-deficiency since there is no intermediate animal model between mice and humans. While our results only neared statistical significance and were limited by a small number of samples, they showed a consistent pattern in which both AAT and KF4 reduced overall lung elastase activity in an AAT-deficient lung.
There are several important limitations of this study. First, we did not evaluate long-term toxicity. The fact that all mice gained weight in this study is reassuring, but it may be that with longer exposures, the effects of digestive dysfunction, liver impairment, or renal injury would become more pronounced. AAT-/- mice were not used for toxicity assessment. While toxicities seen in wild-type mice could be safely assumed to also affect AAT-/-, it may be that the altered immune or oxidative state of AAT-/- animals would make them more sensitive to toxicities. However, the dose at which injury was observed was 10-fold higher than the therapeutic dose, and it may be that KF4 is effective at even lower doses. KF4 and AAT treatment could have directly impacted the degree of injury as stated above. Lastly, the combination of KF4 and AAT replacement could have caused some amount of injury associated with a higher osmotic load obscuring potential benefit of combination therapy.
In conclusion, we found that the KF4 anti-CELA1 antibody and purified human AAT were similarly effective at preventing emphysema in a mouse model of AAT-deficient emphysema but that there was no benefit to combined therapy.
Acknowledgements
Author contributions: AJD, NJS, and BMV made substantial contributions to the conception or design of the work. AJD, NJS, RJ, BBP, JD, ANV, EMG, QF, and BMV made substantial contributions to the acquisition, analysis, or interpretation of the data. AJD, BZ, and BMV drafted the manuscript or revised it critically for important intellectual content. All authors gave final approval of the version of the manuscript to be published and agree to be accountable for all aspects of the work ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data sharing statement: The data used in manuscript presentation is publicly available at 10.6084/m9.figshare.25403464
We would like to thank Mark Brantly at the University of Florida for providing purified, recombinant human alpha-1 antitrypsin.
Declaration of Interests
AJD, NJS, RJ, BBP, JD, ANV, EMG, QF, and BZ have nothing to declare. Brian Varisco and Cincinnati Children’s Hospital Medical Center hold patent WO2021108302A1 Cela-1 inhibition for treatment of lung disease.