Running Head: Visceral Fat and Myocardial Infarction in COPD
Funding Support: This work was supported by National Institutes of Health Grants: COPDGene, R01HL089897, R01HL089856; Dr. San José Estépar, 1K25HL104085 and R01 HL116473; Dr Washko, R01 HL116473 and R01 HL107246; Dr. Diaz, K01HL118714-01 and the Brigham and Women’s Hospital Minority Faculty Career Development Award.
Date of Acceptance:August 14, 2014
Abbreviations: visceral adipose tissue, VAT; subcutaneous andipose tissue, SAT; myocardial infarction, MI; computed tomography, CT; Global initiative for chronic Obstructive Lung Disease, GOLD; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; General Electric, GE; first lumbar vertebrae, L1; liver to spleen attenuation, LSA; low attenuation areas, LAA; percent of low attenuation areas, %LAA; standard deviation, SD; intraclass correlation coefficient, ICC; body mass index, BMI; interleukin-6, IL-6
Citation: Diaz AA, Young TP, Kurogol S, et al. Abdominal visceral adipose tissue is associated with myocardial infarction in patients with COPD.
Chronic Obstr Pulm Dis. 2015; 2(1): 8-16. doi:
http://doi.org/10.15326/jcopdf.2.1.2015.0127
Introduction
Cardiovascular diseases are frequent and linked to increased risk of hospitalization and mortality in patients with chronic obstructive pulmonary disease (COPD).1,2 Nearly 50% of the deaths in COPD are due to cardiovascular diseases.3 Although the mechanisms linking COPD and cardiovascular diseases are unclear, smoking, lung inflammation, and reduced lung function are thought to be involved.2 In the general population, increased content of adipose tissue depots including abdominal fat,4 abdominal visceral fat,5 and liver fat6,7 have also been found to be associated with coronary artery disease by potentially promoting a systemic proatherogenic and inflammatory state.
Computed tomography (CT) is a non-invasive technique which allows measurement of various fat depots.5-9 Prior investigation has shown that individuals with obstructive lung disease have an increased amount of visceral adipose tissue (VAT) measured on CT scans.10 Further high VAT content has been associated with low-grade systemic inflammation in patients with COPD,11 which in turn may increase the risk of cardiovascular disease. The potential role of other fat compartments such as subcutaneous adipose tissue (SAT) and liver adipose tissue on the cardiovascular risk in COPD patients remain to be explored. Based on these findings, we hypothesize that body fat depots are associated with MI in patients with COPD. We examined CT measures of VAT, SAT, and liver adipose tissue in patients with a wide range of COPD from the COPDGene study to test this hypothesis.
Methods
We used data from the COPDGene Study,12 which was designed to assess the genetic and epidemiological associations with COPD in non-Hispanic white and African-American smokers aged 45-80 years. Individuals with active lung diseases other than COPD, emphysema, and asthma were excluded. COPDGene was approved by the internal review board at each participating center, and all patients provided written, informed consent. The current analysis was approved by the Partners HealthCare Research Committee (2007P-000554).
Patient Selection
In this analysis, we selected smokers with COPD from the first 2500-data set who had Global initiative for chronic Obstructive Lung Diseases13 (GOLD) stages I-IV COPD, which is defined as a post-bronchodilator ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) <0.7.
Outcome and Covariates Evaluation
We used the Medical History Questionnaire to assess the primary outcome: self-reported physician-diagnosed myocardial infarction. Patients who responded yes to the question “Have you ever been told by a physician that you have had a heart attack (MI)?” were coded as MI. High cholesterol was coded as present if the individual either self-reported high cholesterol or was on any lipid modifying drug. Diabetes was defined based on either self-reported, physician diagnosis of the condition or diabetes medication use. Hypertension was defined as follows: a) systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or b) if the patient self-reported history of hypertension and was using a blood pressure modifying medication. Medications were extracted from the Medication Questionnaire. Other variables including age, gender, and smoking history were extracted from the Eligibility Questionnaire and Respiratory Disease Questionnaire. The questionnaires are available at www.copdgene.org.
Lung Function Assessment
Spirometric measures of lung function were performed before and after the administration of albuterol according to American Thoracic Society recommendations.14 Postbronchodilator FEV1 and FVC were expressed as percent of predicted values.15
CT Evaluation
All participants underwent volumetric CT scanning of the chest without contrast in the supine position at coached full inspiration and relaxed exhalation. Data collected from inspiratory CT scans were used to measure adipose tissue compartments. Images were acquired with the following CT protocol: for General Electric (GE) LightSpeed-16, GE VCT-64, Siemens Sensation-16 and -64, and Philips 40- and 60-slice scanners: 120kVp, 200mAs, and 0.5s rotation time. Images were reconstructed using a standard algorithm at 0.625mm slice thickness and 0.625mm intervals for GE scanners; using a B31f algorithm at 0.625 (Sensation-16) or 0.75mm slice thickness and 0.5mm intervals for Siemens scanners; and using a B algorithm at 0.9mm slice thickness and 0.45mm intervals for Philips scanners.16
CT Assessment of Fat Depots
Measures of VAT area, SAT area, and liver adipose tissue were performed with in-house software by trained readers who were blinded to patients’ data (Figure 1).
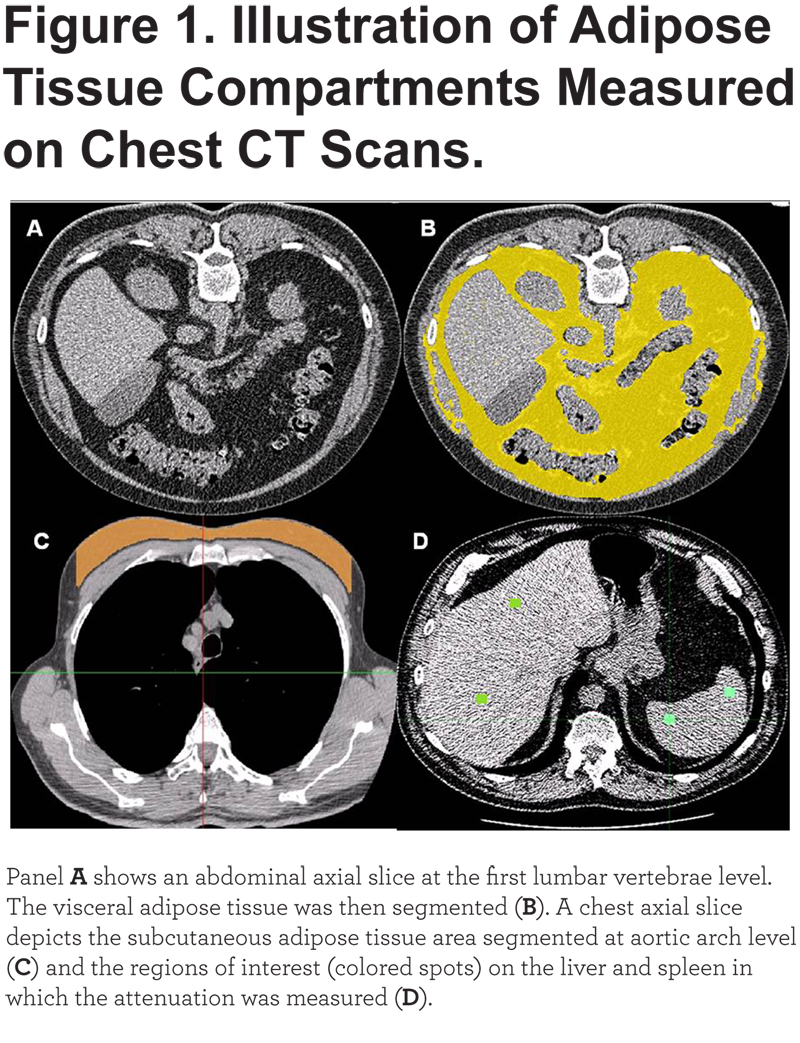
VAT was measured on a single axial slice of the CT scan at the inferior edge of the transverse process of the first lumbar vertebrae (L1). This anatomical reference was selected because it was easy to identify and could be replicated across a large cohort of participants. The abdominal visceral fat was then identified on the selected slice and manually segmented using a pre-defined attenuation range of -250 and -50 Hounsfield Units.17 SAT was measured on a single slice between the pectoralis muscles and skin surface at aortic arch level. We used this site to measure SAT area because the CT field of view was smaller than the circumference of the chest. Additionally, both the aortic arch and the pectoralis muscle are easily identified in non-contrast CT scans. The reader visually identified the first axial image above the superior aspect of the aortic arch and then manually segmented the subcutaneous fat using a pre-defined attenuation range of -200 and 0 Hounsfield Units. The interior of the segmented VAT and SAT were then filled automatically and the software provided the areas of these fat depots, which are reported in cm2. CT measures of VAT and SAT have been previously validated and used in clinical studies.8,18,19 Liver and spleen attenuation on CT scans were measured in 4 and 2 homogenous appearance regions of interest (1 x 1 cm), respectively. Spleen attenuation was used as a reference. The values obtained were averaged to calculate both a patient’s mean attenuation value and ratio of liver to spleen attenuation (LSA). A low ratio indicates greater fat infiltration of the liver. Fatty liver was defined as a ratio of liver to spleen attenuation equal to or less than 1.10.20 In random samples of 80 and 60 patients the inter-reader reproducibility of VAT and SAT was assessed, respectively.
CT Quantification of Emphysema
CT measures of low attenuation areas (LAA) were performed with open source software, Airway Inspector. Emphysema was defined as percent LAA (%LAA) less than -950 Hounsfield Units on the CT scan.21
Statistical Analysis
Data is presented as mean ± standard deviation (SD) and median interquantile range as appropriate. Inter-reader reproducibility was assessed with the intraclass correlation coefficient (ICC) test and Bland-Altman analysis. For visual purposes, regression line plots are shown. Contingency tables and Fisher exact test was performed for comparison of categorical data. Continuous variables were compared with Wilcoxon rank-sum test. The association between MI and adipose tissue depots was assessed with logistic analyses. The VAT area and SAT area distributions did not follow normality and these measures were used as binary variables, upper tertile vs. lower tertiles. We used the upper tertiles as the frequency of MI peaked in this segment of the distribution. Covariates in the model included age, gender, pack years of smoking, obesity (defined as body mass index [BMI]≥30), high cholesterol, high blood pressure, and diabetes. A P value <0.05 was considered significant. Analyses were performed with SAS 9.3 (SAS Institute, Cary, NC).
Results
Out of the 2500 first COPDGene participants, 1272 had COPD. We then excluded 5 patients in whom VAT could not be measured (3 patients had no images available or the CT images were truncated above L1; 2 patients had a severe spinal deformity, which led to the inclusion of lung on L1 level), leaving a final sample size of 1267 patients. Myocardial infarction was reported by 83 (6.6%) COPD patients and their characteristics by MI status are shown in Table 1.
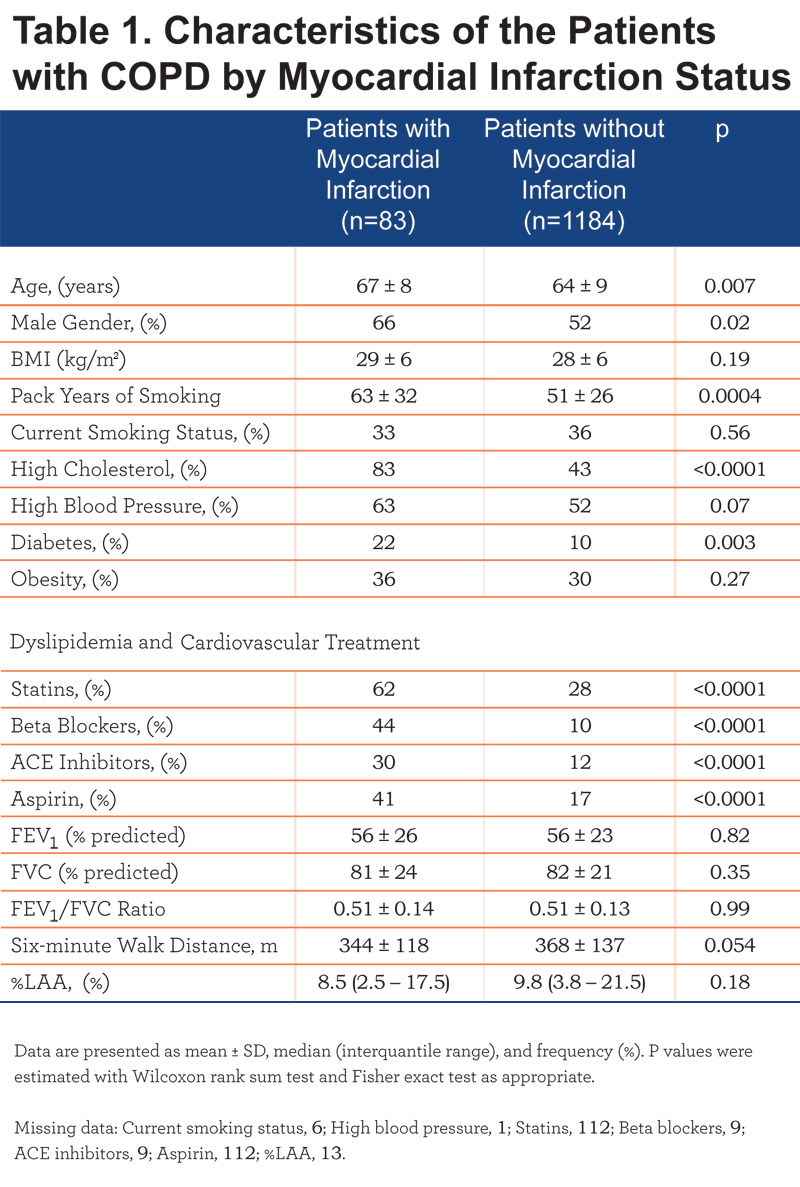
Compared to patients with no history of MI, those with a prior MI were more likely to be older and male, and report higher frequency of cardiovascular risk factors including high cholesterol, high blood pressure, and diabetes. They also reported higher frequency of therapy for cardiovascular disease and dyslipidemia. No differences in BMI, obesity frequency, lung function, and %LAA were found.
Reproducibility of VAT and SAT Measurements
The ICC of the inter-reader reproducibility of both VAT area and SAT area was excellent: 0.99. The Bland-Altman analysis did not show a systemic bias across the range of VAT area and SAT area values (Figure 2).
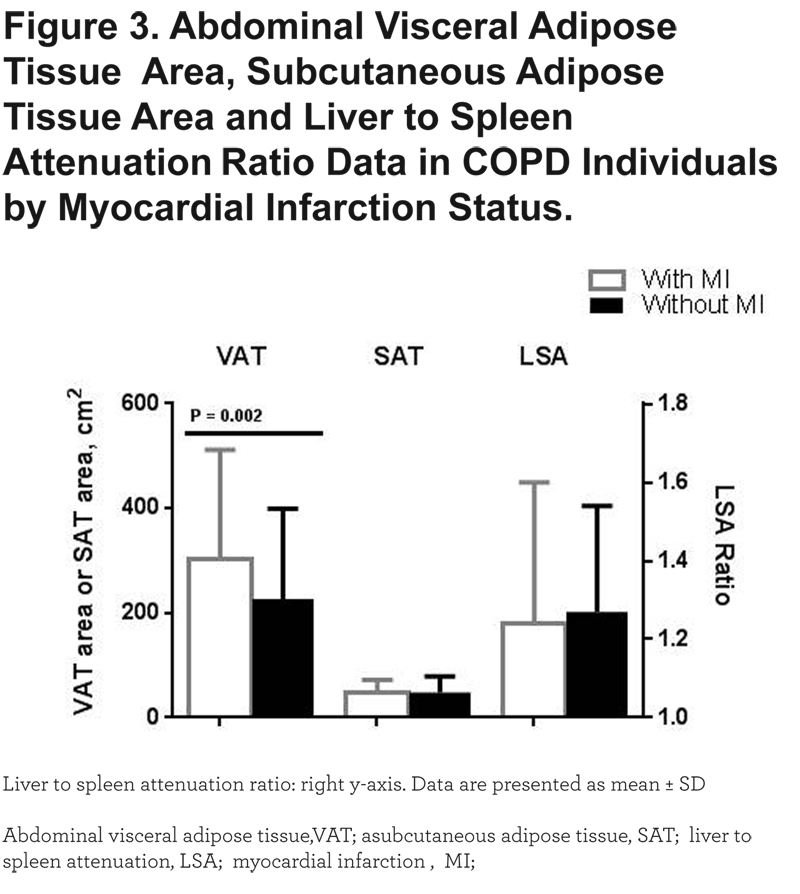
Association Between Myocardial Infarction, Fat Depots, and Cardiovascular Risk Factors
Compared to patients with no history of an MI, those who reported an MI had a higher VAT area (303.4 ± 208.5 vs. 226.8 ± 172.6 cm2; P=0.002). In contrast, there was no difference in the SAT area and LSA ratio between the 2 groups (Figure 3).
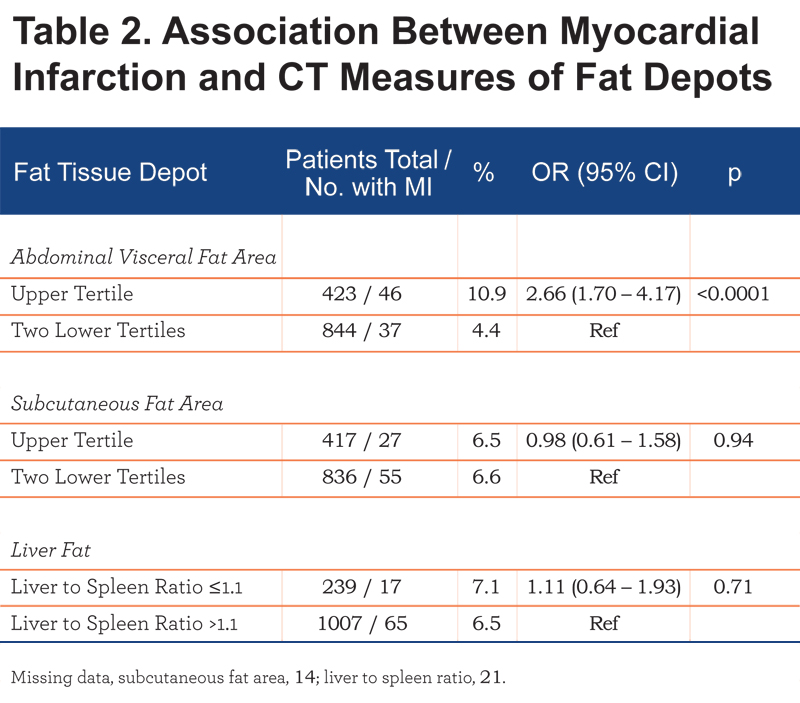
Patients in the upper tertile of the VAT area had a higher frequency of MI compared to those who were in the lower 2 tertiles as well as increased odds of MI (odds ratio [OR], 2.66 [95% confidence interval (CI) 1.70 – 4.17]). No association between MI and either SAT area or fatty liver (LSA ratio ≤1.1) was found (Table 2).
We then used VAT as predictor along with other covariates for MI in multivariate regression.
In univariate analyses for MI, increasing age, increasing pack years of smoking, male sex, high blood pressure, high cholesterol, and diabetes increased the odds of MI by a varying degree (Table 3).

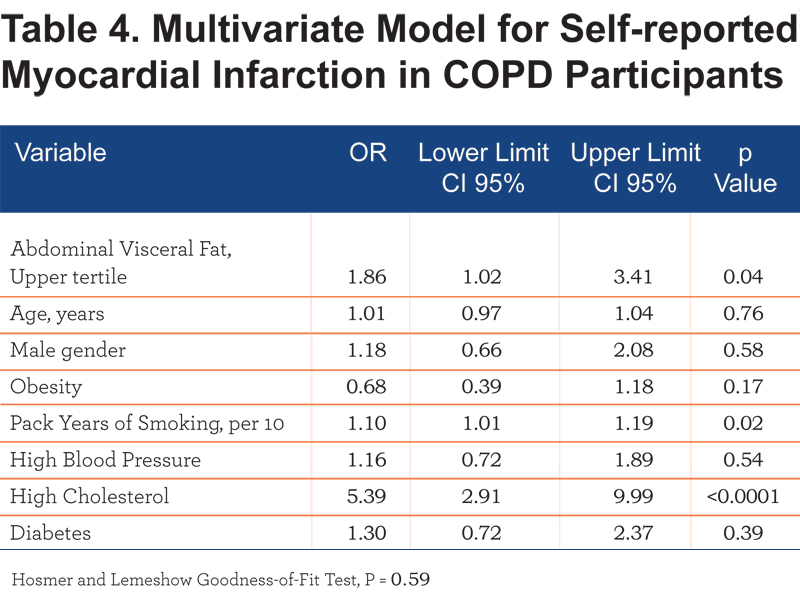
After adjustment for other cardiovascular risk factors, the association between MI and VAT area was attenuated but remained significant (OR 1.86, [1.02 – 3.41]) (Table 4).
In an additional model to account for exercise and lung function as confounding the relationship between MI and VAT area, we included age, gender, BMI, six-minute walk, and FEV1% predicted. In this model, the relationship between MI and VAT area was significant (OR 2.78, [1.45 – 5.30]; P=0.002).
Discussion
In this study we collected CT measures of abdominal visceral fat and subcutaneous fat in patients with COPD to assess the relationship between these adipose tissue depots and MI. We found that the patients who had a history of an MI had larger abdominal visceral fat content than those who did not report this event. After adjustment for relevant covariates, patients within the upper tertile of VAT had increased odds of having an MI.
Several studies have documented that an increased amount of visceral fat, fatty liver disease, and lower CT attenuation of abdominal visceral fat and subcutaneous fat are related to cardiometabolic risk and cardiovascular diseases in different populations.5,6,19,22 In patients with COPD, an altered body composition has been observed with a relative increase in fat mass and visceral fat.10 Also in COPD patients, an increased epicardial adipose tissue has been documented and found to be directly related to coronary artery calcification,23 which is used for cardiovascular risk assessment. We have expanded this prior knowledge by demonstrating that stable COPD patients with history of an MI had higher CT VAT content and that the highest VAT content was associated with increased odds of MI.
In our study, we found that the association between reported MI and CT measures of VAT area was independent of other known cardiovascular risk factors including age, sex, high blood pressure, high cholesterol, smoking intensity, obesity, and diabetes, and of lung function and exercise capacity. This finding is consistent with a prior study in which increased visceral fat was related to higher odds of CT-based coronary artery disease.5 However, a recent study24 did not find such a relationship. Differences in population demographic, cardiovascular risk profile and outcome ascertainment between the latter and our study may explain this discrepancy. The question is then why abdominal visceral fat can increase the odds of MI in patients with COPD beyond the factors included in our modeling.
VAT is considered a proatherogenic fat depot and a source of mediators of inflammation.11 For example, increases in visceral fat have been associated with increased interleukin-six (IL-6) levels in patients with COPD.11 A low-grade systemic inflammation is in turn associated with cardiovascular diseases including ischemic heart disease25 and IL-6 blood levels were found to be higher among COPD patients with heart disease.26 Additionally, visceral fat was positively related to levels of plasminogen activator inhibitor type 1, a fibrinolytic regulation factor.27 While we do not have data on these biomarkers, we speculate that a COPD patient’s VAT content might be a unique pathogenic fat depot potentially involved in those biomarker pathways.
We found no association between MI, subcutaneous fat area and fatty liver measured on CT scans. This finding might reflect differences in the biological activity related to proatherogenic or inflammatory mediators among the adipose tissue depots we assessed. Omental fat releases more IL-6 than subcutaneous fat28 and while VAT was associated with the level of plasminogen activator inhibitor type 1, subcutaneous fat was not.27 Our findings are in agreement with the notion that among these assessed adipose tissue compartments visceral fat seems to have a unique pathogenic role on cardiovascular diseases, but more data are needed to understand the complex relationship between these various adipose tissue depots and cardiovascular outcomes. Since VAT content can be modified by diet and exercise,29 our findings may have clinical implications. COPD patients with a high risk for MI can benefit from interventions aimed to reduce VAT content.
Addressing the mechanisms leading to an increased VAT content in COPD patients with a history of an MI is out of the scope of our study. Other investigators have demonstrated several potential mechanisms of fat accumulation in humans including inflammation, hypertryglyceridemia, and reduced glucose utilization.30
Several limitations should be noted. There may be a selection bias due to the inclusion of patients who survived a heart attack and thus our estimates should be interpreted with caution. The outcome of interest and cardiovascular risk factors such as high blood pressure, cholesterol level, and diabetes was a mixing of self-reported physician diagnoses and self-reported patient medication use. Additionally, the timing of these diagnoses was not recorded by the COPDGene study. Self-reporting cardiovascular diseases has an inherent recall bias.31 However, as expected, high cholesterol and diabetes were higher among individuals with a history of an MI. We used a single cross-sectional measure of adipose tissue from existing chest CT scans. While this approach avoids additional exposure to radiation and is similar to that used in prior studies, it does not provide information about the heterogeneity of adipose tissue compartments. Despite this limitation, we observed a strong relationship between our measure of VAT and BMI (r=0.68, P<0.0001), which was comparable to that from abdominal CT scans.9 Similarly, SAT was also associated with BMI (r=0.71, P<0.0001). Finally, our study is a cross-sectional analysis of the data and determining causality of the relationship of interest is not possible. The relationship we found is the basis for more research in the emerging field of extrapulmonary manifestations of COPD.
In summary, in this study we found that COPD patients with an MI had higher abdominal visceral fat content than those with no prior history of an MI. Higher abdominal visceral fat content was independently associated with increased odds of a history of an MI. Further studies are warranted to assess the potential mechanisms linking MI and abdominal visceral fat in this population.
Acknowledgements
Author contributions: Conception and design of this study and creation, revision, and final approval of this manuscript: AAD, TPY, SK, EE, NM, JKC, GLK, JCR, RSJ, RH, JBS, MB, RPB, JH, GRW; Analysis and interpretation: AAD, JBS, RPB, GRW; Acquisition and process of the data: AAD, TPY, SK, EE, NM, JKC, JCR, RH; Drafting the manuscript for important intellectual content: AAD, RPB, JH, GRW. Dr. Diaz takes full responsibility for the content of the manuscript, including the data and analysis. The sponsor had no role in designing, analyzing, writing, or reporting this work.
Declaration of Interest:
Drs. Diaz, Kurugol, San José Estépar, Harmouche and Misters Young, Eckbo, Chapman, Ross, and Miss Muralidhar have no conflicts of interest to disclose. Dr. Washko has received consulting fees from Spiration, Inc, and his spouse is an employee of Merck & Co, Inc.