Running Head: GOLD-adherent Prescribing and Resource Utilization
Funding Support: This study was supported by Novartis Pharmaceuticals Corporation.
Date of Acceptance: April 2, 2015
Abbreviations: electronic health records, EHR; Global initiative for chronic Obstructive Lung Disease, GOLD; health care resource utilization, HCRU; International Classification of Diseases, Ninth Revision, Clinical Modification, ICD-9-CM; emergency department, ED; odds ratio, OR; National Heart, Lung, and Blood Institute, NHLBI; National Institutes of Health, NIH; World Health Organization, WHO; inhaled corticosteroids, ICSs; forced expiratory volume in 1 second, FEV1; Health Insurance Portability and Accountability Act, HIPPA; interquartile range, IQR; long-acting beta-agonist,LABA; long-acting muscarinic antagonist, LAMA; modified Medical Research Council Dyspnea scale, mMRC; phosphodiesterase-4, PDE4; short-acting beta agonist, SABA; short-acting muscarinic agonist, SAMA; cardiovascular disease, CVD; chronic kidney disease, CKD
Citation: Mannino DM, Yu TC, Zhou H, Higuchi K. Effects of GOLD-Adherent prescribing on COPD symptoms burden, exacerbations and health care utilization in a real-world setting. Chronic Obstr Pulm Dis. 2015; 2(3): 223-235. doi: http://doi.org/10.15326/jcopdf.2.3.2014.0151
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent, progressive airflow limitation associated with enhanced inflammatory responses of the airways and lungs.1 COPD affects approximately 26.8 million Americans2 and is a leading cause of death2 and disability.3 The annual direct and indirect economic costs of COPD management were estimated to be approximately $50 billion in the United States alone in 2010.2 In an effort to provide state-of-the-art information to clinicians on strategies to prevent and treat COPD, the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), and World Health Organization (WHO) launched the Global initiative for chronic Obstructive Lung Disease (GOLD).1 GOLD publishes periodic updates to its report Global Strategy for the Diagnosis, Management, and Prevention of COPD, a widely recognized guideline.1
Pharmacologic therapy is the mainstay of COPD treatment, and GOLD guidelines1 recommend specific drug therapy protocols based on symptoms and risk for exacerbations. For example, short-acting bronchodilators are recommended for treatment of intermittent symptoms in patients with mild COPD, but persistent symptoms in moderate, severe, and very severe patients should be treated with 1 or more long-acting bronchodilators. GOLD guidelines recommend prescribing inhaled corticosteroids (ICSs) in combination with long-acting bronchodilators in patients with severe and very severe COPD, who by definition are at high risk for exacerbations.
The guidelines also emphasize the use of spirometric measures of lung function (i.e., forced expiratory volume in 1 second [FEV1]) in both the diagnosis and evaluation of treatment effectiveness in COPD. However, in patients with COPD, FEV1 may not accurately reflect disease impact.4 Thus, a comprehensive appreciation of the effects of pharmacotherapy requires that spirometry be accompanied by other assessments, including quality of life, symptoms, exacerbations, and health care resource utilization (HCRU) patterns and costs. This study used an electronic health record (EHR) database to assess the effect of adherence and nonadherence to GOLD prescribing guidelines on COPD symptom burden, exacerbations, and HCRU.
Methods
Patients
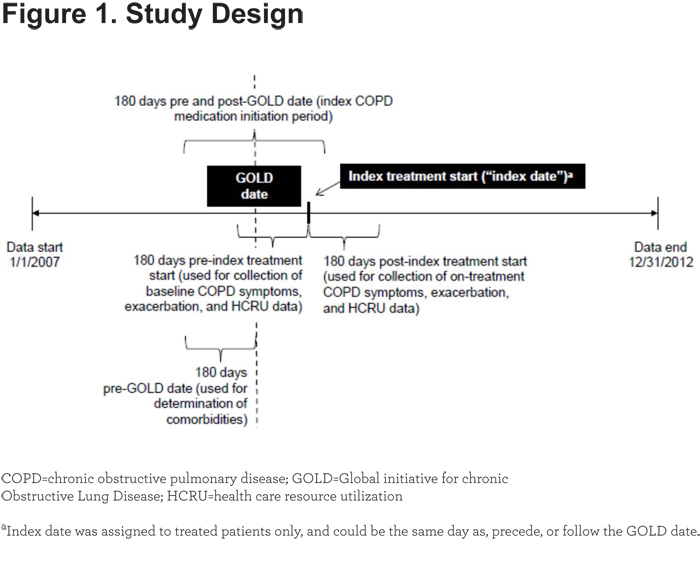
This study used data from the Humedica EHR database from January 1, 2007, to December 31, 2012 (Figure 1).
Humedica’s data acquisition model starts with the providers of care, including many prominent integrated delivery networks. Humedica aggregates EHR data directly from providers, integrating multiple EHRs from across the continuum of care, both inpatient and ambulatory. These data capture a comprehensive clinical picture that includes medications, lab results, vital signs, physician notes, diagnoses, procedures, demographics, hospitalizations, and outpatient visits. Once aggregated, Humedica normalizes, validates, and statistically de-identifies these data for use in clinical investigations. The data are certified as de-identified by an independent statistical expert following Health Insurance Portability and Accountability Act (HIPPA) statistical de-identification rules, and managed according to Humedica’s customer data use agreements.
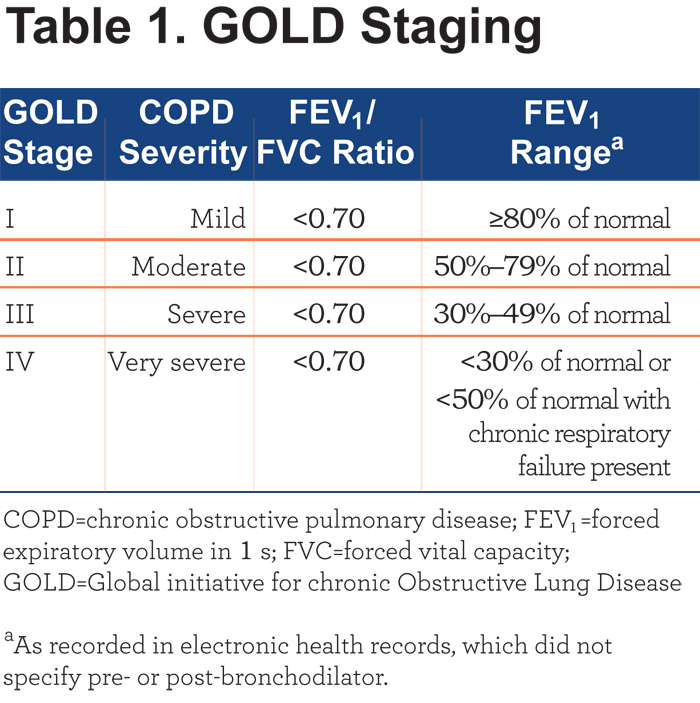
Included patients had COPD (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 490.xx, 491.xx, 492.xx, 496.xx), a valid GOLD stage (i.e., stages I–IV; Table 1) within the study period, and were 40 to 90 years of age (inclusive) at the time of the first GOLD staging.
If there were multiple valid GOLD stages between the date of first GOLD stage (“GOLD date”) and the date of index COPD treatment start (“index date”), the most recent GOLD stage was used. Index COPD treatment was the first COPD treatment (i.e., inhaled beta-agonists or muscarinic antagonists, ICSs, ICS/long-acting beta-agonist [LABA] combination, or phosphodiesterase-4 inhibitor) given during the period 180 days post-GOLD date. If a patient did not receive COPD treatment during the period 180 days post-GOLD date but did receive treatment during the 180 days prior to the GOLD date, the most recent treatment in the pre-GOLD period was the index COPD treatment. Untreated patients were those who had received no COPD treatment 180 days prior to and 180 days following the GOLD date. The GOLD stage (and date) for untreated patients was the first GOLD stage assigned within the study period. The first month active (defined as the earliest month with a recorded health care activity event in the Humedica data set) was required to be ≥12 months before the GOLD date, and the last month active was required to be ≥12 months after the GOLD date.
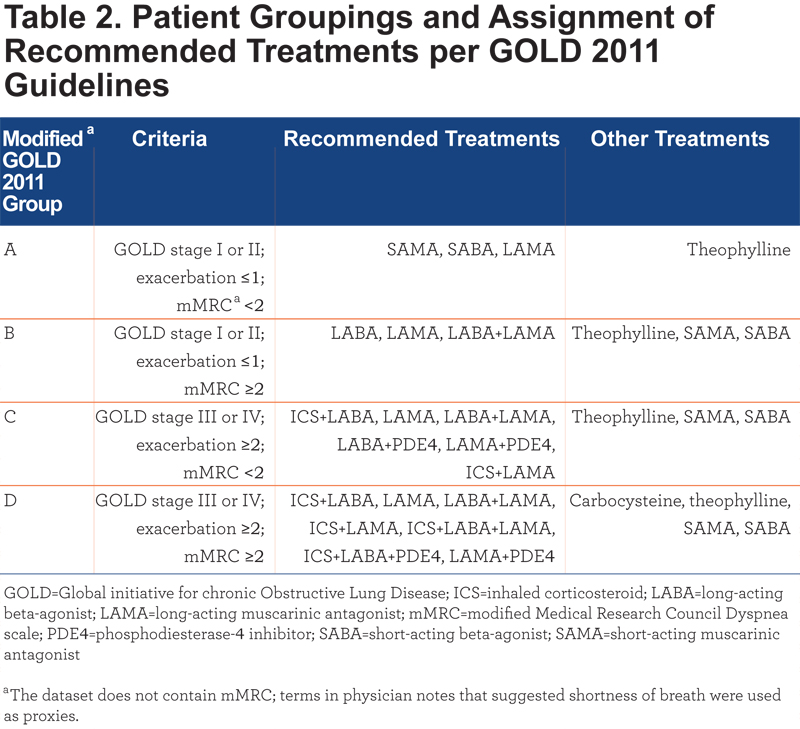
Patients who had a valid GOLD stage and treatment during the period 180 days pre- through 180 days post-GOLD date were assigned to groups A, B, C, and D according to a modified system based on GOLD 2011 guidelines (Table 2).5 Untreated patients could not be assigned an index date and were retained for evaluation of baseline characteristics only. Patients with missing or unknown sex, birth year, or region were excluded.
Study Design
This was a retrospective study. Adherence or nonadherence to GOLD-defined5 prescribing was based on COPD medication prescribed within 180 days of the GOLD date (Table 2). Because this study reflected real-world prescribing behavior, 25% to 50% of patients were prescribed a COPD medication before spirometry tests were conducted, and thus, before a COPD diagnosis could be confirmed. For these reasons, a timeframe of 180 days before and 180 days after GOLD staging was used to identify the index COPD treatment and to determine adherence or nonadherence to GOLD-defined prescribing. COPD symptoms, exacerbations, and HCRU-related variables were quantified during the 180-day period following index treatment start.
Outcomes and Statistical Analyses
Patient and clinical characteristics of interest included age, sex, race, region, and GOLD stage at GOLD date as well as COPD-related symptoms (e.g., shortness of breath, coughing, wheezing, fewer activities), exacerbations (i.e., oral/intravenous steroids with antibiotic use, COPD-related emergency department (ED) visit, and COPD-related hospitalization), and HCRU (all-cause and respiratory-specific hospitalizations, ED visits, office visits, other outpatient visits [i.e., any EHR not occurring in the office]) during the 180-day period before the index treatment start. COPD symptoms, exacerbations, and HCRU during the period 180 days following index treatment start were compared for patients receiving GOLD-adherent treatment versus those receiving GOLD-nonadherent treatment. Odds ratios for categorical/dummy variables were analyzed using chi-square test (or Fisher exact test when ≥20% of cells had an expected value <5). For continuous variables, mean differences were analyzed using 2-sample t test with unequal variance.
Patients receiving nonadherent prescribing were divided into undertreated and overtreated groups and compared with patients receiving GOLD-adherent prescribing. Undertreatment and overtreatment were defined using the GOLD 2011 guidelines for pharmacotherapy summarized in Table 2. For example, a patient in group C prescribed only LABA was undertreated, and a patient in group B prescribed ICS plus LABA was overtreated. Because the goal of the study was to assess the impact of adherence versus nonadherence to GOLD prescribing guidelines, patients with no COPD treatment during the period 180 days pre- and 180 days post-GOLD date were not included in comparative analyses. However, their characteristics during the period 180 days before the GOLD date were summarized descriptively to provide additional insights into COPD treatment patterns.
Results
Patient Characteristics
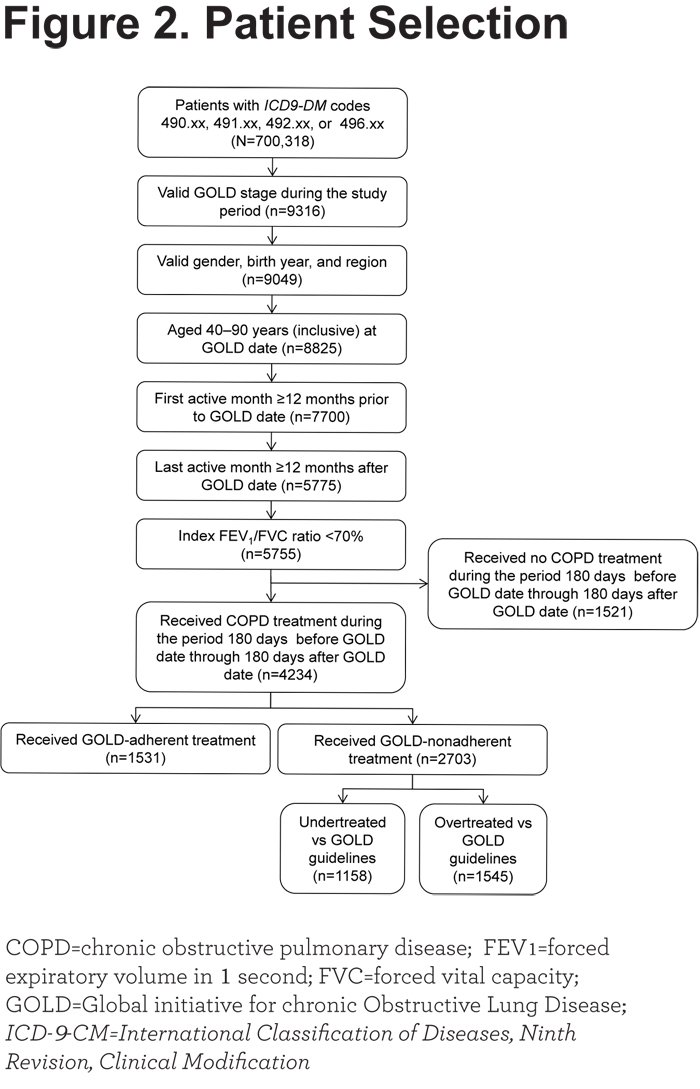
A total of 700,318 patients with a COPD diagnosis based on ICD-9-CM codes were identified, but only 9316 (1.3%) had spirometry that resulted in a valid GOLD stage during the study period. After the remainder of inclusion and exclusion criteria were applied, 4234 treated and 1521 untreated patients were eligible (Figure 2). A total of 1678 treated patients had a GOLD stage but did not fit into groups A, B, C, and D as defined in Table 2 (i.e., they were GOLD stage III or IV with ≤1 exacerbation or GOLD stage I or II with ≥2 exacerbations). These patients were assigned to group C (shortness of breath absent). Most patients (82%) started index treatment after the GOLD date (mean, 32 days after); 18% started index treatment before the GOLD date (mean, 57 days before).
Our patient sample was generally older, and more likely to be Caucasian than the general COPD population (87% versus 67%). Baseline characteristics of patients receiving GOLD-adherent prescribing (n=1531), GOLD-nonadherent prescribing (n=2703 [undertreated, n=1158; overtreated, n=1545]), and no treatment (n=1521) are summarized in Table 3 and Table S1 in the online data supplement.
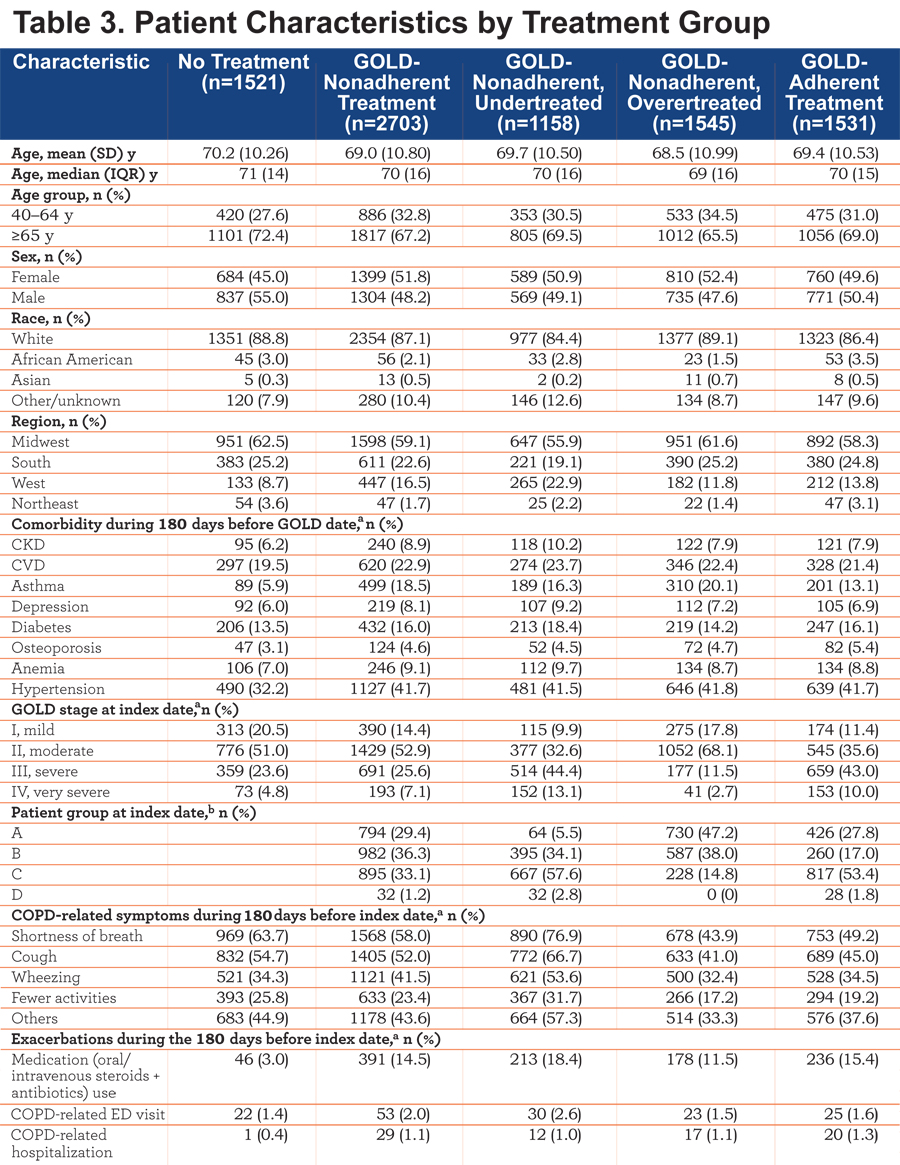
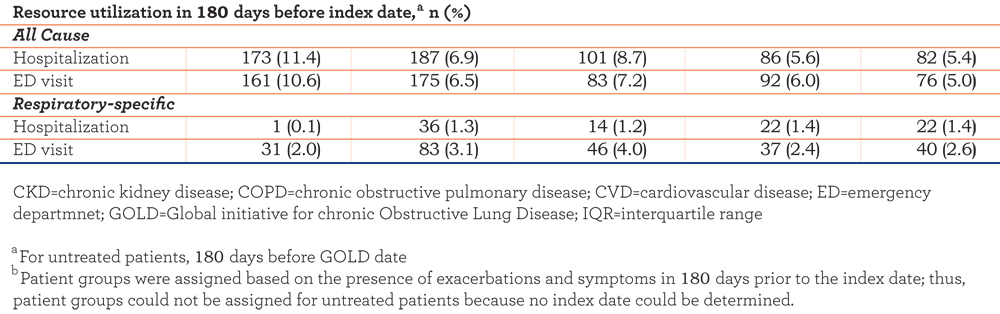
GOLD-adherent and -nonadherent groups had similar demographics. When compared with patients who received GOLD-adherent prescribing, greater proportions of patients in the GOLD-nonadherent group had asthma during the 180 days before the GOLD date, had COPD symptoms during the 180 days before index treatment start, and had milder COPD, based on GOLD stage. Patients receiving no COPD treatment had a low prevalence of asthma (5.9%) during the 180 days before the GOLD date and had predominantly moderate (51.0%) or severe (23.6%) COPD, based on GOLD stage, and generally higher prevalence of COPD symptoms than patients who received GOLD-adherent or -nonadherent treatment. Undertreated patients had mostly moderate (32.6%) or severe (44.4%) COPD based on GOLD staging, and had a higher prevalence of COPD symptoms and a lower prevalence of comorbid asthma (16.3% versus 20.1%) than overtreated patients.
Impact of GOLD-adherent Versus GOLD-nonadherent Prescribing on COPD Symptoms and Exacerbations
Nearly all outcomes showed a numerical benefit of GOLD-adherent over GOLD-nonadherent prescribing. Patients in the GOLD-adherent group had lower odds of experiencing all COPD-related symptoms during the 180 days following index treatment start than those in the GOLD-nonadherent group (Figure 3).
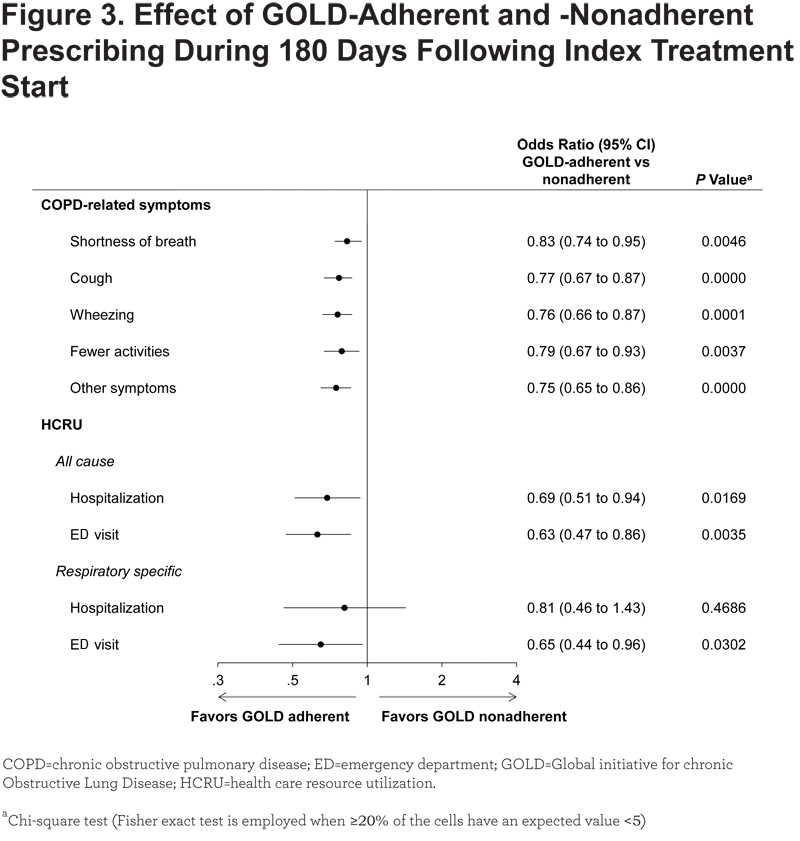
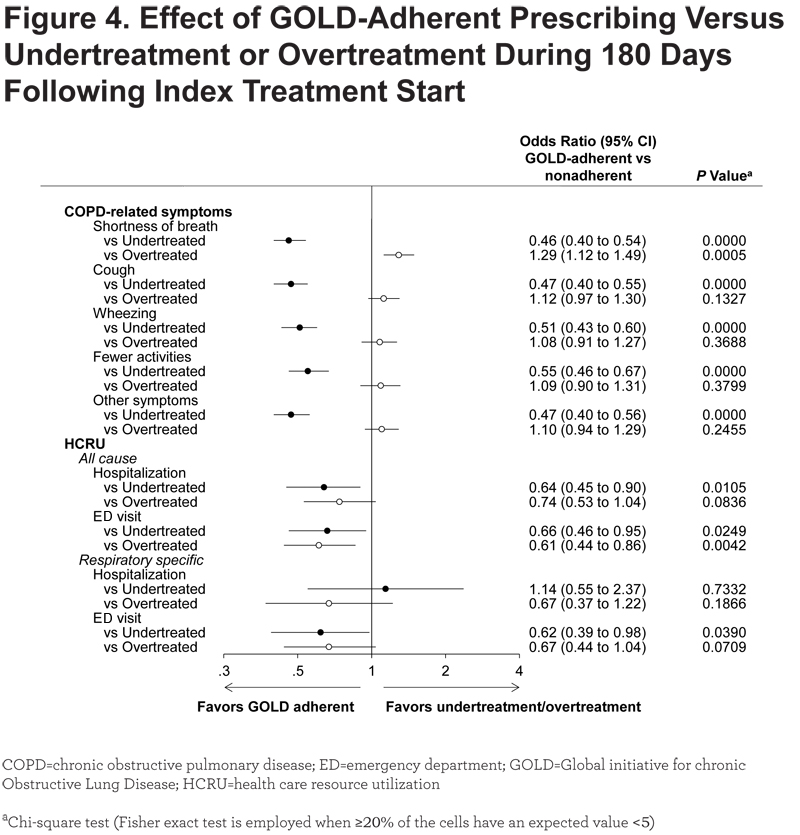
Although the magnitude of the absolute reductions was small (4%–6%), odds ratios ([ORs]; 0.75–0.83) indicated relative reductions that were statistically significant for all symptoms evaluated. GOLD-adherent prescribing was associated with significantly lesser proportions of patients with COPD-related symptoms during the 180 days following index treatment start compared with undertreatment (all ORs, 0.46–0.55; P<0.0001) and with a significantly higher proportion of patients with shortness of breath compared with overtreatment (OR, 1.29; P=0.0005; Figure 4).
Relatively few patients experienced study-defined exacerbations during the study period, and incidences and frequencies of exacerbations were not statistically different between GOLD-adherent and -nonadherent groups (Table S2, online supplement). However, GOLD-adherent prescribing was associated with a significant decrease in patients using oral/intravenous steroids and antibiotics compared with undertreatment (OR, 0.78; P=0.0338; Table S3, online supplement) and a significant increase in this endpoint compared with overtreatment (OR, 1.32; P=0.0256;Table S4-online supplement).
Impact of GOLD-adherent Versus GOLD-nonadherent Prescribing on All-cause HCRU
GOLD-adherent prescribing was associated with significant reductions in proportions of patients with all-cause hospitalizations and ED visits compared with GOLD-nonadherent prescribing (OR, 0.69 and 0.63, respectively), although only 4% to 6% of patients in both groups experienced these events during the 180 days following index treatment (Figure 3). Compared with undertreatment, GOLD-adherent prescribing was associated with a significant reduction in all-cause hospitalizations and ED visits (OR, 0.64 [P=0.0105] and 0.66 [P=0.0249], respectively; Figure 4). Similarly, GOLD-adherent prescribing was accompanied by a significant decrease in all-cause ED visits compared with overtreatment (OR, 0.61; P=0.0042; Figure 4).
Significant benefits of GOLD-adherent versus -nonadherent prescribing were also observed for per-patient frequency of all-cause hospitalizations, ED visits, and office visits, but means were low in both groups (Table S2). GOLD-adherent prescribing was associated with reduced per-patient frequencies of all-cause hospitalizations, ED visits, and office visits compared with undertreatment (mean differences, –1.455 to –0.0308; all P<0.05; Table S3, online supplement) and reduced per-patient frequency of all-cause ED visits versus overtreatment (mean difference, –0.0312; P=0.026; Table S4, online supplement).
Impact of GOLD-adherent Versus GOLD-nonadherent Prescribing on Respiratory-specific HCRU
A significant reduction in the proportion of patients with respiratory-specific ED visits was also observed with GOLD-adherent versus –nonadherent prescribing (2.3% versus 3.5%), but the proportion of respiratory-specific other outpatient visits (i.e., visits not occurring in the office) was greater versus the GOLD-nonadherent group (44.7% versus 40.9%; Table S2, online supplement and Figure 3). GOLD-adherent prescribing was associated with significant reductions in the proportions of patients with respiratory-specific ED visits and office visits compared with undertreated patients (OR, 0.62 [P=0.039] and 0.74 [P<0.001]), but respiratory-specific other outpatient visits were increased (OR, 1.31; P<0.001; Table S2, online supplement). Proportions of patients with respiratory-specific HCRU with GOLD-adherent prescribing were generally similar to those observed with overtreatment (Figure 4, Table S4, online supplement).
Numeric benefits of GOLD-adherent prescribing were observed for the per-patient frequencies of respiratory-specific hospitalizations, ED visits, and office visits but did not reach statistical significance versus GOLD-nonadherent prescribing (Table S2, online supplement). A reduction in the frequency of respiratory-specific office visits was associated with GOLD-adherent prescribing versus undertreatment (mean difference, –0.2392; P=0.0031; Table S3, online supplement). Respiratory-specific other outpatient visits were significantly increased with GOLD-adherent versus nonadherent prescribing (mean difference, 0.1366; P=0.0019; Table S2, online supplement). GOLD-adherent prescribing was associated with significant increases in per-patient frequency of respiratory-specific other outpatient visits versus undertreatment (mean difference, 0.1647; P=0.0017; Table S3, online supplement) or overtreatment (mean difference, 0.1155; P=0.0180; Table S4-online supplement).
Discussion
The current study used real-world data obtained from an EHR database to assess the effects of GOLD-adherent prescribing on symptoms, exacerbations, and HCRU in patients with COPD. The results suggest that only ~36% of patients with COPD are prescribed GOLD-adherent pharmacotherapy. Furthermore, GOLD-adherent prescribing delivered moderate benefits with respect to COPD symptoms and HCRU, with no significant benefit on study-defined exacerbations. Compared with GOLD-adherent prescribing, undertreatment was associated with significant increases in a number of COPD symptoms, and both undertreatment and overtreatment were associated with increases in a number of HCRU endpoints. Our findings are a meaningful complement to measures of lung function, on which approvals of COPD pharmacotherapies are based, but which represent only 1 component of disease impact.
Nonadherence to GOLD prescribing guidelines may occur for a number of reasons, and any discussion of drivers of nonadherence must take into account that we cannot know the prescriber’s intention and the full clinical picture of the individual patient. The finding that GOLD-adherent therapy is often not prescribed is consistent with the published literature. A review of medical records for 169 randomly selected patients with COPD from 12 U.S. communities showed that adherence to GOLD-recommended prescribing was 56%.6 A questionnaire-based study of Swiss general practitioners revealed that prescribing of LABAs or ICSs conformed to GOLD 2007 guidelines in nearly two thirds of patients with GOLD stage III or IV disease, but only 31% of patients with GOLD stage I and 18% of patients with GOLD stage II disease.7 A survey of 154 primary care physicians in New York also showed suboptimal adherence to GOLD guidelines for prescribing LABAs (54%) and ICSs (41%) in patients with COPD.8 Physicians cited lack of familiarity with specific recommendations and low self-efficacy for performing specific recommendations as key barriers to GOLD-adherent prescribing. Low perceived benefit of GOLD recommendations did not play a substantial role in GOLD-nonadherent prescribing.
Our descriptive analysis of baseline characteristics of patients who received no treatment, undertreatment, overtreatment, or GOLD-adherent prescribing sheds further light on drivers of nonadherence to GOLD guidelines. For example, overtreated patients had a higher prevalence of asthma comorbidity than patients receiving GOLD-adherent prescribing (20.1% versus 13.1%), and patients with no COPD treatment had the lowest prevalence of asthma of any group (5.9%). However, patients receiving no COPD treatment or undertreatment were more likely to experience COPD symptoms during the 180 days before the index date than were overtreated patients or those receiving GOLD-adherent prescribing. Undertreated patients had predominantly moderate and severe COPD, based on GOLD staging, whereas overtreated patients had predominantly moderate COPD. These data suggest that the level of COPD treatment may often be based on asthma symptoms rather than on objective spirometric thresholds specified in the GOLD guidelines. However, speculation regarding drivers of GOLD-adherent versus -nonadherent prescribing should take into account that HCRU data collection took place from 2007 through 2012. Thus, GOLD 2011 guidelines, on which our analyses were based, were not available to physicians for a large portion of the data collection period. Furthermore, application of our inclusion criteria likely led to a cohort rich in patients with an index date earlier in the study period. Truncated look-forward adherence and health care activity periods may have disallowed the inclusion of some patients with an index date toward the end of the 2012.
The moderate reductions in symptoms and HCRU observed with GOLD-adherent prescribing have a number of important implications. Although quality of life was not assessed in this analysis, Blinderman et al9 reported strong inverse correlations between quality of life and total symptom distress among patients with advanced COPD. Thus, the moderate reductions in COPD symptom burden observed with GOLD-adherent prescribing may have improved the quality of life in this study population. Significant reductions in proportions of patients with all-cause hospitalizations and ED visits, as well as reductions in per-patient frequencies of these endpoints observed with GOLD-adherent prescribing, suggest that GOLD-adherent prescribing may also reduce health care costs. Asche et al10 reported that adherence to GOLD prescribing guidelines for long-acting muscarinic antagonist (LAMA) plus LABA/ICS, LAMA plus LABA, and LABA plus ICS were all associated with substantial cost savings in patients with moderate to severe COPD.
The lack of a significant effect of GOLD-adherent prescribing on exacerbations in this study is concordant with the findings of the aforementioned Swiss study,11 in which guideline adherence failed to significantly affect exacerbation rates, symptom prevalence, or lung function decline after 1 year of follow-up. It is possible that both results are attributable to a small number of events observed during the study periods. A longer period of observation may have revealed a beneficial effect of GOLD-adherent prescribing. In our study, exacerbations may also have been captured as all-cause or respiratory-specific ED visits, which were significantly less prevalent with GOLD-adherent versus GOLD-nonadherent prescribing.
Finally, the primary goals of COPD pharmacotherapy, as outlined by GOLD, are to relieve symptoms, improve health status, prevent exacerbations, and improve exercise tolerance.1 Because GOLD prescribing guidelines are based on the latest medical evidence, it is unclear why GOLD-adherent prescribing did not have a greater effect on the COPD outcomes assessed. The lack of more pronounced benefits may be a reflection of patient noncompliance with pharmacotherapy, possibly attributable to lack of insurance coverage or high copays. It is also possible that GOLD-defined prescribing may not be appropriate for all patients with COPD. Indeed, shortness of breath was significantly less prevalent in overtreated patients than in those receiving GOLD-adherent therapy.
The strengths of our study include the real-world setting in which it was performed. Consistent with clinical practice, however, more than 98% of patients did not have a GOLD stage before the index treatment start because spirometry is not often used in the primary care settings, which is where many patients initially present with symptoms. Thus, some GOLD-nonadherent index treatments may have been prescribed before spirometry was performed and a COPD diagnosis was confirmed. Inclusion of the 1521 patients who received no COPD treatment in the undertreated group may have strengthened the analysis of HCRU endpoints. Patients receiving no treatment were not included in the analyses of COPD symptoms, exacerbations, or HCRU because they did not have an index treatment start date on which to base capture of data during the specified period (i.e., 180 days following index treatment start).
The results should also be interpreted in light of the strengths and limitations of EHR data. EHR allows collection of a patient’s symptoms, GOLD stage, untreated comorbidities, and other important clinical information not captured in claims databases. However, EHR systems lack enrollment eligibility. Thus, unlike claims data, where the lack of a claim assumes no utilization, the lack of a record in an EHR system may represent no disease, no treatment, or a missing record, as in instances in which the patient is treated at a health care facility that does not contribute records to the same EHR system. As a result, EHRs might not capture the entire medical history of a patient and may underestimate HCRU. As a proxy for eligibility, we only included patients with EHR records ≥12 months before and ≥12 months after the first valid GOLD stage. This provides some assurance of consistency of care (and data capture) within the same EHR system over 2 years. It should also be noted that the EHR database includes drug prescription information, but not information on fills or patient compliance with prescribed medication. Finally, our EHR dataset did not contain modified Medical Research Council Dyspnea scale (mMRC) scores, so terms in physician notes that suggested shortness of breath were used as proxies. For this reason, our assignments of patients to GOLD-recommended pharmacotherapy groups A, B, C, and D may have been different than assignments performed using the mMRC.
As previously mentioned, the small number of events may have weakened some comparisons, particularly with regard to the incidence and frequency of COPD-specific and respiratory-specific ED visits and hospitalizations. Longer follow-up may better reflect the effect of GOLD-adherent prescribing on these outcomes. Adjustment for multiple comparisons in the analyses comparing HCRU between GOLD-adherent and -nonadherent groups was also precluded by small patient numbers and a large number of endpoints. The degree of uncertainty imparted by the assignment of 40% of the included patients into group C for determination of adherent versus nonadherent treatment must also be acknowledged. One patient could be assigned to group C based on lung function and another based on exacerbations, yet the pharmacotherapy recommendations for determination of GOLD adherence were the same. Additional research is necessary to better understand the large number of COPD patients who did not fit the patient groups (i.e., A, B, C, D) defined by GOLD for prescribing of COPD pharmacotherapy, as well as those patients who did not undergo spirometry.
In summary, the current study supports GOLD-adherent prescribing as a means for reducing symptom burden and HCRU among patients with COPD. Physicians must bear in mind that GOLD-adherent prescribing is only 1 component of optimal COPD care. GOLD recommendations regarding the use of spirometry, nonpharmacologic therapies, and management of comorbidities should also be heeded. These nonpharmacologic measures may include rehabilitation therapy, smoking cessation, and vaccinations. It is also important to stage patients with COPD early so that appropriate medication can be initiated early in the disease course.
Acknowledgments
This study was supported by Novartis Pharmaceuticals Corporation. Editorial support for the preparation of this manuscript was provided by Melinda Ramsey, PhD, and Erica Wehner, RPh, CMPP of Complete Healthcare Communications, Inc. and was funded by Novartis Pharmaceuticals Corporation. All authors contributed to the study concept and design, the statistical analysis, interpretation, writing, and critical review of the manuscript. All authors read and approved the final manuscript. The authors thank anonymous reviewers for their comments and suggestions.
Declarations of Interest
D. Mannino has received consulting fees from GlaxoSmithKline plc, Novartis Pharmaceuticals, Pfizer, Inc., Boehringer-Ingelheim, AstraZeneca PLC, Forest Laboratories, Inc., Merck, Amgen, and Creative Educational Concepts. Furthermore, he has received royalties from Up-to-Date and is on the Board of Directors of the COPD Foundation. K. Higuchi and T.C. Yu are employees and stockholders of Novartis Pharmaceuticals Corporation. H. Zhou is an employee of KMK Consulting (providing services to Novartis Pharmaceuticals Corporation).