Running Head: UPLIFT® COPD: Results by GOLD Grading System
Funding Support: Funded jointly by Boehringer Ingelheim and Pfizer.
Date of acceptance: April 13, 2014
Abbreviations: Understanding Potential Long-term Impacts on Function with Tiotropium, UPLIFT®; Global initiative for chronic Obstructive Lung Disease, GOLD; emergency department, ED; forced expiratory volume in 1 second, FEV1; St George’s Respiratory Questionnaire, SGRQ; confidence interval, CI; chronic obstructive pulmonary disease, COPD; modified Medical Research Council, mMRC; COPD Assessment Test, CAT; health-related quality of life, HRQoL; forced vital capacity, FVC; major adverse cardiac events, MACE; inhaled corticosteroids, ICS; long-acting β2-agonists, LABA; rate ratio, RR; coronary artery disease, CAD; cardiovascular, CV; standard deviation, SD
Citation: Halpin DMG, Tashkin DP, Celli BR, Leimer I, Metzdorf N, Decramer M. Effect of tiotropium on outcomes in patients with COPD, categorized using the new GOLD grading system: Results of the UPLIFT randomized controlled trial. Chronic Obstr Pulm Dis. 2015; 2(3): 236-251. doi: http://doi.org/10.15326/jcopdf.2.3.2014.0142
Introduction
Tiotropium is a once-daily, inhaled anticholinergic that provides at least 24 hours of improvement in airflow and hyperinflation in patients with COPD.1,2 The Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT®) trial was undertaken to assess the long-term efficacy and safety of tiotropium compared to placebo (control) with the primary outcome being the rate of decline in forced expiratory volume in 1 second (FEV1) in patients with COPD (primary endpoint was not met).3 Patients in both groups were allowed to use other COPD medications, including short- and long-acting respiratory medications, but no other inhaled anticholinergics. Patients who participated in the UPLIFT® study were carefully observed over a period of 4 years.
In 2011, the Global initiative for chronic Obstructive Lung Disease (GOLD) published updated guidelines on the diagnosis, management, and prevention of chronic obstructive pulmonary disease (COPD),4 which were revised further in 2013.5 One of the most significant changes was the introduction of a new combined assessment scheme that attempts to “assess the severity of the disease, including the severity of airflow obstruction, the impact on the patient’s health status and the risk of future events (such as exacerbations, hospital admissions, or death)” in order to guide therapy. The scheme uses the degree of postbronchodilator FEV1 impairment and the frequency of exacerbations in the previous 12 months to assess risk, as well as the modified Medical Research Council (mMRC) dyspnea score6 or COPD Assessment Test (CAT) score7 to assess symptoms. Four groups (A, B, C, and D) of patients are identified by this scheme. Recommendations for first choice, alternative choice, and other possible treatments for pharmacologic therapy5 have been made following assessment of patients using this scheme, although the clinical trials on which these recommendations were based did not grade patients using the new scheme and its cut-off points.
In the present work, we have performed a retrospective analysis of UPLIFT® trial data, grading patients into the GOLD groups A, B, C, and D (2013 update), to examine the effects of tiotropium compared to usual care on lung function, health status, and exacerbations according to these groupings.
Methods
We performed a post-hoc analysis of data collected during the UPLIFT® study (ClinicalTrials.gov number: NCT00144339). Details of the study design and results on the primary and secondary endpoints have been previously reported.3,8 All patients gave written informed consent. The study was approved by local ethical review boards and conducted in accordance with the Declaration of Helsinki.
Study Design
The UPLIFT® study was a 4-year, randomized, double-blind, placebo-controlled, parallel-group trial in patients with COPD. The primary endpoints were the yearly rate of decline of pre- and postbronchodilator FEV1 until completion of the double-blind treatment period. Secondary outcomes included other lung function measures, health-related quality of life (HRQoL) as measured by the St. George’s Respiratory Questionnaire (SGRQ) total score, COPD exacerbations, and related hospitalizations, as well as safety and mortality.
Patients were recruited from 490 investigational centers in 37 countries. Criteria for participation included diagnosis of COPD, aged ≥40 years, smoking history of ≥10 pack years, and postbronchodilator FEV1 ≤70% of the predicted normal value and FEV1 ≤70% of forced vital capacity (FVC). Postrandomization clinic visits occurred at 1 and 3 months, and then every 3 months throughout the 4-year treatment period.
The treatment arms were tiotropium 18 µg once daily or matching placebo (control), delivered via the HandiHaler® inhalation device (Boehringer Ingelheim Pharma GmbH and Co KG, Ingelheim, Germany). All respiratory medications other than inhaled anticholinergics were permitted during the trial. At 4 years (approximately Day 1440), all patients were asked to stop the trial drug and were instructed to take open-label ipratropium, 2 actuations (40 µg) 4 times daily, and then to return for a final assessment after a 30-day study drug termination (approximately Day 1470), defined as the washout period.
Spirometry
Spirometry was performed according to American Thoracic Society guidelines9: at randomization, at 30 days, and every 6 months throughout the treatment period; then at a follow-up visit approximately 30 days after the end of study treatment. The study drug was administered immediately after prebronchodilator spirometry and just before short-acting bronchodilator administration. All study sites were provided with identical spirometry equipment and study-specific software. A centralized quality assurance review of all spirometry data was performed during the study.8
St. George’s Respiratory Questionaire
Patients were not assessed using the mMRC dyspnea scale nor the CAT test, but they did complete the SGRQ health status questionnaire. Jones et al10 have shown that there is a tight correlation between SGRQ and CAT scores (r = 0.84; p < 0.001). The regression equation for the relationship was: CAT score = 1.54 + 0.36 x SGRQ. Thus, a CAT score of 10 corresponds to an SGRQ of 23.5; but there is considerable scatter in the relationship and, for this analysis, we have used an SGRQ score of 25 as an alternative for the GOLD symptom threshold CAT score of 10.
Exacerbations
Exacerbations were defined as an increase in, or the new onset of, >1 respiratory symptom (cough, sputum, sputum purulence, wheezing, or dyspnea) lasting ≥3 days and requiring treatment with an antibiotic or a systemic corticosteroid. Moderate exacerbations were defined as those requiring a health care provider visit (e.g., home visit or a visit to an outpatient facility or emergency department [ED]) but not requiring hospital admission). Data regarding exacerbations and related hospitalizations were collected on study-specific case-report forms at every visit. Two recorded exacerbation events separated by less than 7 days were considered as 1 single event. At study entry, patients also reported the number of courses of antibiotics or systemic corticosteroid treatment for breathing problems that they had received in the previous 12 months, as well as the number of hospitalizations and ED visits for COPD in the previous 12 months.
Grading of Patients into GOLD Groups
Using the baseline postbronchodilator FEV1 values, SGRQ scores and reported exacerbation frequency over the preceding 12 months, patients randomized into the UPLIFT® study were retrospectively graded into the GOLD groups as follows: Group A, fewer symptoms (SGRQ total score at baseline <25) and low risk (defined as postbronchodilator FEV1 ≥50% and ≤1 course of antibiotics, ≤1 course of systemic corticosteroids and no COPD-related hospitalization); Group B, more symptoms (SGRQ total score at baseline ≥25) and low risk (postbronchodilator FEV1 ≥50% and ≤1 course of antibiotics, ≤1 course of systemic corticosteroids and no COPD-related hospitalization); Group C, fewer symptoms (SGRQ total score at baseline <25) and high risk (defined as postbronchodilator FEV1 <50% or >1 course of antibiotics or >1 course of systemic corticosteroids or ≥1 COPD-related hospitalization); Group D, more symptoms (SGRQ total score at baseline ≥25) and high risk (postbronchodilator FEV1 <50% or >1 course of antibiotics or >1 course of systemic corticosteroids or ≥1 COPD-related hospitalization).
Safety Analysis
Major adverse cardiac events (MACE) were recorded in the UPLIFT® study and were analysed across the GOLD groups A–D.
Statistical Analysis
Time to first exacerbation was compared between treatment groups using log-rank tests, and Cox regression was used to derive hazard ratios separately for each of the GOLD A–D subgroups. To obtain the GOLD group–treatment interaction p-value, a subgroup–treatment interaction term was added to the model. Kaplan-Meier curves of the probability of no exacerbation were calculated and displayed together with the results of the log-rank test. Number of events was compared between treatment groups using Poisson regression, including the factors subgroup and subgroup-by-treatment interaction, with correction for treatment exposure and over dispersion.
Forced expiratory volume in 1 second, FVC values, and SGRQ total score were compared between treatment groups separately for each of the GOLD A–D subgroups using repeated-measures analysis of covariance without imputation of missing values with fixed, categorical effects of treatment subgroup, month, treatment-by-month interaction, as well as the continuous, fixed covariates of baseline score and baseline score-by-visit interaction. To obtain the subgroup-by-treatment interaction p-value, a subgroup-by-treatment interaction term was added to the model. All reported p-values are 2-sided and not adjusted for multiple testing.
A sensitivity analysis was performed to demonstrate the effects of using different cut-offs in the SGRQ total score on the split between GOLD groups with fewer/more symptoms (A/B, and C/D, respectively).
Deaths (fatal adverse events), fatal MACE and MACE data by GOLD group were summarized using descriptive statistics (number and percentage of patients; incidence rate per 100 patient years).
Results
Study Population
Data were available for 5713 of the 5992 patients randomized and treated in the UPLIFT® trial to allow for classification into GOLD Groups A, B, C, and D. Of these, 357 (6.2%) were in GOLD Group A, 1421 (24.9%) were in Group B, 299 (5.2%) were in Group C, and 3636 (63.6%) were in Group D.
Patient baseline characteristics are shown in Table 1. Although classified on the basis of higher SGRQ scores, patients in Group D also had lower mean FEV1 than those in Group C, but there was no difference in lung function between Groups A and B. In all, 30.4% of patients in Group C and 21.7% of patients in Group D had FEV1 ≥50% predicted and were classified as high risk based on exacerbation history only.
The proportions of patients receiving inhaled anticholinergics and inhaled and oral corticosteroids at baseline are also shown in Table 1. Overall, 171 patients (47.9%) in Group A, 777 (54.7%) in Group B, 192 (64.2%) in Group C, and 2401 (66.0%) in Group D were receiving inhaled corticosteroids (ICS) at baseline; 127 patients (35.6%) in Group A, 592 (41.78%) in Group B, 152 (50.8%) in Group C, and 1915 (52.7%) in Group D were receiving a combination of ICS and long-acting β2-agonists (LABA), either as separate components or as a fixed-dose combination (Table 1).
There was minimal difference between the groups in the proportion of patients with any cardiac disorder, including coronary artery disease, or the proportion receiving cardiovascular medication (Table 1).
By the end of the study, a total of 2344 patients had discontinued. Of these, 74 (3.2%) patients in Group A had discontinued prematurely (39 [3.7%] in the tiotropium group and 35 [2.7%] in the control group), 471 (20.1%) in Group B (223 [21.3%] in the tiotropium group and 248 [19.1%] in the control group), 94 (4.0%) in Group C (46 [4.4%] in the tiotropium group and 48 [3.7%] in the control group), and 1705 (72.7%) in Group D (739 (70.6%) in the tiotropium group and 966 (74.5%) in the control group).
Lung Function
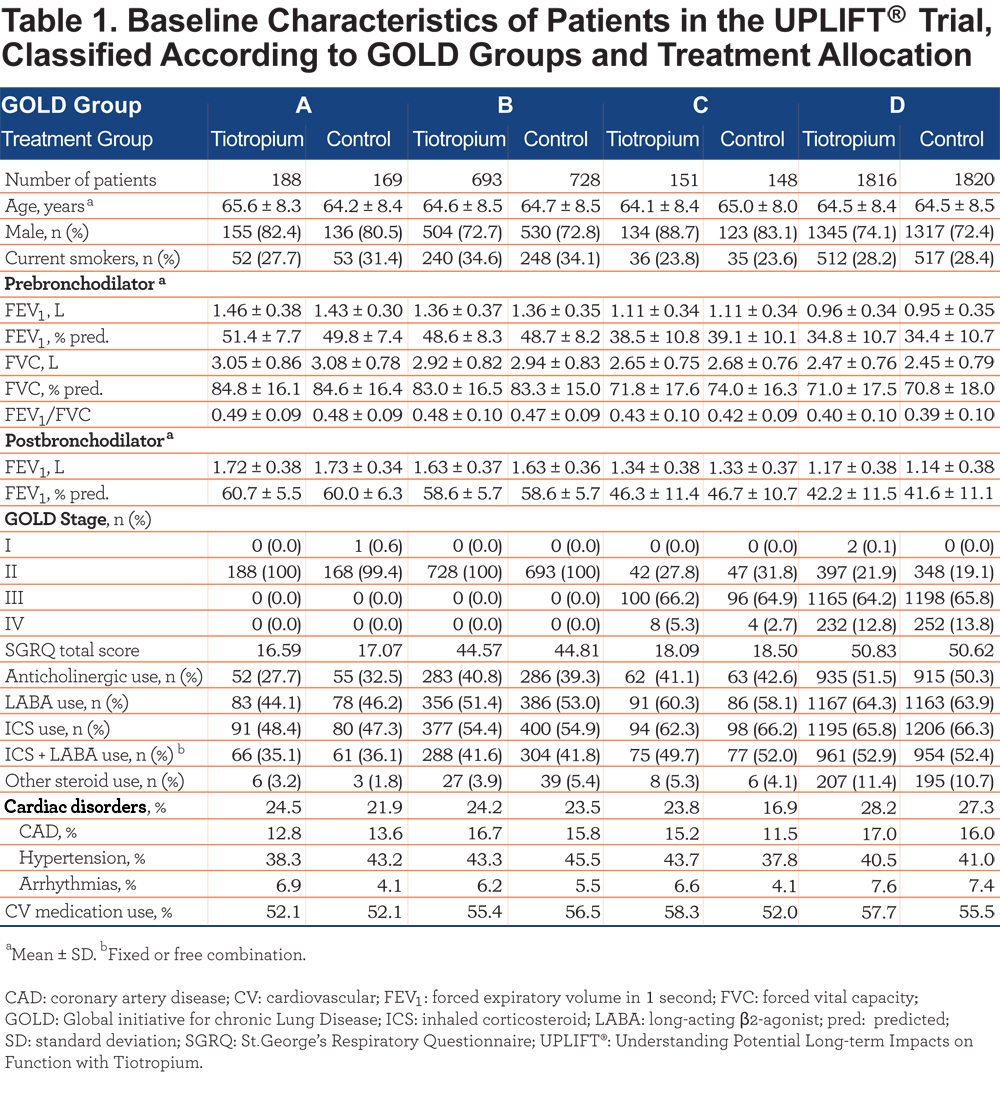
Mean values of prebronchodilator FEV1 were consistently higher with tiotropium than control in all GOLD groups at all post-baseline time points during the trial, with differences by Year 4 of 160 mL for Group A (95% CI: 110, 210; p < 0.0001), 90 mL for Group B (95% CI: 59, 122; p < 0.0001), 100 mL for Group C (95% CI: 30, 162; p = 0.0046) and 80 mL for Group D (95% CI: 63, 104; p < 0.0001) (Figure 1). Over the course of the study, between-treatment group differences in prebronchodilator FEV1 ranged from 110 to 180 mL in Group A, 90 to 120 mL in Group B, 100 to 150 mL in Group C, and 80 to 100 mL in Group D (Figure 1). The p-value for treatment–subgroup interaction term was 0.0196. For the postbronchodilator FEV1 (after inhalation of salbutamol plus ipratropium in both groups), differences between treatment groups ranged from 70 to 140 mL in Group A, 50 to 70 mL in Group B, 50 to 110 mL in Group C, and 40 to 60 mL in Group D (treatment subgroup interaction p-value: 0.1503) (Figure 1).
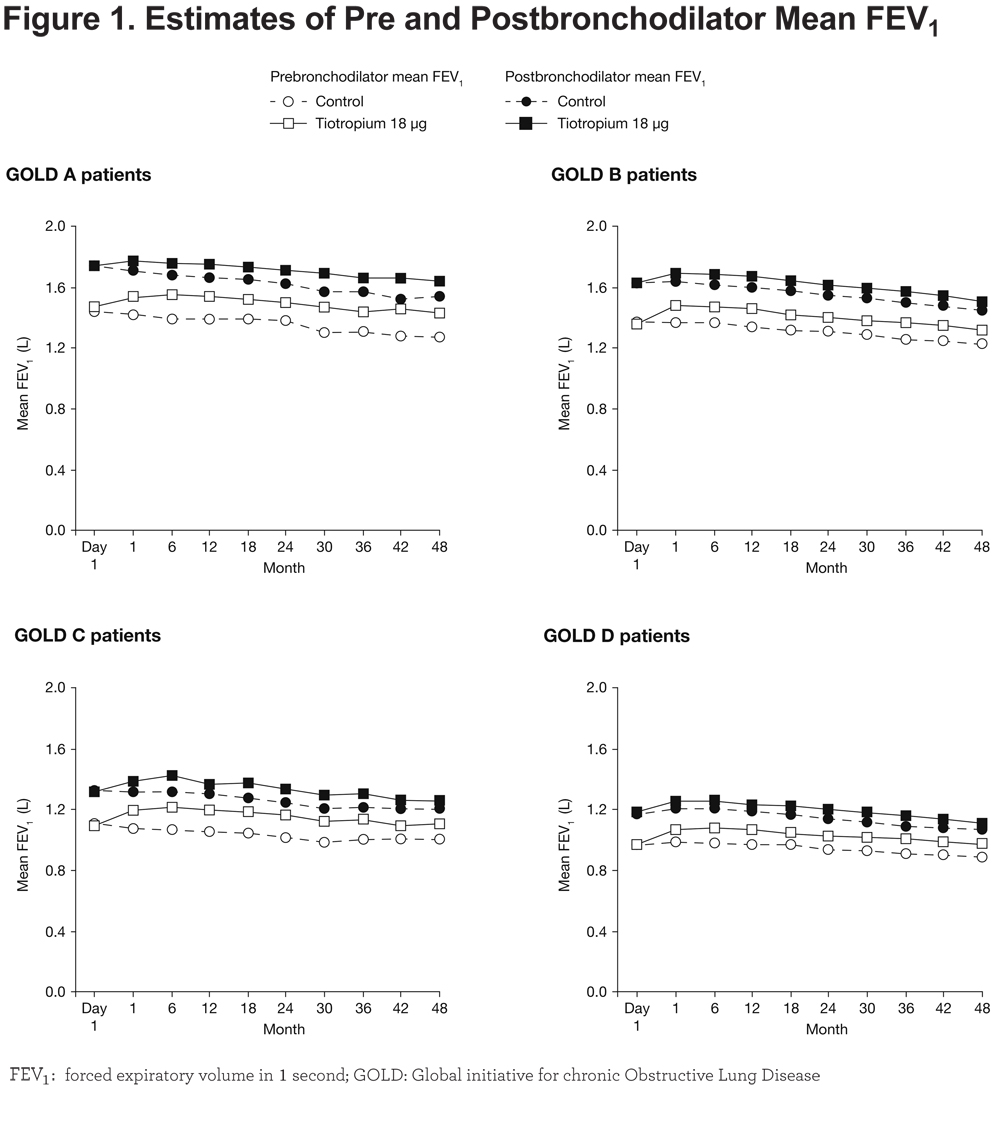
Prebronchodilator FVC improved significantly for patients receiving tiotropium versus control, with differences by Year 4 of 210 mL for Group A (95% CI: 115, 314; p < 0.0001), 170 mL for Groups B (95% CI: 112, 222; p < 0.0001) and D (95% CI: 127, 216; p < 0.0001), and 200 mL for Group C (95% CI: 75, 321; p = 0.0017) (Figure 2). Differences between the tiotropium and control groups in prebronchodilator FVC throughout the study duration ranged from 170 to 250 mL in Group A, 160 to 200 mL in Group B, 180 to 310 mL in Group C, and 170 to 220 mL in Group D. The model including the treatment–subgroup interaction term showed a non-significant interaction (p = 0.4644) (Figure 2). Differences in postbronchodilator FVC throughout the study duration ranged from 30 to 100 mL in Group A, 30 to 60 mL in Group B, 10 to 110 mL in Group C, and 40 to 80 mL in Group D. Also, here the investigation of the GOLD group*treatment interaction showed no significance (p = 0.8806) (Figure 2).
Health Status
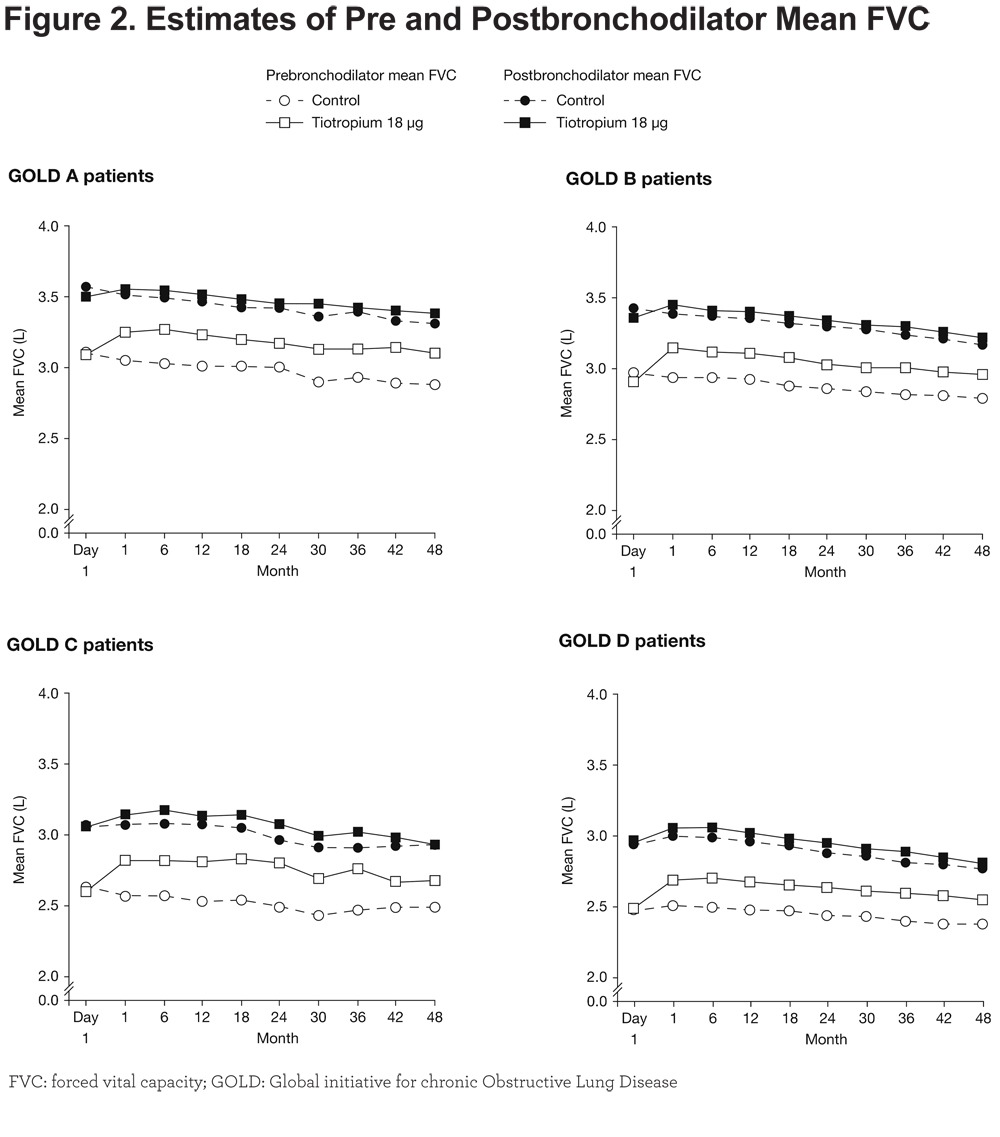
In the fewer-symptom groups (A and C), SGRQ scores increased (worsened) progressively from baseline, but less so in patients treated with tiotropium; conversely, in Groups B and D, SGRQ total scores decreased (improved) relative to baseline within the first 6 months (Figure 3).
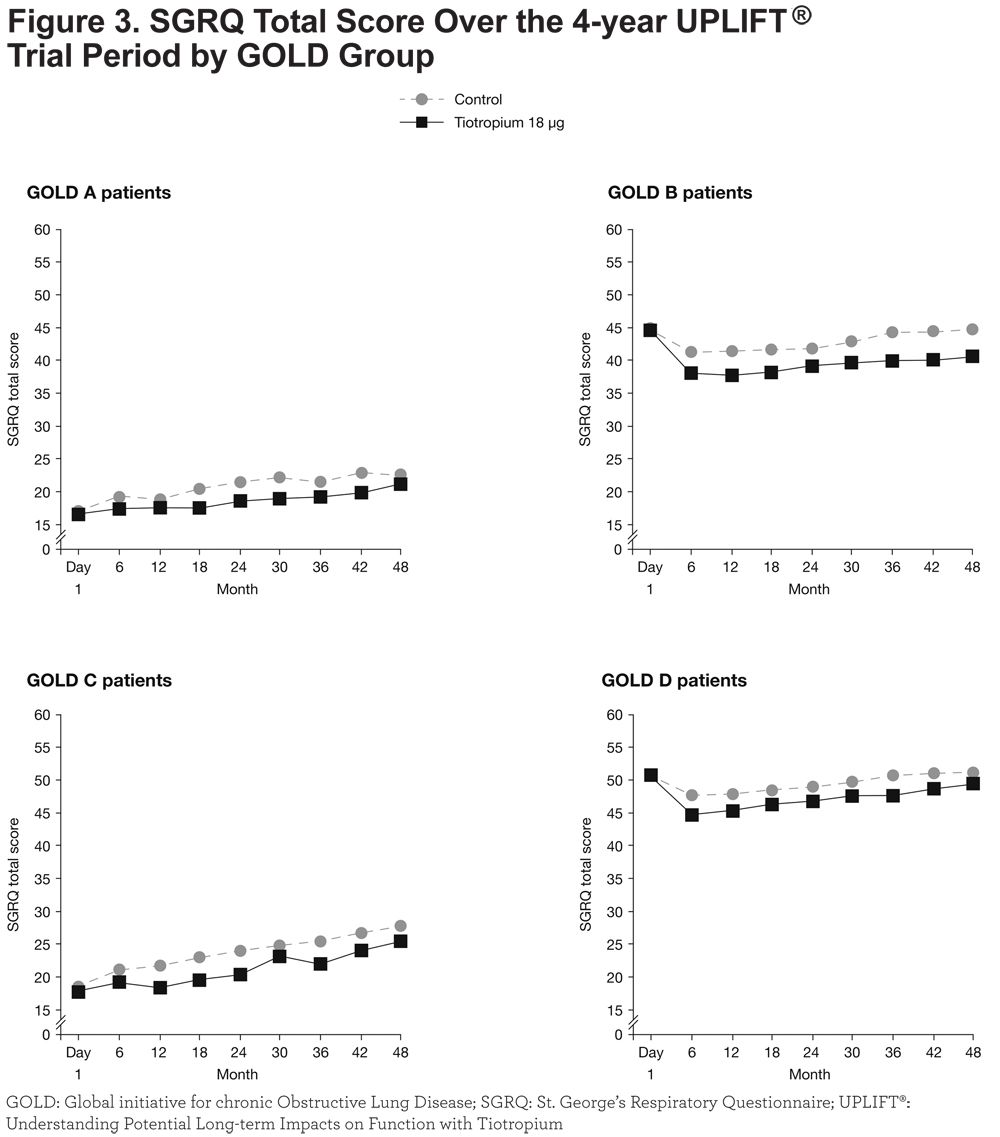
Differences in SGRQ scores between patients treated with tiotropium compared to the control groups ranged from 1.29 to 3.12 (p ≤ 0.05 at all time points except at 12, 36 and 48 months) in Group A, 2.71 to 4.36 (p ≤ 0.001 at all time points) in Group B, 1.68 to 3.70 (p < 0.05 at 12, 18, 24 and 36 months) in Group C, and 1.73 to 3.08 (p < 0.01 at all time points) in Group D (treatment subgroup interaction p-value: 0.6484). Improvements (95% CI) in SGRQ total scores for tiotropium compared with control by Year 4 were –1.33 (–4.22, 1.56), –4.03 (–5.89, –2.18), –2.46 (–6.44, 1.52), and –1.73 (–3.02, –0.44) for GOLD Groups A, B, C, and D, respectively. The differences were all in favor of tiotropium throughout the course of the trial.
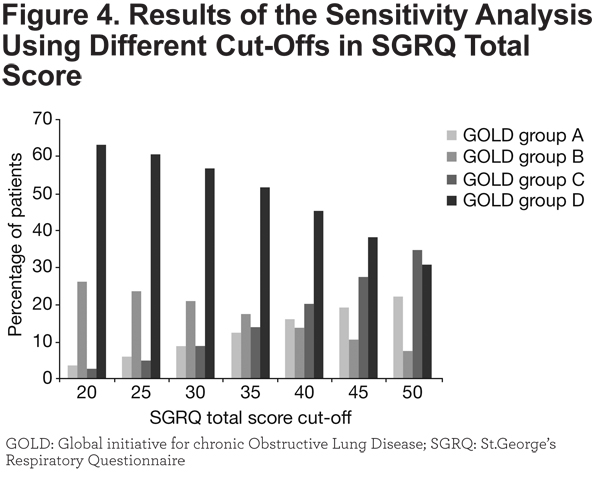
A sensitivity analysis was performed to examine the effects of using different cut-offs in the SGRQ total score (between 20 and 50) on the distribution of patients among the GOLD groups (Figure 4). As patients with more symptoms (Groups B and D) generally had a higher SGRQ score, increases in the cut-off value had a greater impact on the relative proportion of patients in Groups A and C (who had fewer symptoms), particularly if low risk (Group A).
Exacerbations
Table 2 shows the numbers and rates of exacerbations in patients in GOLD Groups A–D. The proportion of patients who experienced at least 1 exacerbation was lower in Groups A and B than in Groups C and D. The number of exacerbations per patient-year was lower in the tiotropium group than in the control group in all GOLD groups and these differences were significant, except in Group C (treatment subgroup interaction p-value: 0.0250).

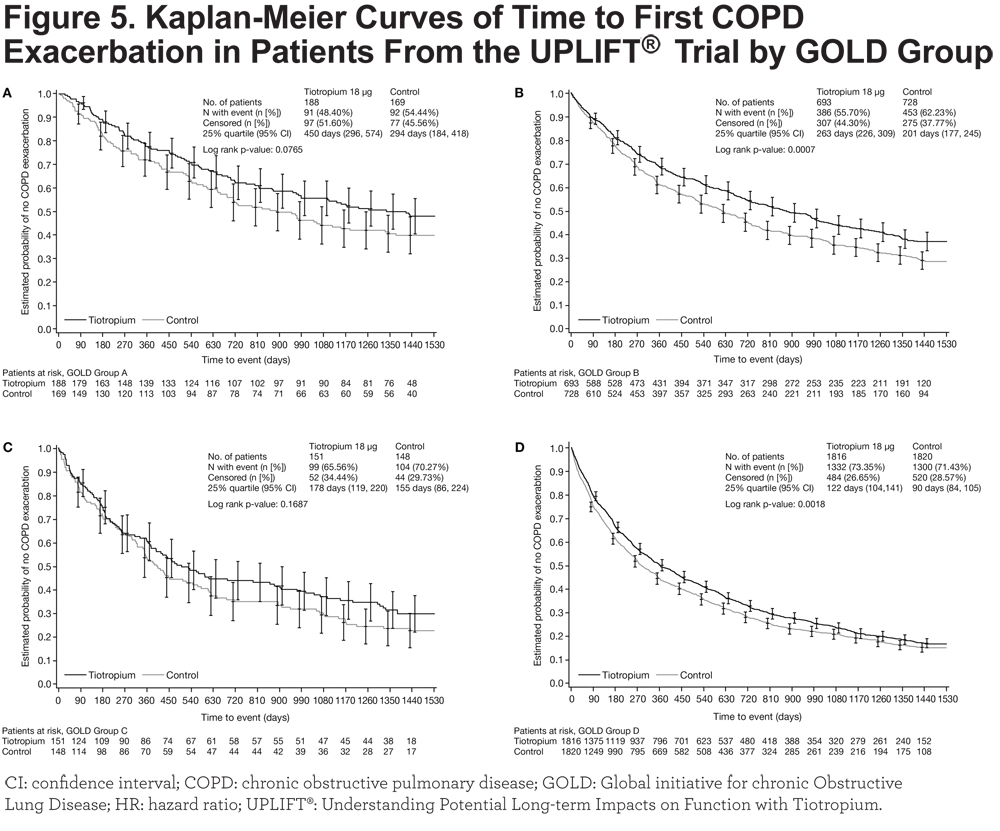
Tiotropium prolonged the time to first COPD exacerbation compared to control, with a consistently reduced exacerbation risk across all GOLD groups (Figure 5); the p-values from the log-rank test for tiotropium versus control were 0.0765, 0.0007, 0.1687, and 0.0018 for Groups A, B, C, and D, respectively. Hazard ratios (95% CI) from the Cox regression were 0.77 (0.58, 1.03), 0.79 (0.69, 0.91), 0.82 (0.63, 1.09), and 0.89 (0.82, 0.96), p-values were very similar to those from the log-rank test. The model containing the treatment–subgroup interaction term showed no significant interaction (p = 0.5231). Statistical significance was reached in Groups B and D in which patient numbers were highest.
Safety Analysis
Rates of death (fatal adverse events) and fatal MACE are shown in Table 3.
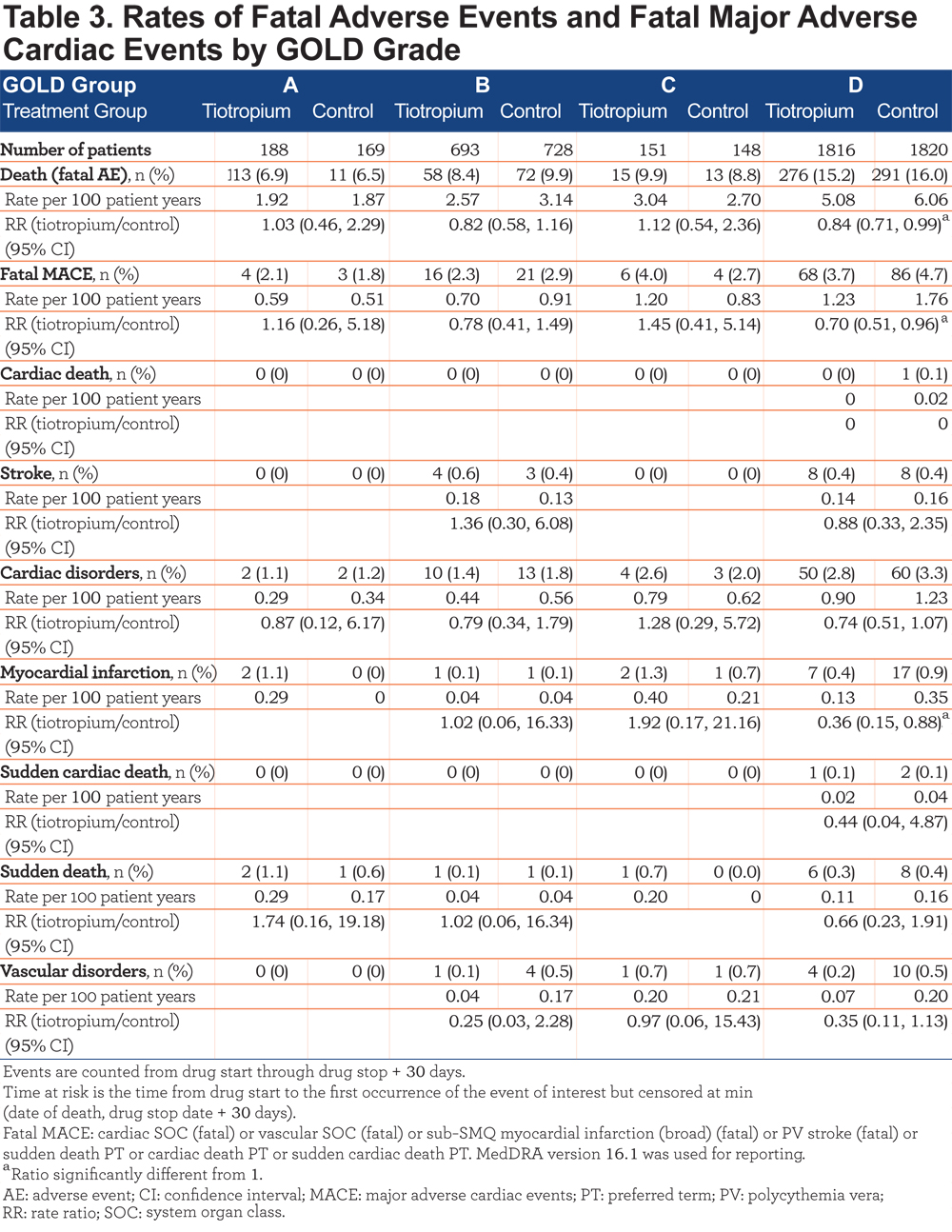
The incidence of death was highest in GOLD Group D (rate per 100 patient years, 5.08 with tiotropium and 6.06 with control) and lowest in Group A (rate per 100 patient years, 1.92 with tiotropium and 1.87 with control). Rates of death, fatal MACE overall, and fatal myocardial infarction were significantly lower with tiotropium than control in Group D (rate ratio [RR] (95% CI) 0.84 (0.71, 0.99), 0.70 (0.51, 0.96), and 0.36 (0.15, 0.88), respectively). No cardiac deaths were reported in GOLD Groups A-C but one cardiac death occurred in group D (control).
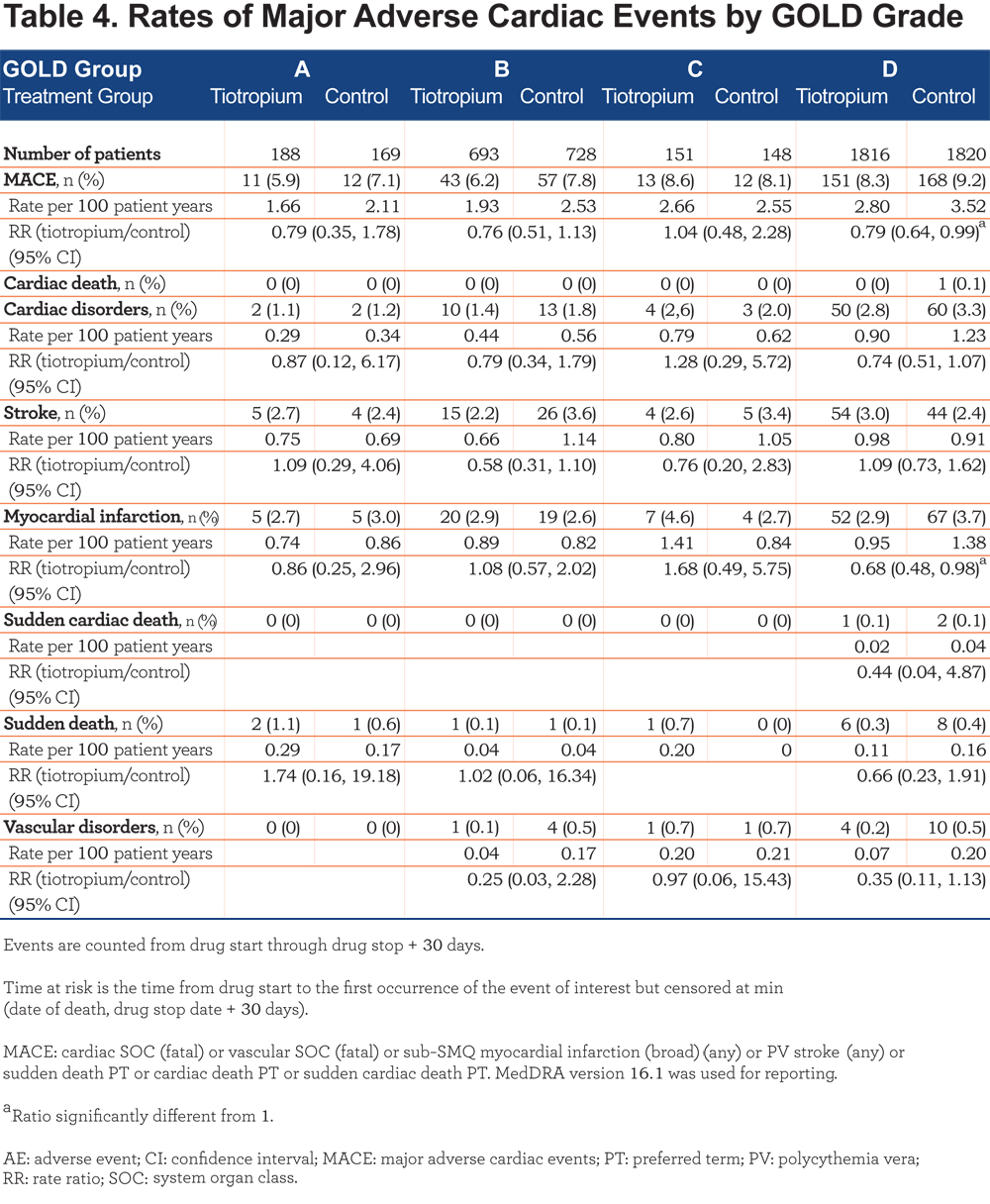
A similar pattern of results was seen for the incidence of MACE (Table 4). The rate per 100 patient years was highest in Group D (2.80 with tiotropium and 3.52 with control) and lowest in Group A (1.66 with tiotropium and 2.11 with control). In Group D, there was a significant difference in favor of tiotropium for rates of MACE overall and rates of myocardial infarction (RR [95% CI] versus control, 0.79 [0.64, 0.99] and 0.68 [0.48, 0.98], respectively). Rates of fatal MACE and MACE were similar in the tiotropium and control groups across GOLD groups A–C.
Discussion
In the UPLIFT® trial, the use of tiotropium was associated with improvements in lung function and quality of life, and a reduction in the mean number of exacerbations by 14% (p < 0.001).3 A subgroup analysis of UPLIFT® showed that in patients with GOLD Stage II (moderate) COPD, lung function, and HRQoL were better in the tiotropium group than in the control group, and tiotropium was more effective in reducing the rate of decline of postbronchodilator FEV1 and the risk of exacerbations.11
Since the publication of these results, GOLD proposed a new approach to the severity assessment of COPD that addresses both the level of symptoms and the risk of exacerbations.4,5 On the basis of this assessment scheme, the GOLD guidelines recommend long-acting anticholinergic therapy as a first-choice option for patients in GOLD Groups B, C, and D. It was, therefore, important to assess the effects of tiotropium in patients in these groups compared to usual therapy with freely prescribed respiratory medications (i.e., inhaled LABAs, ICS, and theophyllines) other than another inhaled anticholinergic agent. The analysis also gives insights into the natural history of patients with COPD, classified according to the GOLD 2013 grading system and closely monitored over 4 years.
Although the criteria for participation in the UPLIFT® trial included a diagnosis of COPD, an age of 40 years or more, a smoking history of at least 10 pack years, a postbronchodilator FEV1 of 70% or less of the predicted value, and an FEV1 of 70% or less of the FVC,3 the majority of patients fell into GOLD Group D. The proportion of patients categorized as Group A or C (i.e., having fewer symptoms) was lower, but data were available for a substantial number of patients (more than 650) in these groups. Higher levels of symptoms were associated with lower FEV1 and FEV1/FVC ratios in high-risk but not low-risk patients. One in 5 patients in Group D had lung function impairment equivalent to GOLD Stage II, illustrating the effects of a multidimensional assessment approach.
A previous analysis of 2 population studies from Copenhagen, Denmark, including 6628 individuals with COPD, used information from medical records regarding hospital admissions and the use of oral corticosteroids and antibiotics to assess the number of exacerbations in the previous year, prebronchodilator FEV1 measurements and mMRC scores to classify patients into the GOLD groups as defined in the 2011 guideline update.12 The authors (Lange et al) found that 77.3% of patients were in Group A, 14.1% in Group B, 4.1% in Group C, and 4.4% in Group D. Perhaps reflecting the fact that the cohorts were recruited nearly 10 years ago, the proportion of patients on inhaled therapy with LABAs, long-acting anticholinergics, or ICS was low, with only 43.4% of patients in Group D on 1 or more of these therapies and only 52.5% on any inhaled therapy in this group. In accord with our findings (which utilized the GOLD 2013 group classification), Lange et al observed that the majority of the patients in Groups C and D were classified into these groups because of low FEV1 level: 77% compared with 78% in UPLIFT®. In this population study, a similar proportion of patients in Groups B, C, and D to the proportions found in patients in the UPLIFT® study had ischemic heart disease/coronary artery disease (20.7% versus 16.3%, 10.3% versus 13.4%, and 19.3% versus 16.5%, respectively). The proportion of patients with ischemic heart disease/coronary artery disease was lower in Group A in the population study than in UPLIFT® patients (7.0% versus 13.2%). It is important to note that, unlike UPLIFT®, the Copenhagen analysis included patients with GOLD Stage I (mild) COPD.
In another study, based on 3633 COPD patients from 11 cohorts in Spain recruited over a period of 20 years and using only hospitalization data to assess exacerbation risk, 33.6% overall were in Group A, 16.3% were in Group B, 17.7% were in Group C, and 32.3% were in Group D based on the GOLD 2011 classification scheme.13 There was, however, significant heterogeneity between the individual cohorts, with 4 having over 50% of their patients in Group D, with similar distributions in these clinical cohorts to our single UPLIFT® cohort (which was based on the GOLD 2013 update).
Among patients in the control group of the UPLIFT® study, exacerbation rates were highest in Group D, but the rates were similar in patients in Groups B and C. Exacerbation rates in Group A were less than half of the rates in Group D. In the Copenhagen population study, the average number of exacerbations per year in Groups A, B, C, and D over 3 years, was 0.0, 0.2, 0.6, and 0.9, respectively.12 The rates we observed were higher in Groups A and B, but similar in Groups C and D; again, this may reflect the fact that GOLD Stage I patients were included in the Copenhagen analysis.
In the Norwegian Genetics of Chronic Obstructive Lung Disease (GenKOLS) study, Johannessen and colleagues compared the 2011 and previous 2007 GOLD grading system for predicting mortality and hospitalization, and concluded that the predictive ability of the new grading system did not differ significantly from the previous one.14 This study followed 912 patients with COPD for 8 years: 20% of patients were classified in Group A, 30% in Group B, 6% in Group C, and 44% in Group D, with more patients classed as Group A and less in Group D than in our UPLIFT® cohort.
Baseline SGRQ total scores in Groups A and C of our analysis (fewer symptoms) were only just above the values found in healthy people of a similar age.15 As shown by the sensitivity analysis, SGRQ scores were higher in patients in Group D compared with those in Group B, suggesting that the impact of exacerbations on health status is greater in patients with higher levels of symptoms, particularly those who are breathless. After at least 6 months of treatment, patients in the tiotropium group also had lower SGRQ scores (indicating better health status) than patients in the control group and these differences were statistically significant. There was a difference in the change in SGRQ scores over time between patients in low- and high-symptom groups, with those in the fewer-symptom groups showing progressive worsening rather than the initial improvement seen in high-symptom patients. This may reflect the fact that it is not possible to reduce symptom levels in patients who have minimal or no symptoms at baseline, whereas those with a higher level of symptoms can experience a reduction in their symptoms, either as a result of taking part in the study or as a result of the addition of tiotropium, which had the greatest effect. Regression to the mean may be an alternative possible explanation for these differences.
Tiotropium significantly improved lung function in patients in all 4 GOLD groups, with similar absolute differences in pre- and postbronchodilator FEV1 and FVC in Groups B, C and D. The greatest level of improvement in FEV1 and FVC was seen in Group A; the disparity (70 mL/year higher at the 4-year time point) in improvement in prebronchodilator FEV1 in Group A versus Group B, which had similar baseline FEV1 values, may have been influenced by the low number of patients in Group A. Patients in all GOLD groups who were treated with tiotropium also had fewer exacerbations than those in the control group, with a significant 11% reduction in high-risk patients in Group D. Low-risk patients also showed large and significant reductions in exacerbation rates: 36% in Group A and 28% in Group B. Bearing in mind the importance of exacerbations in the natural history of COPD, our results suggest that therapy with tiotropium has an important role even in fewer-symptom, low-risk patients. This is supported by the recent findings of a randomized, placebo-controlled trial, in which tiotropium HandiHaler® improved lung function and patient-reported outcomes in maintenance therapy-naïve patients with GOLD stage II COPD.16
Previous evidence suggested an increased risk of mortality from cardiovascular disease in patients stratified into GOLD group B and D (versus A and C).12 We observed that the incidence of death (fatal adverse events), fatal MACE or MACE was highest in Group D, although within this group, the rates were significantly lower in patients treated with tiotropium versus control.
A potential fundamental limitation of this study is that between 47% and 66% of patients were receiving ICS at baseline, albeit at varying doses, and between 35% and 53% of patients were receiving a combination of ICS and LABAs. Thus, although patients in Group A were classified as having fewer symptoms and being at low risk, some of them may have had few symptoms or less than 1 exacerbation because of the effects of their therapy rather than being intrinsically at low risk. However, despite this, if the GOLD assessment scheme is used to decide on therapy in previously treated as well as treatment-naïve patients, the results of this analysis appear valid.
In addition to the above limitation, patients’ levels of symptoms at baseline were classified using SGRQ scores rather than mMRC or CAT scores (as recommended by GOLD); however, in view of the close correlation between CAT scores and SGRQ and the large number of patients included in the trial, we do not believe that this has significantly influenced the results. The CAT score is a more appropriate tool than the SGRQ for use in routine clinical practice, but SGRQ is well suited for use in clinical trials and both scores have good longitudinal validity.15,17 Using different cut offs for the SGRQ results in a widely different distribution of patients among the groups, but the original intent of the GOLD revision was to emphasize a gradation across the symptom axis rather than a validated dichotomous cut-off at a particular CAT score. Classification of risk was based both on postbronchodilator FEV1 values and the number of courses of antibiotics and/or systemic corticosteroids as recalled by the patients. It is possible that their recollection was incorrect, but other studies have shown that, generally, it is accurate and appropriate to use this as an alternative to relying on health records.18
Finally, the patients who took part were selected for involvement in a clinical trial and, therefore, may not be fully representative of patients seen in practice, particularly with regard to the distribution between GOLD groups and the presence of comorbidities. It should be noted, that the inclusion and exclusion criteria were relatively liberal, recruitment included a broad selection of patients with COPD with multiple comorbidities, and patients were permitted to continue to use all prescribed respiratory medication except for anticholinergics, thus mimicking real-world conditions for pharmacotherapy. Other preliminary analyses of patient populations have shown similar distributions of patients into the GOLD groups and similar levels of cardiovascular comorbidity.12-14
In conclusion, this analysis suggests that once-daily tiotropium improved lung function, HRQoL and exacerbation outcomes in patients in the UPLIFT® trial across all 4 GOLD 2013 severity grades (Groups A, B, C, and D).
Acknowledgments
The authors would like to thank Dr. Katrin Kupas for statistical support. Editorial assistance was provided by Natalie Dennis and Sarah J. Petit from PAREXEL, which was funded jointly by Boehringer Ingelheim and Pfizer. All authors contributed to drafting the article and critically revising the content of the manuscript, and made the decision to submit this work for publication. All authors read and approved the final manuscript.
Declaration of Interest
Dr. Inge Leimer and Dr. Norbert Metzdorf are employees of Boehringer Ingelheim. Dr. Halpin has received personal fees from Almirall, AstraZeneca, Boehringer Ingelheim, GSK, Intermune, Pfizer, Novartis, and non-financial support from Boehringer Ingelheim and Novartis. Dr. Tashkin has received grants or personal fees for consulting, speaking or acting on the advisory board from Boehringer Ingelheim, Novartis, Pfizer, AstraZeneca, Forest, Sunovion, GlaxoSmithKline, and Theravance. Dr. Tashkin received grants and advisory board fees from Boehringer Ingelheim during the course of the UPLIFT study. Dr. Celli has received fees for consulting from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, and Medimmune. Dr. Celli was a principal investigator in the UPLIFT® study, and received grants from the St. Elizabeth's Medical Center. Dr. Decramer has received research grants or fees for consulting or speaking from Novartis, Nycomed, Boehringer Ingelheim, GlaxoSmithKline, Altana, and AstraZeneca. Editorial assistance was provided by Natalie Dennis and Sarah J. Petit from PAREXEL, which was funded jointly by Boehringer Ingelheim and Pfizer.