Running Head: Peak Expiratory Flow Rate and COPD Exacerbation
Funding Support: Supported in part by the Maxine Gitlin COPD Research Fund
Date of Acceptance: September 23, 2015
Abbreviations: chronic obstructive pulmonary disease, COPD; peak expiratory flow rate, PEFR; forced expiratory volume in 1 second, FEV 1 ; 6 minute walk distance, 6MWD; Pennsylvania Study of COPD Exacerbations , PA-SCOPE; Health Insurance Portability and Accountability Act, HIPAA; Global initiative for chronic Obstructive Lung Disease, GOLD; forced vital capacity, FVC; residual volume, RV; total lung capacity, TLC; diffusing capacity of the lungs for carbon dioxide, DLCO; peak expiratory flow rate, PEFR; myocardial infarction, MI; congestive heart failure, CHF; coronary artery disease, CAD; gastroesophageal reflux disease/peptic ulcer disease, GERD/PUD; cerebrovascular accident, CVA
Citation: So JY, Lastra AC, Zhao H, Marchetti N, Criner GJ. Daily peak expiratory flow rate and disease instability in chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2016; 3(1): 398-405. doi: http://doi.org/10.15326/jcopdf.3.1.2015.0142
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive and debilitating disease, and one of world’s leading causes of mortality.1 Frequent exacerbations of COPD have been associated with poorer quality of life and worse morbidity and mortality.2,3 (However, it has been difficult to prevent and identify those patients at risk of frequent or severe exacerbations. Currently, monitoring of COPD is limited mainly to pulmonary function testing using office- or laboratory-based spirometry, without effective tools to objectively monitor disease activity at home on a day-to-day basis.
In asthma patients, peak expiratory flow rate (PEFR) measurement has been promoted as a useful tool for assessing airway obstruction and titrating therapy. It is not only cheap, but has high reproducibility and user compliance rates, making it a useful tool for ambulatory monitoring of asthma.4 In COPD, however, the utility of PEFR monitoring remains unclear. Some authors have shown that PEFR monitoring is a reasonably precise measurement tool for measuring airflow obstruction in COPD patients.5 In addition, the compliance rate of daily PEFR use in COPD patients has been >90% in recent studies, making it a potentially useful monitoring modality.6 A good correlation between forced expiratory volume in 1 second (FEV1) and PEFR has been reported in COPD, further validating the possible use of home PEFR as a surrogate for office-based or laboratory spirometric monitoring.7 A study by Donaldson et al illustrated a positive relationship between exacerbation frequency and daily PEFR variations in measurements of the alpha value in detrended fluctuation analysis, validating the use of PEFR measurements as a potential monitoring tool in COPD patients.13
In an attempt to assess the utility of PEFR monitoring in COPD management, we analyzed daily PEFR variability in patients with severe and very severe COPD as a marker of disease instability and its impact on patient-related outcomes. We hypothesized that individuals with greater variability in daily PEFR would signal an unstable patient population with worse outcomes.
Methods
Study Design
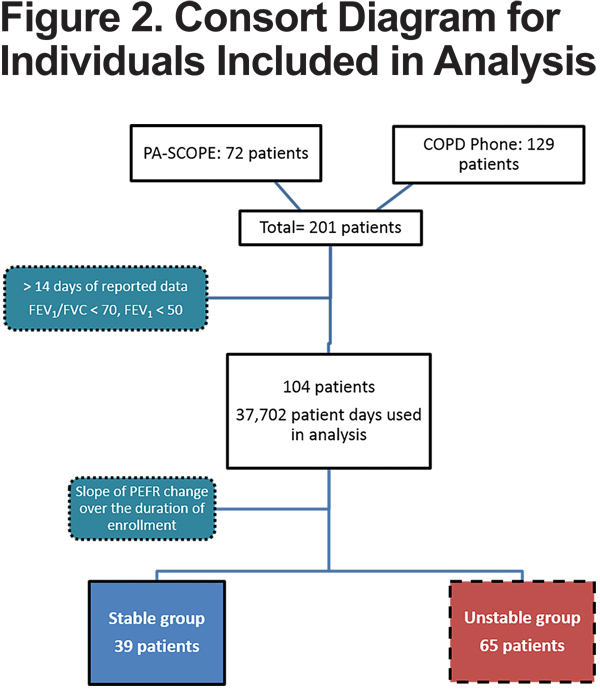
This is a retrospective analysis of prospectively collected data from June 2005 to May 2011. Data from 201 patients was available and 104 patients met the inclusion criteria with 37,702 total patient-days available for analysis.
The data was obtained from 2 prospective studies previously done at our institution. A detailed description of the study designs for the Pennsylvania Study of COPD exacerbations (PA-SCOPE) and the COPDphone Telemedicine Program has been published.8 Briefly, PA-SCOPE was a randomized, multicenter prospective study to evaluate the efficacy of daily monitoring of symptoms and peak expiratory flow rate for the early treatment of symptoms heralding an impending COPD exacerbation using an electronic diary (PHT, Boston, Massachusetts).8 The COPDphone Telemedicine Program recruited patients with COPD with previous acute exacerbation of COPD episodes or chronic use of oxygen who reported daily symptoms (dyspnea, sputum production, coughing, wheezing, nasal congestion, fever and sore throat) and peak flow measurements using a cell or land-based phone or via a Health Insurance Portability & Accountability Act (HIPAA) compliant password protected internet website. Our institutional review board approved both study protocols.
For both studies, we enrolled patients 40 years old and older with severe to very severe COPD defined by Global initiative for chronic Obstructive Lung Disease (GOLD) criteria (FEV1/ forced vital capacity [FVC] <70% and FEV1 < 50% predicted) and more than 14 days of reported data. Daily PEFR, dyspnea, sputum (quantity, color, and thickness), cough, wheezing, sore throat, nasal congestion and body temperature were recorded. Data on demographics, baseline spirometry measurements, 6 minute walk distance (6MWD), comorbidities, and oxygen and medication use were collected for analysis.
Outcome Measures
The primary outcome was exacerbation-free days. A daily score was calculated by semi-quantitating symptoms of breathlessness, sputum (quantity, color and consistency), temperature over 100ºF, cough, wheeze, sore throat, and nasal congestion (G.J. Criner, 2004, U.S. Copyright TX7-170-236). We used a modified symptom score by eliminating PEFR measurement from the scoring system. An exacerbation was considered if the modified symptoms score was greater than 1, as illustrated in Table 1.
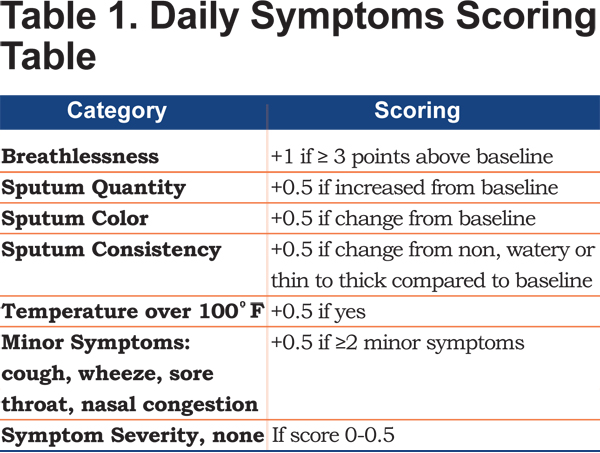
Secondary outcomes included time to first hospitalization, hospitalization rate, length of hospitalization, and all-cause mortality.
Data Analysis
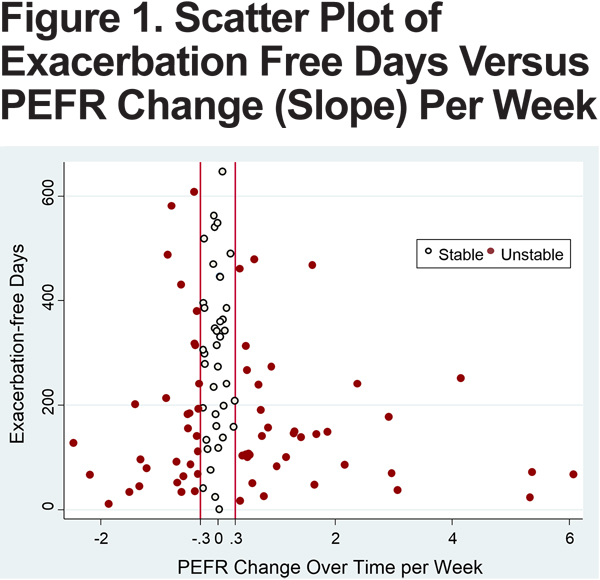
A weekly average of daily PEFR rate was calculated for each patient. The slope of the change over the duration of enrollment was then determined using linear regression. Patients were divided into 2 groups (stable versus unstable) based on the magnitude of the slope: stable group (slope between -0.29 to 0.29) and unstable group (slope <-0.3 or >0.3). The cutoff point of 0.3 was determined by the distribution of slopes resembling a bell curve with clustering of slopes around 0.3 (Figure 1). Individuals with slopes <-0.3 and >0.3 had relatively large variability in slope and were grouped together.
Univariate comparisons of baseline characteristics and outcomes were conducted using a two-sample t-test for continuous data and a chi-square test for categorical data. Log-rank tests were used to compare all-cause mortality and time to first hospitalization between stable and unstable groups and illustrated using Kaplan-Meier survival curves. Statistical significance was declared if the P value was < 0.05. Statistical analysis was performed using STATA13.0 (College Station, Texas) and SAS 9.3 (Cary, North Carolina).
Results
Participant Characteristics
There were a total of 104 patients observed for 37,702 patient-days. These patients were divided into the stable (39 patients) and unstable (65 patients) (Figure 2).
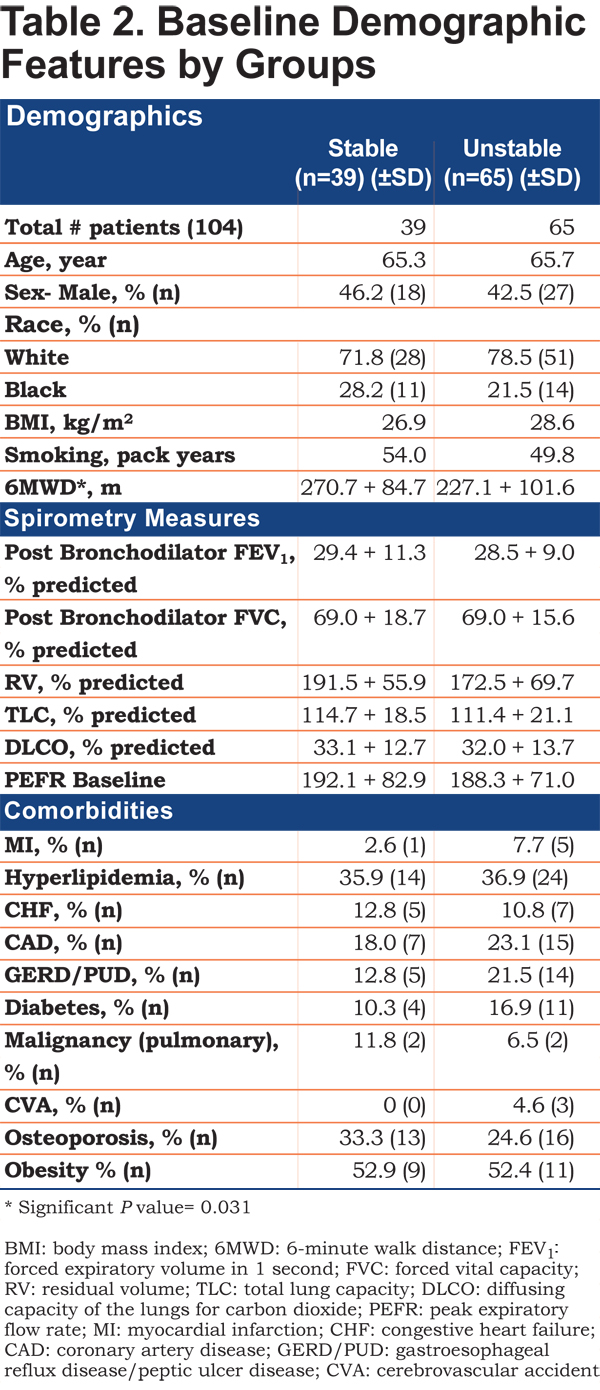
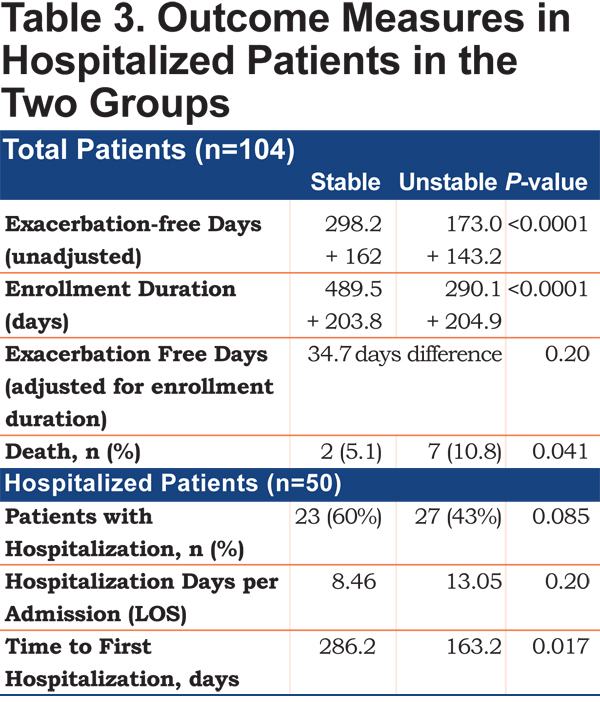
There were no differences in baseline demographic features, spirometric measures, oxygen use or comorbidities between the 2 groups (Table 2). Medication usage at enrollment, including short- and long-acting bronchodilator, steroids, theophylline, anti-acids, antibiotics, and statin, as well as baseline PEFR measurements, were also similar. However, more patients in the unstable group were prescribed inhaled steroids compared to the stable group (unstable [97%] versus stable [82.1%], p<0.01). The unstable group also had significantly shorter 6MWD (227.1 [SD 101.6] versus 270.7 [SD 84.7] meters, p=0.031) and had shorter enrollment duration (290.1 [SD 204.9] versus 489.5 days [SD 203.8], p<0.01) compared to the stable group.
Primary Outcome
The absolute exacerbation-free days were significantly lower in the unstable group compared to the stable group (173 versus 298 days, p<0.01). When adjusted for the duration of the study, the unstable group had 34.7 less exacerbation-free days compared to the stable group (p= 0.20) (Table 3). Exacerbation-free days, instead of the rates, were used as the availability of daily symptom measurements and allows us to quantify the exact number of days with worse symptoms, rather than grouping them into 1 event, obscuring duration and severity of the symptoms.
Secondary Outcomes
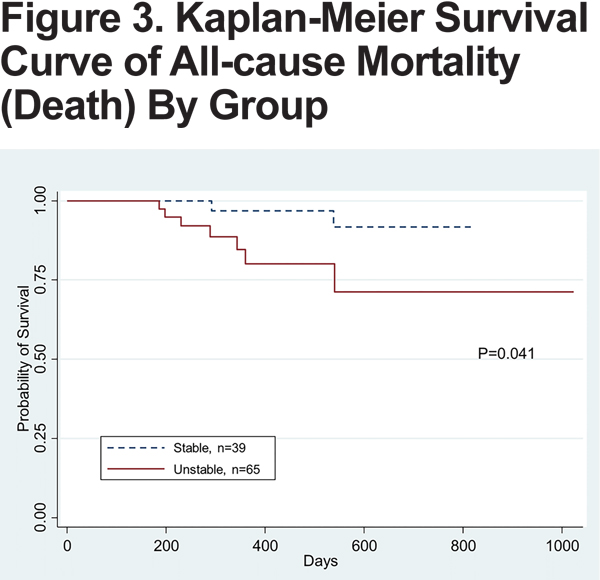
All-cause mortality was higher in the unstable group compared to the stable group (10.8% versus 5.1%, p= 0.04), with the death rate per person year of 0.14 versus 0.038 in the unstable versus stable groups (Figure 3). The unstable group also had longer hospitalization duration per admission compared to the stable group (8.5 versus 13.1 days, p=0.2).
Hospitalization
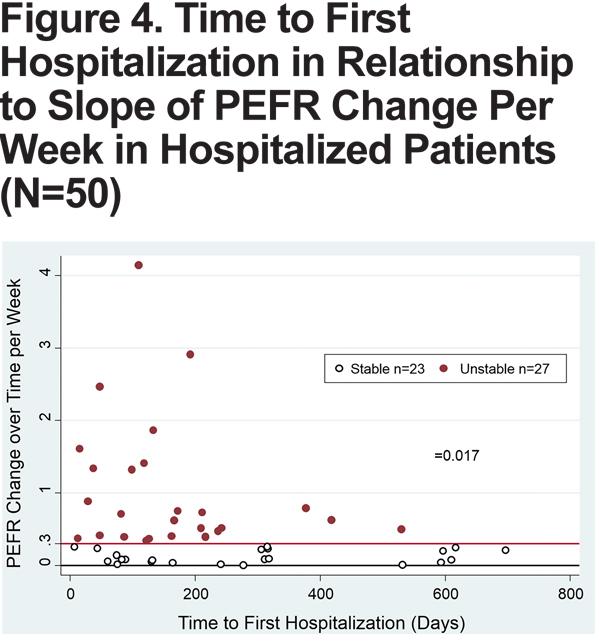
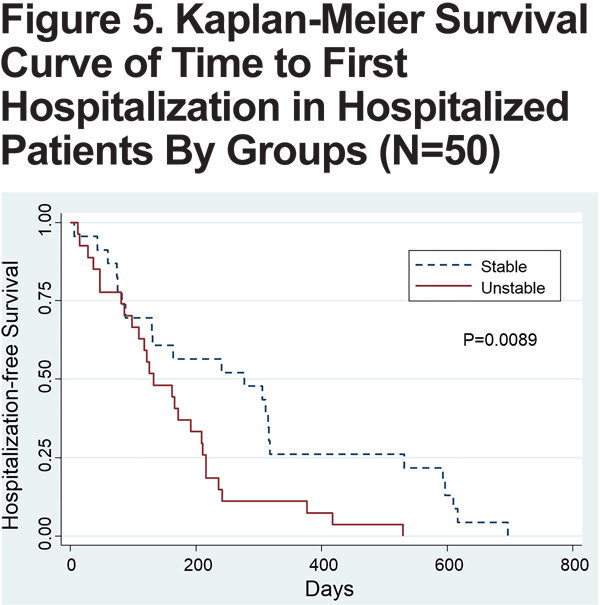
Of 104 patients, a total of 50 patients had at least 1 hospitalization. Forty-three percent (n=23) of patients in the stable group and 60% (n=27) in the unstable group comprised this group of hospitalized patients. Of these patients, the mean time to first hospitalization was 163 days in the unstable group, compared to 286 days in the stable group (p=0.017) (Figure 4), also seen on the Kaplan-Meier curve (Figure 5). Patients in the unstable group also had a significantly higher rate of hospitalizations per year (2.64 versus 1.66, p=0.03), and stayed in the hospital longer with each admission (13.05 versus 8.46 days, p=0.20).
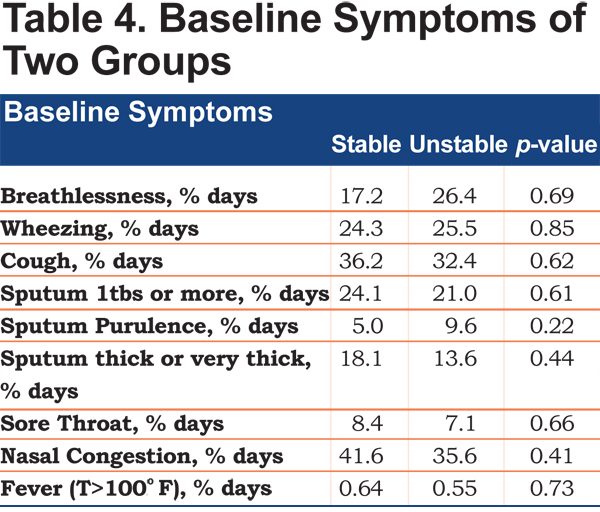
Symptoms
There were no statistically significant differences in the prevalence of daily symptoms, including breathlessness, wheezing, cough, sputum quantity, purulence and thickness, sore throat, nasal congestion and body temperature between the stable and unstable groups (Table 4). However, patients in the unstable group had more days with breathlessness (26.4% versus 17.2% days) and sputum purulence (9.6% versus 5% days).
Discussion
Our study shows that patients with severe to very-severe COPD who have greater changes in the daily PEFR (unstable group) have less stable disease. These patients had significantly shorter 6MWD and a higher prevalence of inhaled corticosteroid use on enrollment. Of the patients who had at least 1 hospitalization, patients in the unstable group had significantly shorter time to first hospitalization, higher rates of hospitalization and longer hospital length of stay compared to those in the stable group. They also had 34.7 fewer days of exacerbation-free days, when adjusted for the study enrollment duration, although this value was not statistically significant. It is important to note that there were no differences in patient demographics, comorbidities, or baseline symptoms between the stable and unstable groups. These findings show that the patients with greater changes in PEFR are sicker, have worse disease and overall functional status, and support monitoring of daily PEFR as a useful risk stratification tool for COPD patients, in addition to GOLD staging and symptom scores.
Few studies have looked at the patterns and changes in daily PEFR measurements in COPD patients. Donaldson et al identified patients with frequent and infrequent exacerbations and found that there was a greater decline in FEV1 and PEFR in those with more frequent exacerbations.9 In our study, we looked at changes in daily PEFR over time and showed that greater increases or decreases in PEFR portend worse outcomes, likely signaling disease instability. Donaldson also examined the alpha value from the detrended fluctuation analysis of the daily diary symptoms and PEFR in moderate to severe COPD patients, and found a positive relationship between self-similarity of daily PEFR and exacerbation frequency.13 The different findings between these studies may be due to differences in the patient populations studied. Donaldson’s study had a mixture of patients who were either followed in an outpatient COPD study cohort and COPD individuals enrolled into a pharmacological trial.13 Our study examined patients who were more ill; they had lower FEV1 and more serious comorbid conditions, and likely more severe exacerbations, such as hospitalizations. Our findings may indicate a patient group that is experiencing dynamic changes in airflow due to chronic inflammation and sputum production. Therefore, there exists a phenotype within severe and very severe COPD patients who experience greater variability in airway obstruction that may portend higher morbidity and mortality.
Studies by Tashkin et al and Ramsdale have shown that COPD patients can have significant bronchodilator responsiveness and airway reactivity, as well as sputum production and changes in airway resistance.10,11 No study has shown direct correlation of the airway inflammation with PEFR changes, but many indirect findings have shown likely correlation between the two.4 A significant increase in CD4 T-lymphocyte activation and eosinophil counts in peripheral blood has been demonstrated in asthma patients with greater coefficient of variation in daily PEFR, suggesting that the unstable group may have enhanced inflammatory cell activation.12 Ramsdale’s study also discussed how increased sputum production could increase airway resistance and affect PEFR.11 These findings shed light onto the possibility that changes in airway reactivity and resistance can be detected by daily PEFR measurements, and can help identify a subgroup of patients with worse chronic bronchitis and dynamic sputum production, with worse outcome.
One of the limitations of our study is the determination of the slope threshold used to define stable and unstable groups. The slope method was used for data analysis because of the ability to detect the degree of changes over time. This method was chosen over the coefficient of variation as it allowed for better discrimination of PEFR changes over a longer period. The cutoff slope of 0.3 was determined after evaluating the distribution of the slopes in each individual, where a clustering of slopes were observed between-0.3 to +0.3.The distribution of slopes resembled a bell curve closely, and our data analysis shows that patients with slopes less than -0.3 or greater than 0.3, indeed, behaved very similarly. For example, patients with a slope less than -0.3 or greater than +0.3 had significantly shorter duration of enrollment and exacerbation free days compared to those between -0.3 and +0.3. One hypothesis for this finding is that the patients in the unstable group have more current active airways dysfunction with greater barriers to participate in the study secondary to worse functional status, compliance with PEFR or unreported adverse outcomes. Further studies are needed to assess the functional status in these patients, and to define a standardized method to prospectively determine a clinically significant slope cutoff point.
This study also excluded patients with mild and moderate COPD, and future studies looking at the significance of PEFR changes in these patients are needed. In addition, assessing the histopathologic characteristics (such as airway wall thickness and smooth muscle mass), prevalence of inflammatory cells and the magnitude and types of emphysema in the unstable compared to the stable daily PEFR group could help to further understand and phenotype these patients.
Conclusion
Our study showed that greater variability in daily PEFR measurements in patients with severe to very severe COPD (with similar comorbidities, age, medication use, and symptoms) could help to objectively identify patients with more unstable disease with a propensity for greater exacerbation and a higher mortality.
Declaration of Interest: none