Running Head: Overall and Cardiovascular Safety of Aclidinium
Funding support: The studies included in this pooled analysis were funded by Almirall S.A., Barcelona, Spain and Forest Laboratories LLC, a subsidiary of Actavis PLC, New York, New York. The pooled analysis was funded by Almirall S.A., Barcelona, Spain.
Date of Acceptance: October 30, 2015
Abbreviations: twice daily, BID; cardiovascular, CV; chronic obstructive pulmonary disease, COPD; adverse event, AE; major adverse cardiovascular event, MACE; serious adverse event, SAE; rate ratio, RR; confidence interval, CI; long-acting muscarinic antagonist, LAMA; Global initiative for chronic Obstructive Lung Disease, GOLD; dry-powder inhaler, DPI; Understanding Potential Long-term Impacts on Function with Tiotropium, UPLIFT trial; TIOtropium Safety and Performance in Respimat, TIOSPIR trial; chronic kidney disease, CKD; fixed-dose combination, FDC; once daily, QD; forced expiratory volume in 1 second, FEV1; Standardized MedDRA Queries, SMQ; preferred term, PT; myocardial infarction, MI; system organ class, SOC; angiotensin-converting enzyme, ACE; calcium ion, Ca2+; standard deviation, SD
Citation: Chapman KR, Beck E, Alcaide D, Garcia Gil E. Overall and cardiovascular safety of aclidinium bromide in patients with COPD: A pooled analysis of six phase III, placebo-controlled, randomized studies. Chronic Obstr Pulm Dis. 2016; 3(1): 435-445. doi: http://doi.org/10.15326/jcopdf.3.1.2015.0148
CORRECTION: Please note that when Volume 3, Issue 1 launched on January 27, an incorrect table was included in the online version of this article (Table 1) (The pdf version was always correct.)This error has been corrected and the article now contains the correct Table 1.
Introduction
International guidelines recommend the use of long-acting muscarinic antagonists (LAMAs) as a first-choice treatment option for patients with chronic obstructive pulmonary disease (COPD) who are in Global initiative for chronic Obstructive Lung Disease (GOLD) Groups B, C and D.1 However, the results of several meta-analyses have highlighted concerns surrounding the safety – particularly cardiovascular (CV) safety – of the LAMA tiotropium when delivered via mist inhaler (Respimat® Soft MistTM) as a therapy for COPD.2-4
A meta-analysis of 5 randomized controlled trials (n=6522) indicated a 52% increased risk of all-cause mortality associated with tiotropium via mist inhaler compared with placebo.4 Similarly, a mixed-treatment comparison meta-analysis (n=52,516) found an increased risk of all-cause mortality associated with tiotropium via mist inhaler compared with placebo and other treatments, including tiotropium via dry-powder inhaler (DPI) (HandiHaler®).2 Whilst the increased risk of all-cause mortality with tiotropium via mist inhaler was particularly pronounced for the unlicensed 10µg dose compared with the licensed 5µg dose, there was a twofold increase in the risk of CV death compared with placebo, irrespective of dose.2 In contrast, for patients randomized to tiotropium via DPI in the 4-year Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT) trial, there was a trend towards reduced mortality compared with placebo.5 TIOtropium Safety and Performance in Respimat (TIOSPIR), a large (n=17,135), randomized safety study intended to address the question of LAMA safety, did not include a placebo arm and therefore only reported no difference in mortality or exacerbation rate among tiotropium formulations.6
Given that CV comorbidities are common in patients with COPD,7,8 further investigation of the CV safety of tiotropium and other LAMAs is essential. Other comorbidities may also need to be taken into account when considering a treatment strategy. For example, there is some evidence that patients with chronic kidney disease (CKD) may be at increased risk of mortality when using tiotropium mist inhaler compared with patients with normal kidney function.9,10
Aclidinium bromide, another LAMA for the treatment of COPD, has high affinity for all 5 human muscarinic receptor subtypes (M1-M5), but a residence time at M3 that is 6 times longer than that at M2, and a half-life for M2 that is 3.22 times shorter than tiotropium.11 This kinetic selectivity may reduce the potential for non-pulmonary adverse events associated with blockade of M2. More significantly, and distinct from other commonly used antimuscarinic bronchodilators, aclidinium is rapidly hydrolyzed in human plasma (Figure 1).12 Preclinical and pharmacological studies with aclidinium have demonstrated low systemic availability and a low propensity to induce cardiac arrhythmias,11-13 and these findings have been reflected in a safety and tolerability profile that is similar to placebo in larger clinical trials.14-19 Due to the rapid hydrolysis of aclidinium in plasma, the kidney plays little role in clearance of the drug and urinary excretion is very low.20 Consistent with this, there is no relationship between the degree of renal impairment and aclidinium plasma exposure. 20
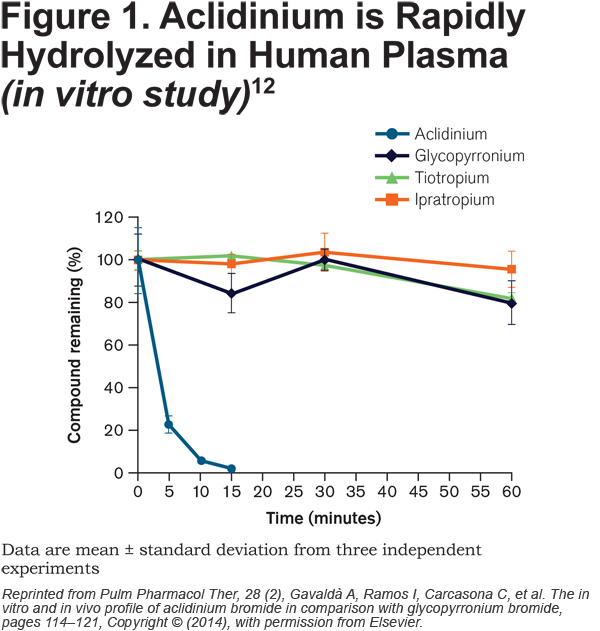
Here, we report data pooled from 6 placebo-controlled Phase III studies of at least 1 month’s treatment duration and evaluate the overall and CV safety of aclidinium 400µg administered twice daily (BID) in patients with moderate to severe COPD.
Methods
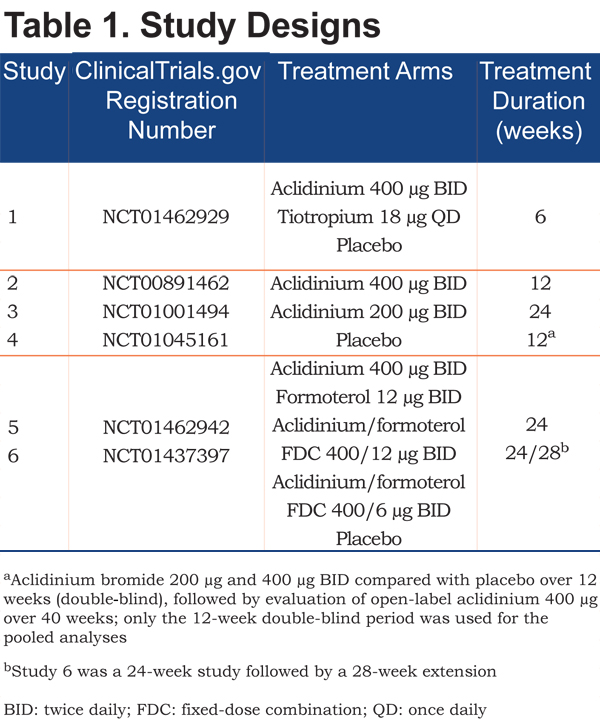
Safety data were pooled from the placebo and aclidinium 400µg BID arms of 6 multinational, multicenter, randomized, double-blind, parallel-group studies designed to assess the efficacy and safety of aclidinium in patients with COPD (Table 1). Aclidinium and placebo were administered in the morning and evening via a breath-actuated multidose DPI (GenuairTM/Pressair®a). All studies were conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization/Good Clinical Practice Guidelines and local regulations. The studies were approved by the relevant institutional review board or independent ethics committee at each study center.
Study Populations
Eligible patients were males or females aged ≥40 years of age who were current or former smokers (≥10 pack years) diagnosed with moderate to severe COPD (post‑bronchodilator forced expiratory volume in 1 second [FEV1]/forced vital capacity ratio <70%; post-bronchodilator FEV1 ≥30% and <80% predicted normal).
Key exclusion criteria included: respiratory infection or COPD exacerbation ≤6 weeks pre-screening (≤3 months if hospitalized for exacerbations); presence or history of clinically significant respiratory conditions other than COPD (e.g., asthma); and presence or history of clinically significant CV conditions, including myocardial infarction (≤6 months), newly diagnosed arrhythmia (≤3 months), unstable angina or unstable arrhythmia requiring changes in pharmacological therapy (≤12 months; Study 6 ≤6 months) and hospitalization for heart failure, functional class III or IV, as per New York Heart Association Guidelines (≤12 months).21
Inhaled salbutamol (100µg/puff) was permitted as rescue medication, provided its use was discontinued 6 hours before planned study visits. Inhaled corticosteroids, oral or parenteral corticosteroids (≤10 mg/day of prednisone or 20 mg every other day), oral sustained-release methylxanthines and oxygen therapy (<15 hours/day) were allowed, provided treatment was stable for ≥4 weeks before screening. The use of other long-acting bronchodilators was prohibited.
Safety Outcomes
Patients were asked to report AEs in a patient diary on a daily basis between scheduled visits. Study physicians assessed the seriousness of the AEs at each study visit. AEs of special interest were analyzed based on the following standard Medical Dictionary for Regulatory Activities queries (Standardized MedDRA Queries [SMQs]): ischemic heart disease (including SMQs of myocardial infarction and other ischemic heart disease), tachyarrhythmia (and the following preferred terms [PTs]: tachycardia, heart rate increase, palpitations), bradyarrhythmia (non-specific terms and the following PTs: sinus arrest and sinus bradycardia), conduction defects and cardiac failure. For anticholinergic events the anticholinergic syndrome SMQ was used in addition to other PTs of relevance, due to the mechanism of action.b Lower respiratory tract infections, including pneumonia, were also investigated (High Level Term of lower respiratory tract and lung infections and additional PT of pneumonia). Additionally, cerebrovascular events were identified from the following SMQs: hemorrhagic and ischemic cerebrovascular conditions. Major adverse CV events (MACE), defined as the composite of CV deaths, non-fatal myocardial infarctions (SMQ: myocardial infarction) and non-fatal strokes (SMQs: hemorrhagic and ischemic cerebrovascular conditions), were also evaluated. Additional safety assessments were clinical laboratory tests, vital signs, electrocardiograms and Holter monitoring.
A sub-analysis of cardiac and cerebrovascular events was performed in patients with CV risk factors (cardiac disease, obesity [body mass index ≥30 kg/m2], hypertension, dyslipidemia or hyperglycemia/new onset diabetes mellitus). Additionally, a sub‑analysis of AEs in patients with renal impairment was performed. In Studies 2–6, the degree of renal impairment was determined based on creatinine clearance (mild to severe: ≤80 mL/min; moderate to severe: ≤50 mL/min). As creatinine levels were not assessed in Study 1, the higher level term ‘renal failure and impairment’ was used to identify patients with renal impairment; these cases were considered moderate to severe because mild cases are difficult to diagnose.
Safety outcomes were analyzed using descriptive statistics in the safety population (all randomized patients who received ≥1 dose of study medication). Rate ratios (RR) were adjusted for exposure time using the Mantel-Hanszel formula.
Results
Patients
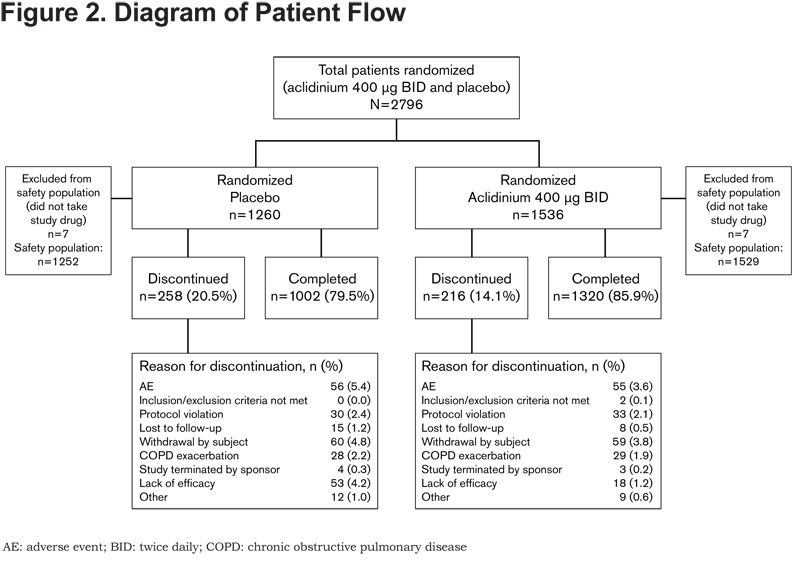
Of 2796 randomized patients, 2781 were included in the pooled safety population. In total, 474 patients (17.0%) discontinued treatment, the primary reasons being withdrawal of consent (4.3%), AEs (4.0%) and lack of efficacy (2.5%). Patient flow and the reasons for discontinuation are presented by treatment group in Figure 2.
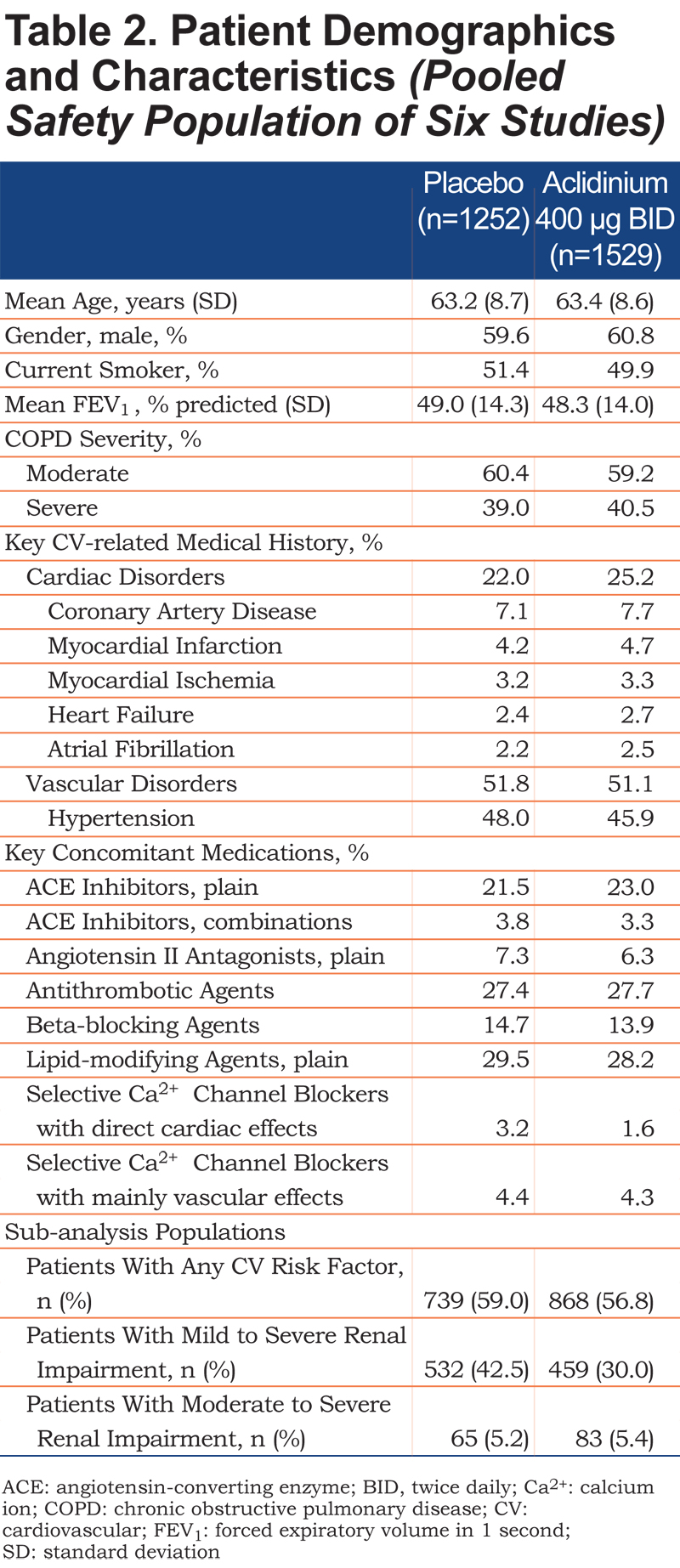
Patient demographics and baseline characteristics were similar between the treatment groups (Table 2). Overall, 23.8% of patients had a history of cardiac disorders and 51.4% had a history of vascular disorders, as expected for this population. In total, 1607 patients had CV risk factors and were included in the CV risk factor sub‑analysis. The sub-analyses in patients with renal impairment included 991 patients with mild to severe renal impairment and 148 patients with moderate to severe renal impairment.
Overall Safety
The incidence of AEs, including cardiac and cerebrovascular AEs, was comparable with aclidinium 400µg and placebo (Table 3). A similar proportion of patients with aclidinium 400µg and placebo reported AEs (including CV and cerebrovascular AEs) leading to discontinuation (Table 3). There were 7 deaths (0.5%) in the aclidinium 400µg group and 4 (0.3%) in the placebo group (Table 3).
Anticholinergic Events
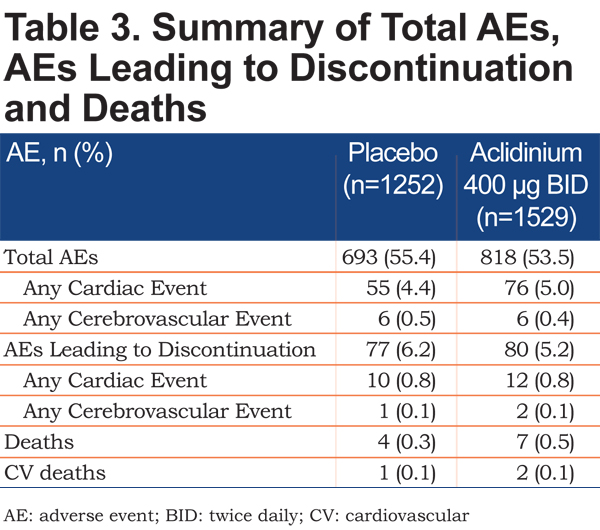
The incidence of possible anticholinergic AEs with aclidinium 400µg was low (<2%) and comparable to placebo (Table 4). The most commonly reported AEs that were potentially anticholinergic in nature were urinary tract infection and oropharyngeal pain.
Major Adverse Cardiovascular Events (MACE)
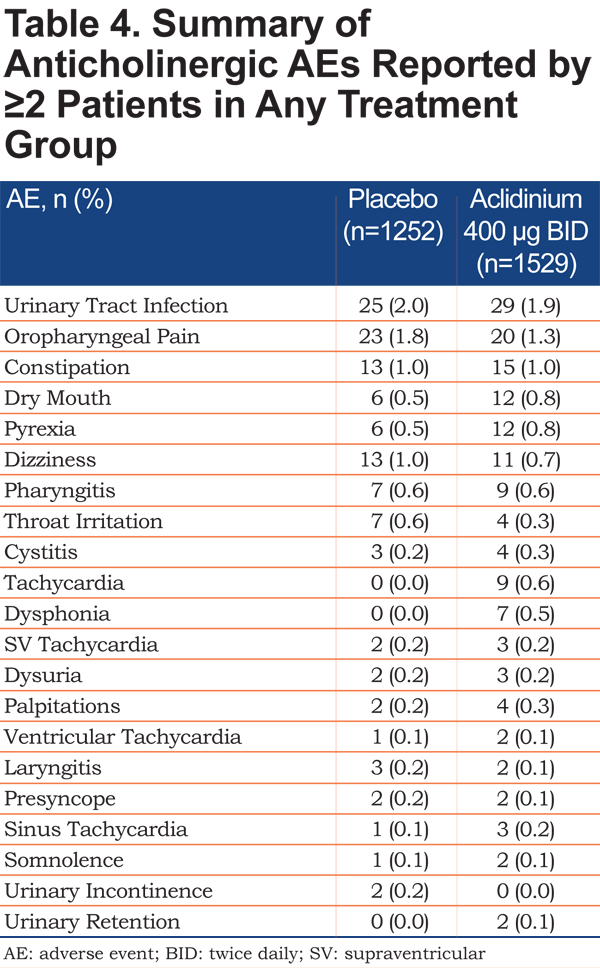
There was no difference between the aclidinium 400μg and placebo groups for the incidence of MACE AEs (Figure 3a) and SAEs (Figure 3b). The total incidence of MACE across treatment groups was low (AEs: 0.7%; SAEs: 0.5%). None of the 3 CV deaths that occurred across the 6 studies were considered to be related to treatment by the investigators.
Cardiac and Cerebrovascular Events
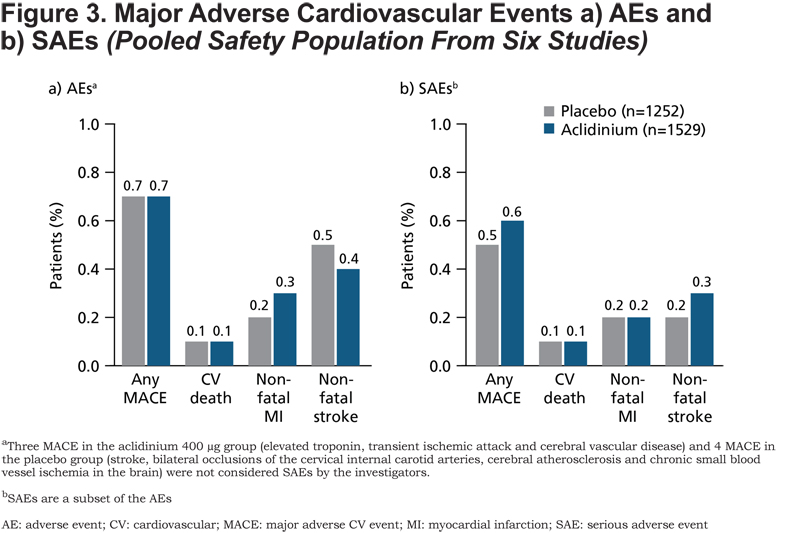
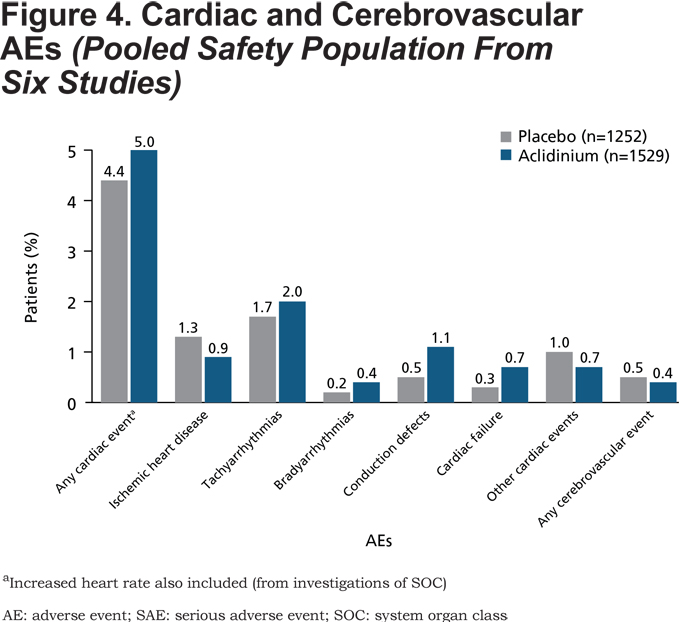
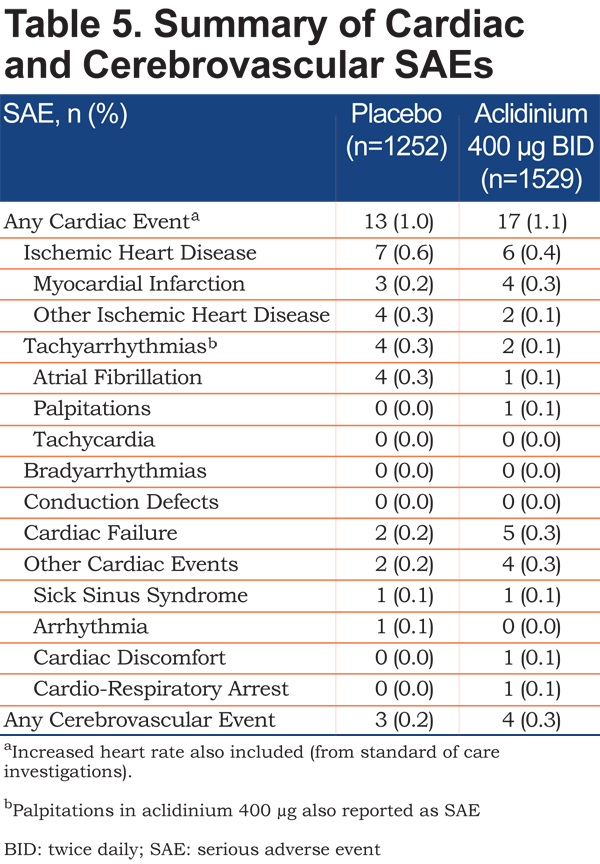
The incidence of cardiac and cerebrovascular events based on SMQ categories was low (AEs: ≤2% in each category; SAEs: ≤0.6% in each category) and comparable between the aclidinium 400µg and placebo groups (Figure 4 and Table 5). Furthermore, the incidence of cardiac and cerebrovascular events was generally similar for patients with CV risk factors who received aclidinium compared with those who received placebo (aclidinium: 67 [7.7%], incidence rate per 1000 patient-years: 182.6; placebo: 53 [7.2%], incidence rate per 1000 patient-years: 191.9; RR [95% confidence interval (CI)]=1.01 [0.74, 1.39]). In the subset of patients with mild to severe renal impairment, the incidence of cardiac events was similar with aclidinium and placebo (aclidinium: 27 [5.9%], incidence rate per 1000 patient-years: 120.5; placebo: 31 [5.8%], incidence rate per 1000 patient-years: 146.6; RR [95% CI]=0.87 [0.56, 1.36]). Similarly, in patients with moderate to severe renal impairment, the incidence of cardiac events was comparable with aclidinium and placebo (aclidinium: 4 [4.8%], incidence rate per 1000 patient-years: 113.2; placebo: 3 [4.6%], incidence rate per 1000 patient-years: 114.7; RR [95% CI]=0.53 [0.17, 1.67]).
Lower Respiratory Tract Infections
The incidence of any lower respiratory tract infection was low and similar between the 2 treatment groups (AEs: aclidinium: 32 [2.1%]; placebo: 36 [2.9%]; SAEs: aclidinium: 6 [0.4%]; placebo: 9 [0.7%]). Similarly, the incidence of pneumonia was comparable with aclidinium and placebo (AEs: aclidinium: 9 [0.6%]; placebo: 11 [0.9%]; SAEs: aclidinium: 6 [0.4%]; placebo: 8 [0.6%])
Discussion
This pooled analysis of 6 placebo-controlled Phase III studies suggests that aclidinium 400µg BID has a goodsafety profile, with no evidence of increased CV or cerebrovascular risk compared with placebo in patients with moderate to severe COPD. Furthermore, sub-analyses showed that the incidence of cardiac and cerebrovascular events was similar with aclidinium and placebo in patients with CV risk factors and there was no evidence for a difference in the incidence of cardiac events between treatment groups in patients with mild to severe renal impairment.
There has been controversy surrounding the CV safety of anticholinergics since a meta-analysis of 17 randomized clinical trials reported a significantly increased risk of major CV events (relative risk 1.58) with inhaled anticholinergics (tiotropium or ipratropium via mist inhaler, DPI or metered dose inhaler) compared with control therapy (placebo or active comparator) in patients with COPD; however, the difference in all-cause mortality with inhaled anticholinergics compared with controls did not reach significance (relative risk 1.26; p=0.06).22 Subsequent reports of tiotropium safety compared with placebo have varied, with a 4-year study of tiotropium via DPI showing a trend towards decreased all-cause mortality and no change in CV events5 and a second meta-analysis of tiotropium via mist inhaler reporting a 52% increase in all-cause mortality and an increased risk of CV death.4 Studies comparing mortality between the 2 tiotropium formulations have been inconclusive, with some studies suggesting mortality is higher with the mist inhaler compared with the DPI2,3 and others finding no difference between the 2 formulations.6
The baseline characteristics of the study population in our pooled analysis are similar to those of the 2011 meta-analysis,4 with comparable mean age (63.2–63.4 years versus 63.0–65.7 years), a slightly lower degree of airflow limitation (mean FEV1 48.3–49.0% versus 37.5–42.0% predicted normal) and proportion of male patients (59.6–60.8% versus 69.0–79.3%), and a slightly greater proportion of current smokers (49.9–51.4% versus 35.0–43.0%).
One of the limitations of this pooled analysis is that since CV safety was not the primary outcome in any of the 6 clinical trials, all of the pooled safety data used in the analysis were collected from patient reports, in which AEs and SAEs were spontaneously reported to the physician by the patient. However, if the study physician observed a clinically relevant abnormality in clinical laboratory tests, vital signs or electrocardiogram/Holter recordings, this was to be reported as an AE/SAE. A further limitation was that none of the studies were enriched with high-risk patients, such as those with clinically significant CV disease or renal insufficiency. Indeed, TIOSPIR also excluded high-risk patients, as have many of the other studies investigating the efficacy and safety of LAMAs.4,6,23,24 A recent post-hoc analysis of the UPLIFT trial, however, found that patients with cardiac comorbidities were not at increased risk of mortality or cardiac SAEs with tiotropium 18µg once daily versus placebo.25 The ongoing Evaluate the Effect of Aclidinium Bromide on Long-term Cardiovascular Safety and Exacerbations in Moderate to Very Severe COPD Patients: ASCENT COPD(NCT01966107) double-blind, randomized, placebo-controlled Phase IV study is investigating the long-term CV safety of aclidinium 400µg BID versus placebo in patients with moderate to very severe COPD and significant CV comorbidities.26 However, there is still a need to conduct longer-term head-to-head studies of LAMAs, including all available formulations, as well as studies in “real-world” patients with common comorbidities, in order to fully evaluate and compare the safety profile.
Although the 2011 meta-analysis raised concerns about the safety of tiotropium via mist inhaler, we have shown that in a similar patient population, aclidinium has an overall and CV safety profile comparable to placebo. To date, there have been no head-to-head comparator studies of aclidinium and tiotropium via mist inhaler. However, aclidinium and tiotropium via DPI have been evaluated in a 6-week, randomized, placebo- and active-controlled Phase III study, in which the incidence of AEs was similar with aclidinium (27.5%) and tiotropium (29.7%).14 The incidence of potentially anticholinergic AEs was <1.5% in both treatment groups.14
In vitro studies have shown that aclidinium is rapidly hydrolyzed into 2 inactive metabolites in human plasma and has a shorter half-life than tiotropium, glycopyrronium and ipratropium (Figure 1).12,13 This property of aclidinium may help to minimize cardiac events. A study in dogs, in which rapid plasma hydrolysis of aclidinium also occurs, showed that aclidinium had a reduced effect on heart rate compared with tiotropium when administered at doses that were 100-fold higher than those needed for bronchodilation.11 Rapid hydrolysis of aclidinium also minimizes systemic exposure and, consequently, the potential for systemic side effects.12,13 Systemic side effects are a particular concern for patients with CKD, as renal impairment can reduce drug clearance and increase systemic exposure. Recent United Kingdom data suggest the prevalence of CKD is higher in the COPD population (Stage 3–5 CKD: 18.2%) than in the general adult population (Stage 3–5 CKD: 8.5%).27 Verhamme and colleagues have shown evidence for increased mortality with tiotropium via mist inhaler compared with tiotropium via DPI in patients with renal impairment, with a 27% increased risk of death in patients who received tiotropium via mist inhaler compared with those who received the drug via DPI.28 When the data were stratified by level of renal impairment, the association between the use of the mist inhaler and mortality remained significant in patients with Stage 3–5 CKD but not in those with normal kidney function.9 Renal clearance of aclidinium is very low in both patients with mild to severe renal impairment and patients with normal renal function.20 In patients with normal kidney function, 0.090% of the aclidinium dose is recovered from urine20 compared with 60.1% of the tiotropium dose.29 The low urinary excretion of aclidinium may make it suitable for patients with CKD. Indeed, the results from this pooled analysis suggest there are no increased safety risks in patients with renal impairment. However, it is important to highlight that in this pooled analysis, the number of patients with renal impairment – particularly those with moderate to severe renal impairment – was relatively low.
In conclusion, aclidinium 400µg BID has a goodsafety profile with no evidence of increased CV or cerebrovascular risk compared with placebo. The safety profile of aclidinium and its rapid plasma hydrolysis suggest it can be used safely in patients with comorbidities, including patients with CV disease and CKD. However, further studies are needed to assess the CV safety of aclidinium 400µg BID in high-risk patients.
aRegistered trademark of AstraZeneca group of companies; for use within the USA as Pressair® and as Genuair® within all other licensed territories.
bSinus tachycardia, supraventricular tachycardia, ventricular tachycardia, heart rate increased, palpitations, angle closure glaucoma, glaucoma, mydriasis, intraocular pressure increased, intraocular pressure test abnormal, papillary reflex impaired, pupils unequal, visual disturbance, accommodation disorder, blindness transient, cyclopegia, constipation, gastrointestinal obstruction, ileus paralytic, urinary tract infection, cystitis urinary retention, urinary incontinence, incontinence, dysuria, urge incontinence, urine flow decreased, bladder irritation, oropharyngeal pain, dysphonia, laryngitis, pharyngitis and throat irritation.
Acknowledgments
The authors would like to thank Rosa Lamarca for assistance with statistical analyses and the study investigators at each of the participating centers for their contribution to the studies.
Declaration of Interest
KRC has received grants from Almirall, Forest Laboratories, Novartis, GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, Roche, Genentech, Amgen, Grifols and CSL Behring. EB has no conflicts of interest to disclose. DA is a former employee of Almirall, S.A., Barcelona, Spain. EGG is an employee of AstraZeneca PLC, Barcelona, Spain and a former employee of Almirall, S.A., Barcelona, Spain. The sponsor, Almirall S.A., Barcelona, Spain, was involved in the design and conduct of the analysis, review of the data, and review and approval of the manuscript; AstraZeneca also reviewed the manuscript. Medical writing support, funded by AstraZeneca, was provided by Suzanne McAllister, PhD, of Complete Medical Communications.