Running Head: Socioeconomic Status and Treatment Response in COPD
Funding support: Funding for this COPD Biomarkers Qualification Consortium working group was provided by AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis and Pfizer.
Date of Acceptance: February 1, 2017
Abbreviations: randomized controlled trials, RCTs; socioeconomic status, SES; chronic obstructive pulmonary disease, COPD; St George’s Respiratory Questionnaire, SGRQ; minimum clinically important difference, MCID; COPD Biomarkers Qualification Consortuim, CBQC; forced expiratory volume in 1 second, FEV1; modified Medical Research Council, mMRC; standard deviation, SD; analysis of covariance, ANCOVA; body mass index, BMI; Global initiative for chronic Obstructive Lung Disease, GOLD
Citation: Jones PW, Gelhorn H, Wilson H, et al. Socioeconomic status as a determinant of health status treatment response in COPD trials. Chronic Obstr Pulm Dis. 2017; 4(2): 150-158. doi: http://doi.org/10.15326/jcopdf.4.2.2017.0132
Introduction
Many clinical trials in chronic obstructive pulmonary disease (COPD) are conducted globally, recruiting patients from multiple countries and representing varied populations, including those with both lower and higher socioeconomic status (SES).1,2 Patients with lower SES have poorer outcomes that is multi-factorial in origin, but is likely to include less access to health care.3 In countries with lower SES, patients entering a clinical trial may move into a system of health care similar to that found in highly developed countries. Increasing numbers of trials are now being performed solely in countries with developing economies, in which recruited patients may be naïve to the class of drug being tested.4 These factors could all impact the measured response to treatment.
Health status instruments such as the St George’s Respiratory Questionnaire (SGRQ)5 are often used to quantify the overall benefit of treatment by providing a standardized measure of the impact of disease on patients’ wellbeing and daily activities.6,7 It can be used across patient groups and was designed to provide a score that is not influenced by the mode of therapeutic action, thereby permitting comparisons of treatment effect across populations and modalities.7 When used together with its minimum clinically important difference (MCID), its scores can provide an indication of whether the treatment effect is worthwhile.8,9 In clinical trials, changes are often seen in the placebo arm across a range of outcomes, particularly those that measure symptoms and health status10,11 The responsible mechanisms are not well understood, but are likely to include better care and a Hawthorne effect,12 in which the patient’s (and perhaps their health care provider’s) behavior changes due to the fact that they are being observed. Such changes could include: better compliance with, and use of, trial-permitted concomitant therapy, earlier treatment of acute episodes, and adoption of a healthier life style including smoking cessation.
The effects due to trial participation, coupled with the possible influences of SES on health outcomes, raise important questions for the interpretation of clinical trial results from countries of differing SES. For example: Do patients recruited from low SES countries experience the same benefit as those in wealthier countries? Does the clinical trial effect differ between countries of high and low SES? Is the size of the measured treatment effect influenced by SES? Can therapeutic data from countries of differing SES be combined to produce a meaningful dataset?
To address these questions in COPD - a common, chronic and worldwide condition13 - we carried out a pooled analysis using individual patient clinical trial data from a newly created comprehensive database using the SGRQ as the outcome measure.5 The SGRQ is particularly suitable for this type of analysis since it is very widely used and available in many linguistically and culturally validated translations.
Methods
Identification of Clinical Trials
Studies where SGRQ was included as an outcome were identified in the COPD Biomarkers Qualification Consortium (CBQC) database of clinical trials in COPD.14 Twenty-one studies were identified with SGRQ scores measured at baseline and during follow-up in each trial arm: 18 randomized controlled trials (RCTs) and 3 observational studies. For the current analysis, RCTs that included an intervention arm with long-acting bronchodilators only (long-acting beta2-agonists and long-acting anti-muscarinic agents) or placebo were evaluated (N=17). The observational studies were not incorporated in this analysis, nor were other treatment arms. Details of the RCTs included in the analysis are described elsewhere.15 All participants in these trials who had a baseline SGRQ score were included in these analyses.
The 17 RCTs were grouped by study duration, categorized as short-term (≤1-year duration) comprising 14 trials with 10802 COPD patients (placebo=3670; bronchodilator=7132), and medium-term (2-4 years in duration) comprising 3 trials with 8963 patients (placebo=4184; bronchodilator=4779). Comparable numbers of study participants are included in each category. The 2 groups of trials were analyzed separately to test for consistency of findings across studies of different duration. The World Health Organization (WHO) socioeconomic grading system was used to group countries into High and Low/Medium SES (the latter 2 combined to achieve a similar balance of patient numbers between groups).16
Outcome Measures
The main outcome of interest was SGRQ total score. A range of other demographic and disease-related measures were available, including forced expiratory volume in 1 second (FEV1), modified Medical Research Council (mMRC) dyspnea score and history of COPD exacerbations.
Statistical Analysis
For each of the short-term and medium-term analysis samples used in the current analysis, demographic characteristics are presented as mean and standard deviation (SD). Analysis of covariance (ANCOVA) models were conducted with treatment, SES status, and treatment x SES status as independent variables, and change from baseline in SGRQ score as the dependent variable to evaluate the impact of SES status on SGRQ. Sex, body mass index (BMI), year of study, smoking status (current versus past), Global initiative for chronic Obstructive Lung Disease (GOLD) FEV1 grade,13 and baseline SGRQ score were included as covariates. Separate models were conducted for different time points. In the short-term dataset, models were conducted with change from baseline to 1, 3, 6 and 12 months. In the medium-term dataset, models were conducted with change from baseline to 6, 12, 24, and 36 months. Scheffe tests were used for post-hoc comparisons.
Results
Baseline Characteristics
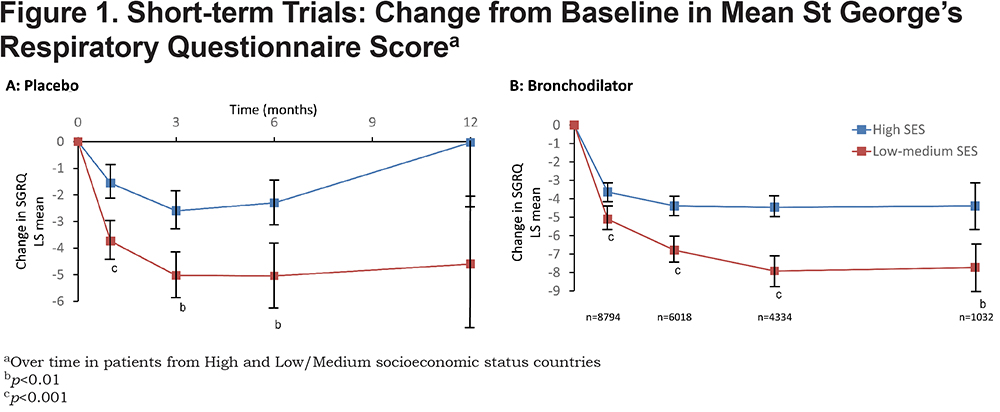
The baseline demographics of the patients were similar between SES groups, although in Low/Medium SES countries the proportion of males was higher and the patients were slightly younger (by approximately 2 years) (Tables 1 and 2). In the short-term trials, patients in Low/Medium SES countries had more severe disease as shown by FEV1, SGRQ score, mMRC dyspnea score, COPD exacerbation rate and hospitalizations due to exacerbation; although generally the differences were not clinically important (Table 1). The 2.0 unit difference in mean SGRQ total score between groups at baseline shows that, on average, the health status of patients from Low/Medium SES countries was worse than in those from High SES countries at entry to the study, but the difference did not reach the MCID.
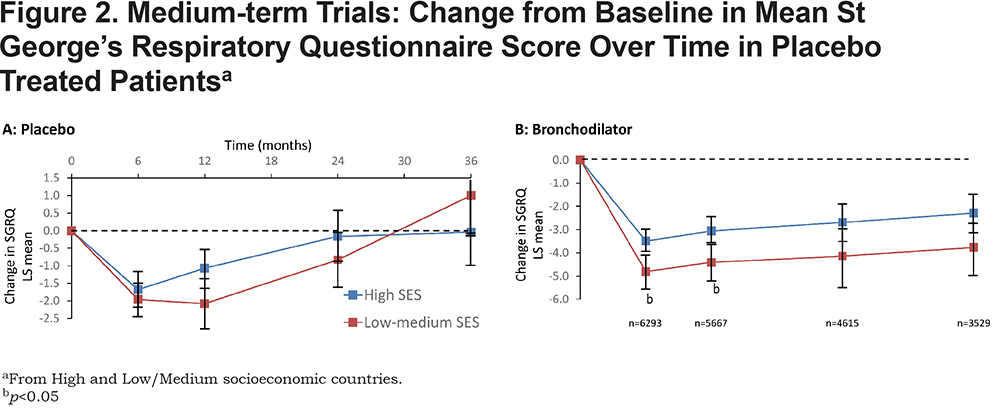
A similar pattern was observed in the medium-term trials, with the exception that the mean difference in SGRQ was 3.5, showing that at study entry, patients from Low/Medium SES countries had health status that was clearly worse than that of participants from High SES countries (Table 2). There was no difference in FEV1 between the SES groups in the medium-term trials, but patients from Low/Medium SES countries reported worse dyspnea grades, more exacerbations and more hospitalizations due to an exacerbation.
Patient Withdrawal Rates
The withdrawal rate in Low/Medium SES countries was almost half that seen in High SES countries. In short-term trials the dropout rates (placebo and bronchodilator combined) were: Low/Medium SES 20% (496/2476); High SES 37% (1828/4927). In the medium-term trials the dropout rates were similar to those seen in the short-term trials: Low/Medium SES 18% (363/2040); High SES 34% (2312/6823).
Response to Placebo
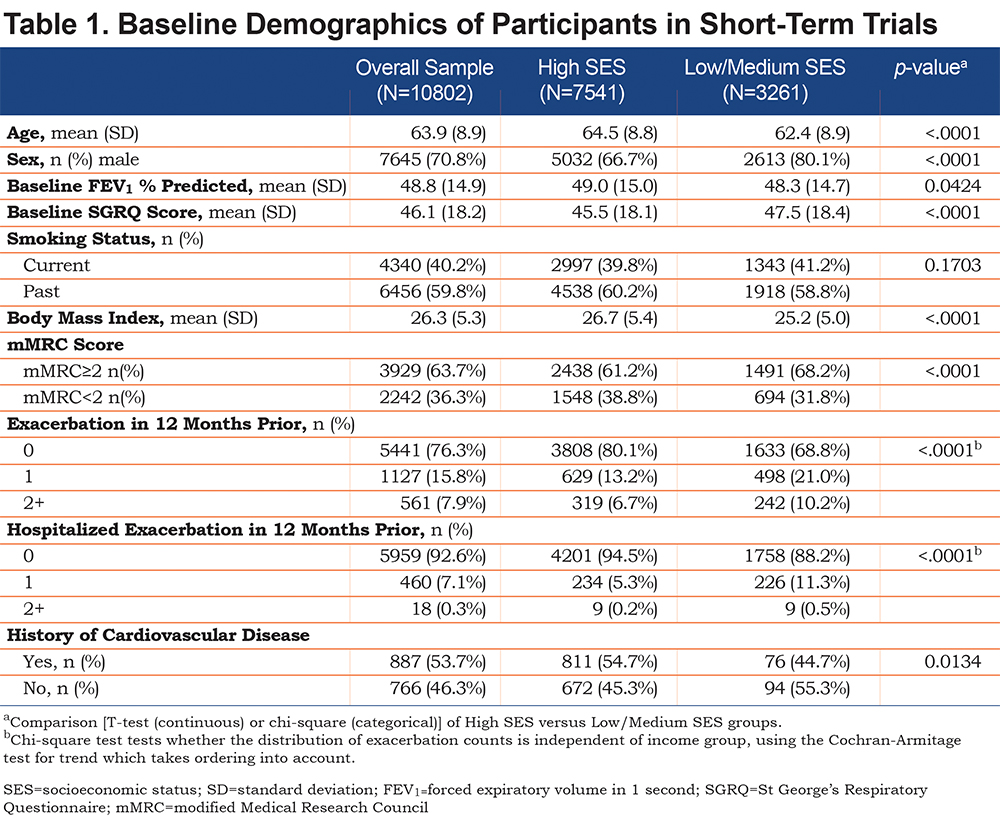
A significant improvement in mean SGRQ score was seen with placebo in Low/Medium and High SES countries in both short-term and medium-term trials (Figures 1A & 2A). The pattern was different between short- and medium-term trials. In the short-term trials (Figure 1A), the improvement developed progressively to be maximal at 3-6 months; it exceeded the 4-unit MCID in countries with Low/Medium SES. The greater response in Low/Medium SES countries was statistically significant, even at 1 month and at 3-6 months it was over 2 units more than that seen in the High SES countries. The wide and overlapping 95% confidence intervals at 12 months was associated with a much smaller number of patients which precludes any firm inferences being drawn at this time point.
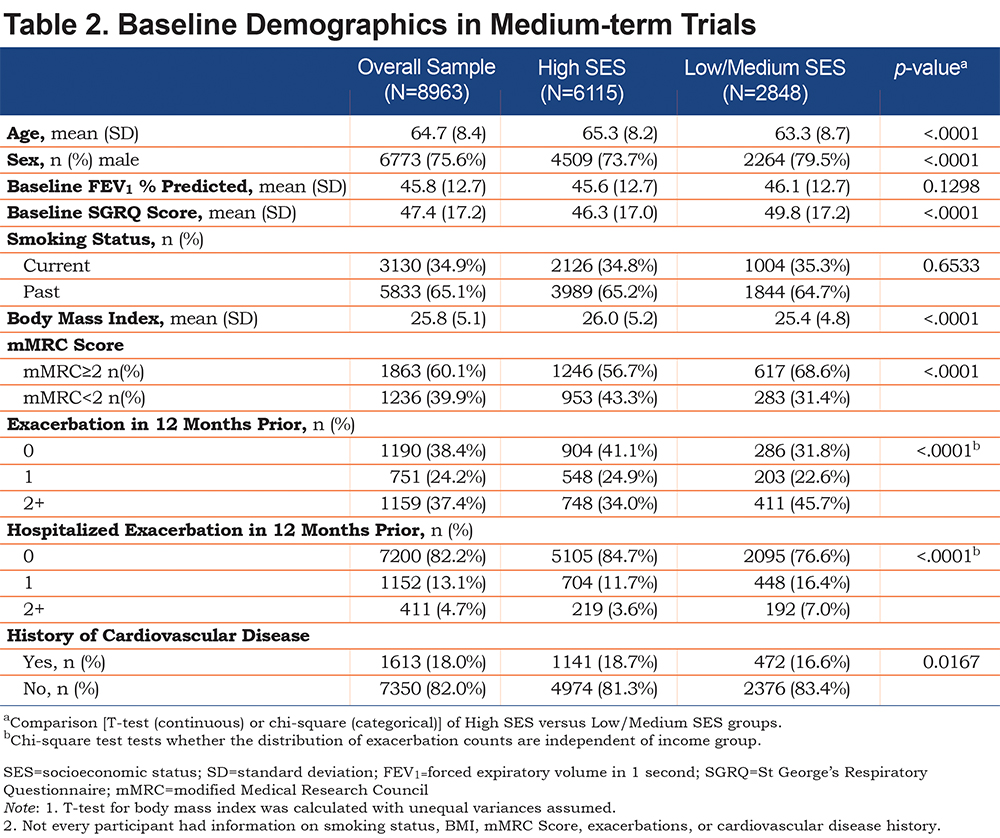
In the medium-term trials (Figure 2A), the improvement was similar in both groups of patients and around 2 units. This effect was maintained for 12 months, but was gone by 36 months in both groups. It is noteworthy that a similar pattern can been seen at 6 and 12 months in both groups of studies. During that interval, there is a trend for the patients in the High SES group to worsen, but for the improvement to be maintained up to 12 months in the Low-Medium SES group
Treatment Response to Long-acting Bronchodilator
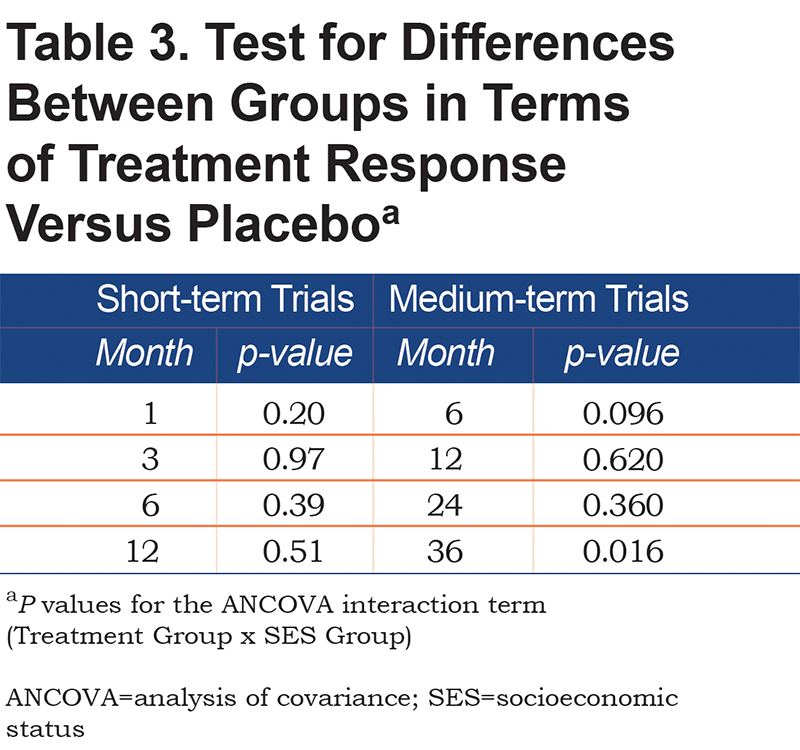
There was a significant main effect for treatment in each of the ANCOVA models for the short-term and medium-term studies (all p values <0.001), with greater improvement in SGRQ over time in bronchodilator relative to placebo. In the short-term trials (Figure 1B), there was a significant improvement from baseline, which either attained (High SES) or well-exceeded (Low/Medium SES) the 4-unit MCID, and in both groups of patients this benefit was maintained for 12 months. The difference in response to bronchodilator between groups increased up to 6 months and was statistically significant at all time points. At 6 and 12 months, the difference was over 3 units. There was a similar pattern of sustained difference over 3 years (Figure 2B), but it was smaller (≈1.5 units) and only statistically significant at 6 and 12 months (Table 3).
Treatment x Socioeconomic Response
The interaction between treatment versus placebo and SES group was not significant in the majority of the analyses, with 1 exception. In the medium-term dataset when evaluating change from baseline to 36 months the difference between bronchodilator and placebo was significantly greater in the Low/Medium SES patients ( Table 3). This may be related to the fact that patients in the Low SES group receiving placebo initially experienced a greater improvement relative to those in the High SES group, but by month 36 they experienced a greater deterioration relative to patients in High SES groups (Figures 2A & B).
Discussion
This is the first major analysis of the effect of socioeconomic status on health status in response to treatment for COPD. It shows that individuals from both High and Low/Medium SES countries experience an improvement in health status on entering a clinical trial, even if they receive placebo. This effect is larger and appears to be sustained for longer in study participants from Low/Medium SES countries, although it is lost after 2 years. With long-acting bronchodilator treatment there is an improvement from baseline that almost reaches (High SES) or exceeds (Low-Medium SES) the 4 unit SGRQ MCID, thereafter the improvement in the High SES patients is relatively small (< 1 unit), but in Low-Medium SES patients it appears to be larger (>2 units).
The greater improvement in Low-Medium SES patients is maintained for 3 years; however, there appears to be no difference in treatment response compared to placebo, since the larger clinical trial effect with placebo is matched by a larger active treatment effect. The only possible exception to this is at 36 months and is due to the greater worsening in placebo treated patients in the Low-Medium SES group. It is known that SGRQ deteriorates over time and the apparently greater worsening in Low-Medium SES patients may be a function of the lower withdrawal rate in this group, since it is known that patients with worse SGRQ scores are more likely to withdraw early from a trial.17 Overall, these results suggest that data from patients in countries of different SES status may be combined and that results from one country should be generalizable to countries of different SES status.
At baseline, study participants in Low/Medium SES countries had slightly more severe COPD across a range of measures, including SGRQ score, but the differences were small and largely consistent between short- and medium-term studies. Previously, we have shown that demographic factors other than SES have a very small effect on SGRQ scores.18 The mean baseline SGRQ scores in the patients analyzed here were in the range 46-48 units, which is in contrast to a mean score of 64.8 reported in an audit of severe COPD patients in a routine care setting in the United States.19 Whereas in an observational study in China, the baseline SGRQ score was 46.3 units20 suggesting that in both higher and lower SES countries, the more severe patients are not recruited to clinical studies.
The onset of the clinical trial effect appeared to follow a relatively consistent pattern across countries, increasing to a maximum at around 6 to 12 months. It was progressive in onset, rather than being apparent from the outset of the trial, which suggests that the benefit was acquired progressively over time, rather than as a step response to entering the study. The effect was larger and sustained for longer in poorer countries. The responsible mechanisms cannot be identified from this analysis, but a Hawthorne effect12 producing changes in health behaviors and treatment compliance on entering a trial is one possible factor. In addition, a patient may have an improved sense of confidence in their ability to manage their disease, since on joining the trial they gain access to free and regular health care, which they may not have experienced previously. In support of this conclusion is the demonstration that more intensive monitoring during a randomized trial was associated with a better outcome compared to that seen in patients who received the same pharmacological treatment, but less intensive monitoring.21
This analysis used individual participant data, rather than a meta-analysis of reported study results, but it does have limitations. All the trials were randomized and double blind, so the treatment arms will have been well-matched at an individual trial level, but differing trial durations and different dropout rates would have led to the treatment groups becoming less well matched over time. However, it is noteworthy that the overall pattern of results was very similar in trials of quite different duration. The assessment time-points differed between trials, so not all patients were assessed at each time point, however; the large sample sizes appear to have minimized this effect at a group level, since the time trends showed quite smooth curves across the assessment points. Another limitation is our use of the World Health Organization classification for determining a country’s health care system, since the proportion of gross domestic product spent on health varies a great deal between countries. More importantly, it treats each patient as having the same SES status. Within all countries there is a wide variation in income and the effect of this on health may be increasing since a widening mortality gap was shown in Canada between 1996/7 and 2012/3 in COPD patients with low SES compared to those who were more affluent.22 Despite this limitation, we have shown, in 2 separate analyses in a large number of individuals, that the pattern in the results appeared remarkably consistent.
These findings have implications for those involved in performing and evaluating clinical trials. Any influence of SES on the size of treatment effect compared with placebo appears to be relatively small. The inclusion of patients from Low/Medium SES countries into multi-national trials with patients from High SES countries should not greatly influence the size of effect compared to a study that recruited patients solely from High SES countries. This conclusion is supported by an analysis of one of the primary trials included here, which showed that world region did not influence the size of treatment effect on SGRQ.23 The current analysis also shows that there may be an advantage to the recruitment of patients from Low/Medium SES countries, since they have lower withdrawal rates, which would reduce the effect of an important uncontrolled bias in the trials.
Finally, this analysis shows that in a chronic disease, such as COPD, a patient’s health may be improved by a substantial amount just through regular clinical review without additional treatment, whether in a higher or lower income country. There is a broader message here for all health care systems
Acknowledgements
The authors would like to thank Debbie Merrill, COPD Foundation, for managing the review process, and the CBQC for their role in aggregating the data. They would also like to thank Thomas Martin of Novartis and Katja Rüdell of Pfizer for their review and oversight through the CBQC Steering Committee, and Pfizer for supporting the CBQC. The authors would like to acknowledge editorial support in the form of copyediting and collating author comments which was provided by Kate Hollingworth of Continuous Improvement Ltd, and funded by the CBQC.
Declaration of Interest
HM, VSB, MT, RTS, PWJ, NK, SM and SIR are employees of the pharma companies who funded this analysis. SIR is an employee of the University of Nebraska Medical Center. HG and HW participated in this project as employees of Evidera, a company which performs work for hire for multiple pharmaceutical and device companies in outcomes research, and were funded by the CBQC consortium. DM has nothing to declare.