Running Head: 2017 Update of COPD Foundation Pocket Guide
Funding Support: not applicable
Date of Acceptance: March 23, 2017
Abbreviations: COPD Foundation Pocket Consultant Guide, PCG; chronic obstructive pulmonary disease, COPD; asthma-COPD overlap syndrome, ACOS; inhaled corticosteroids, ICSs; Global initiative for chronic Obstructive Lung Disease, GOLD; spirometry grade 0, SG0; spirometry grade 1, SG1; spirometry grade 2, SG2; spirometry grade 3, SG3; spirometry grade undefined, SGU; forced expiratory volume in 1 second, FEV1; computed tomography, CT; modified Medical Research Council, mMRC; COPD Assessment Test, CAT; phosphodiesterase 4 inhibitor, PDE-4; long-acting muscarinic antagonist, LAMA; long-acting beta2-agonist, LABA; forced vital capacity, FVC
Citation: Yawn B, Thomashow B, Mannino DM, et al. A statement of the COPD Foundation: The 2017 update to the COPD Foundation COPD Pocket Consultant Guide. Chronic Obstr Pulm Dis. 2017; 4(3): 177-185. doi: http://doi.org/10.15326/jcopdf.4.3.2017.0136
Introduction
Chronic obstructive pulmonary disease (COPD) continues to be a common condition among primary care patients and has risen to become the third leading cause of death in the United States.1 Yet it is still often diagnosed late and may be under or even untreated when it is recognized.2 For many practicing physicians and other clinicians, aggressive diagnosis and management of COPD was not a significant part of their training. Spirometry remains underused as a diagnostic and follow-up assessment and is often not easily accessible to primary care clinicians and patients.3-5
To supplement limited COPD-focused training and provide updated information, guidelines and care recommendations have been produced by both national and international organizations. These recommendations and guidelines are often not in a format to facilitate implementation or point-of-care use. For example, the 132 page 2017 Global initiative for chronic Obstructive Lung Disease (GOLD) recommendations6 provide an important update and review of the evidence and recommendations for COPD management, but are lengthy.
The COPD Foundation produced its first brief, point-of-care COPD Foundation Pocket Consultant Guide (PCG) in 2007 with major updates in 20127 and in November 2016. The new PCG is a 4″ by 6″ tri-fold card to be used at the point of care (office, emergency department or hospital) and is supplemented with online versions (www.copdpraxis.com) and a phone-friendly app for Apple products (search “COPD Foundation” in the App Store). An app for Android users will be released later in 2017. This tool is an expert opinion, consensus document that relies on published evidence but is not an evidence-based review of all elements of COPD diagnosis and management. It is designed to incorporate best practices and serve as a short summary to guide major clinical decisions during visits to the office, emergency department or hospital. It is the work of the authors with input from many primary care health professionals who have reviewed and commented on its content.
Highlights of the 2017 Update
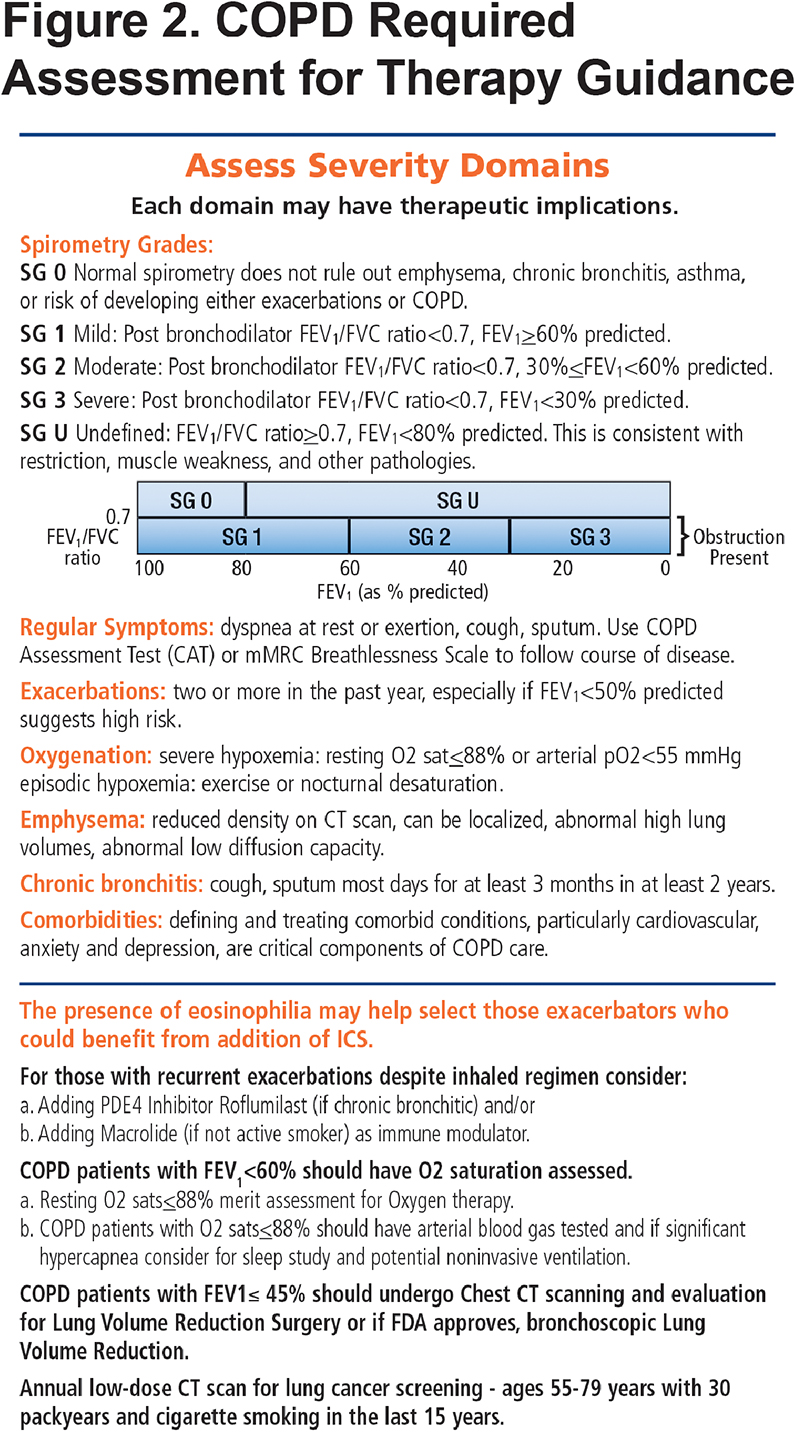
The new PCG begins with a practice flow diagram (Figure 1) to illustrate steps in diagnosing, assessing and grading COPD for management and ongoing care. Additionally, the PCG stresses that optimal COPD care requires evaluation of all the severity domains: spirometry, symptoms, exacerbation frequency, oxygen requirements, presence of emphysema, chronic bronchitis and comorbidities (Figure 2). The flow diagram is color coded (blue for assessments, green for available tools or metrics and orange for therapies) to remind clinicians that several important tools and tests are available for comprehensive COPD assessment. Furthermore, repeated re-evaluation of patients is key and may be used to guide both pharmacotherapy and other COPD treatment decisions. The chart highlights the importance of smoking cessation, vaccinations, exercise, pulmonary rehabilitation, testing for Alpha-1 antitrypsin deficiency and again, evaluating comorbid conditions.
Spirometry is the basis for confirmation of a COPD diagnosis. The PCG continues to provide guidance for categorizing spirometry results, which fall into 3 major groups: normal, spirometry grade 0 (SG0); obstructive lung disease, spirometry grades 1-4 (SG1-4); and reduced lung function without obstruction, spirometry grade undefined (SG-U) (Figure 2). This panel also highlights the other roles of spirometry: to identify those with lower forced expiratory volume in 1 second (FEV1) who may benefit from evaluation of hypoxemia at rest or with exercise (FEV1 below 60% of predicted are candidates for pulse oximetry evaluation) and for those with FEV1<45% predicted in whom you should consider computed tomography (CT) evaluation for emphysema that may be treatable with lung volume reduction surgery.
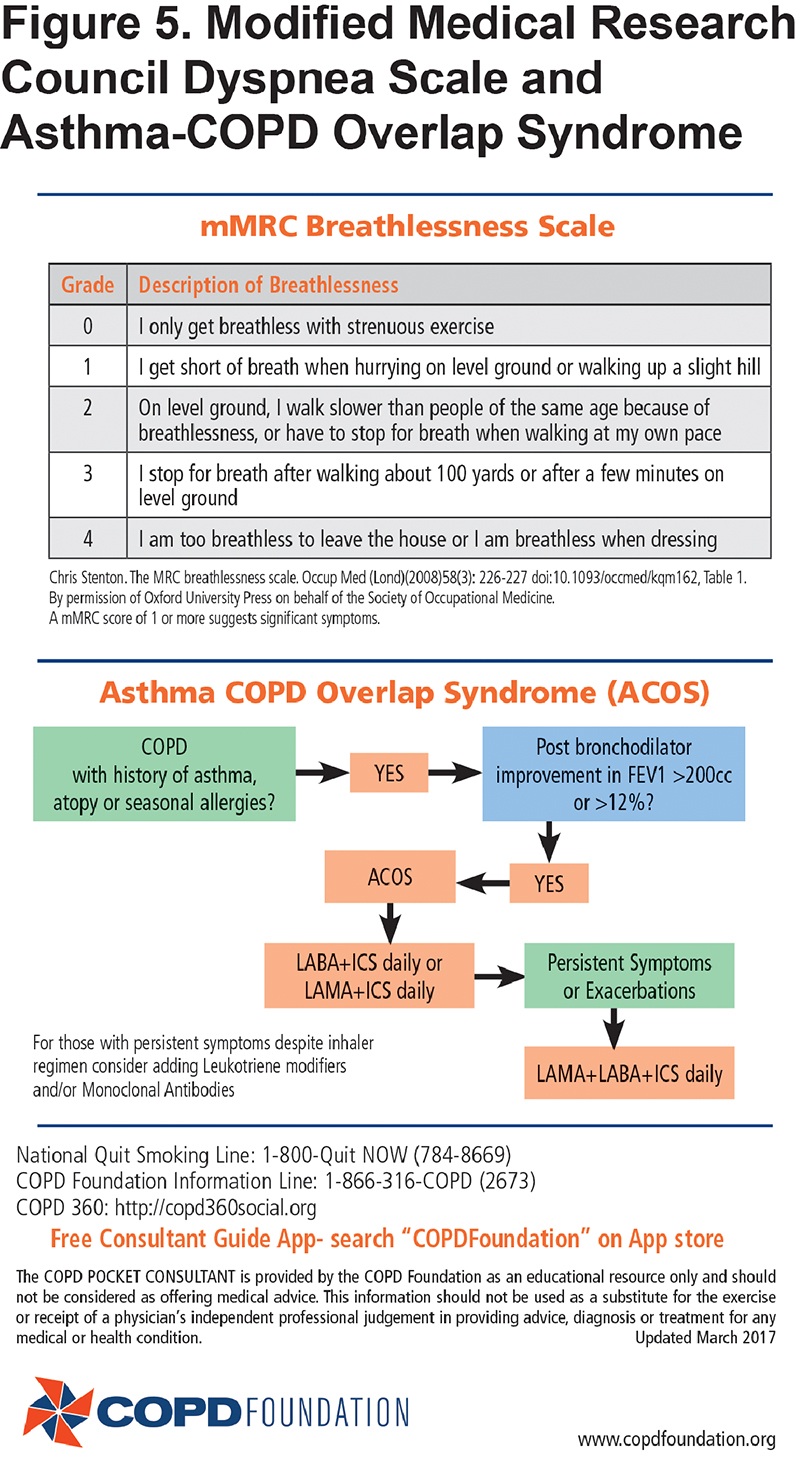
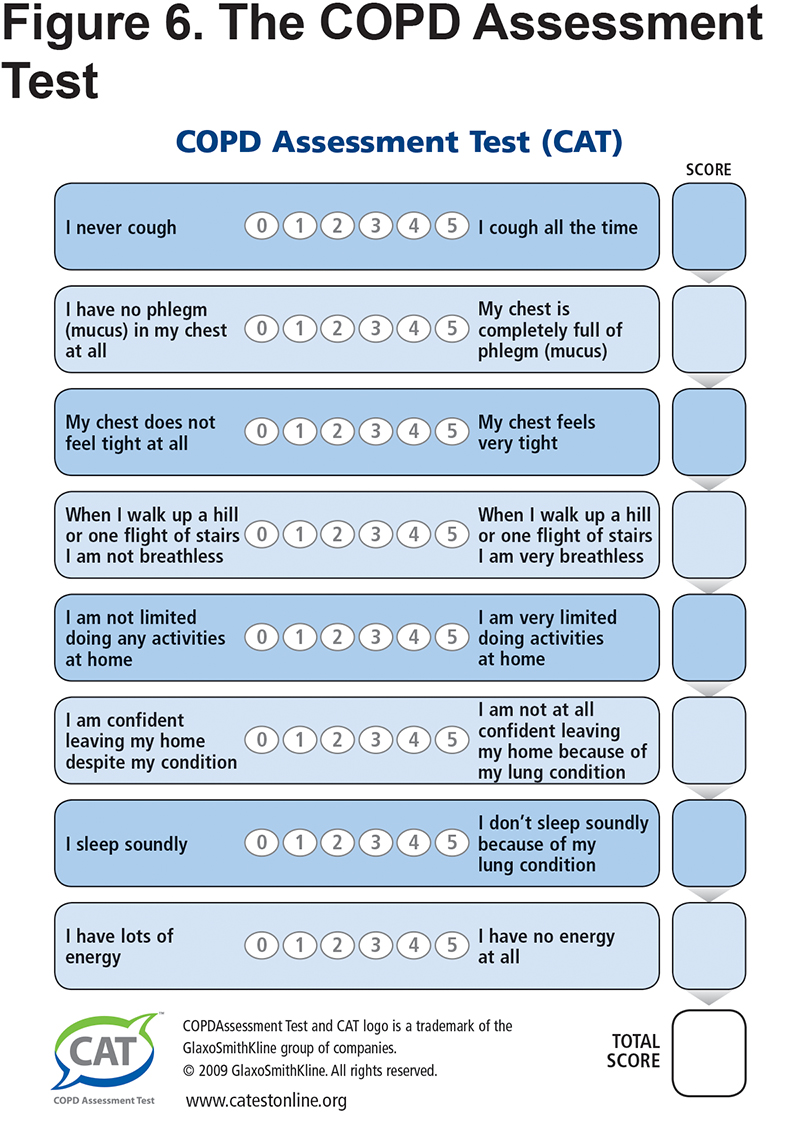
While not all patients fit into any 1-page algorithm, the simplified PCG fits most patients by grouping them into those with lower and higher symptom and functional status burdens using the modified Medical Research Council (mMRC) and the COPD Assessment Test (CAT)8 scales which are short and feasible additions to routine primary care practice. The mMRC is a quick way to determine the activity and functional capacity of patients. The CAT incudes a broad range of questions across 8 domains including activity, sleep, fatigue, and symptoms such as daily cough. The care flow diagram adds the frequency of exacerbations to the symptom burden with the caveat that before diagnosis, COPD exacerbations are often referred to as recurrent and prolonged “bad” colds or episodes of "bronchitis."9
The flow chart (Figure 1) recognizes that COPD is progressive and that therapy is not static, requiring updates and enhancements as the patient’s status changes. The medications can be thought of as a hierarchy centered around the current mainstay of COPD therapy, bronchodilators. Steps go up from “as needed short-acting bronchodilator” to “daily long-acting bronchodilators.” Dual bronchodilator therapy is acknowledged as a newly recommended, optional starting choice for all COPD patients requiring daily therapy and a likely appropriate starting choice for those who are more symptomatic (e.g., mMRC >2 or CAT >10). The presence of 2 or more exacerbations a year is addressed by use of dual bronchodilator therapy plus consideration of the addition of daily inhaled corticosteroids (ICSs) if needed (e.g., “triple therapy”). The current controversy regarding when to start and continue ICS therapy for prevention of exacerbations is noted at the bottom of the list of domain assessments (Figure 2). Specifically, the presence of eosinophilia may help select those whose exacerbations are most amenable to ICS therapy.
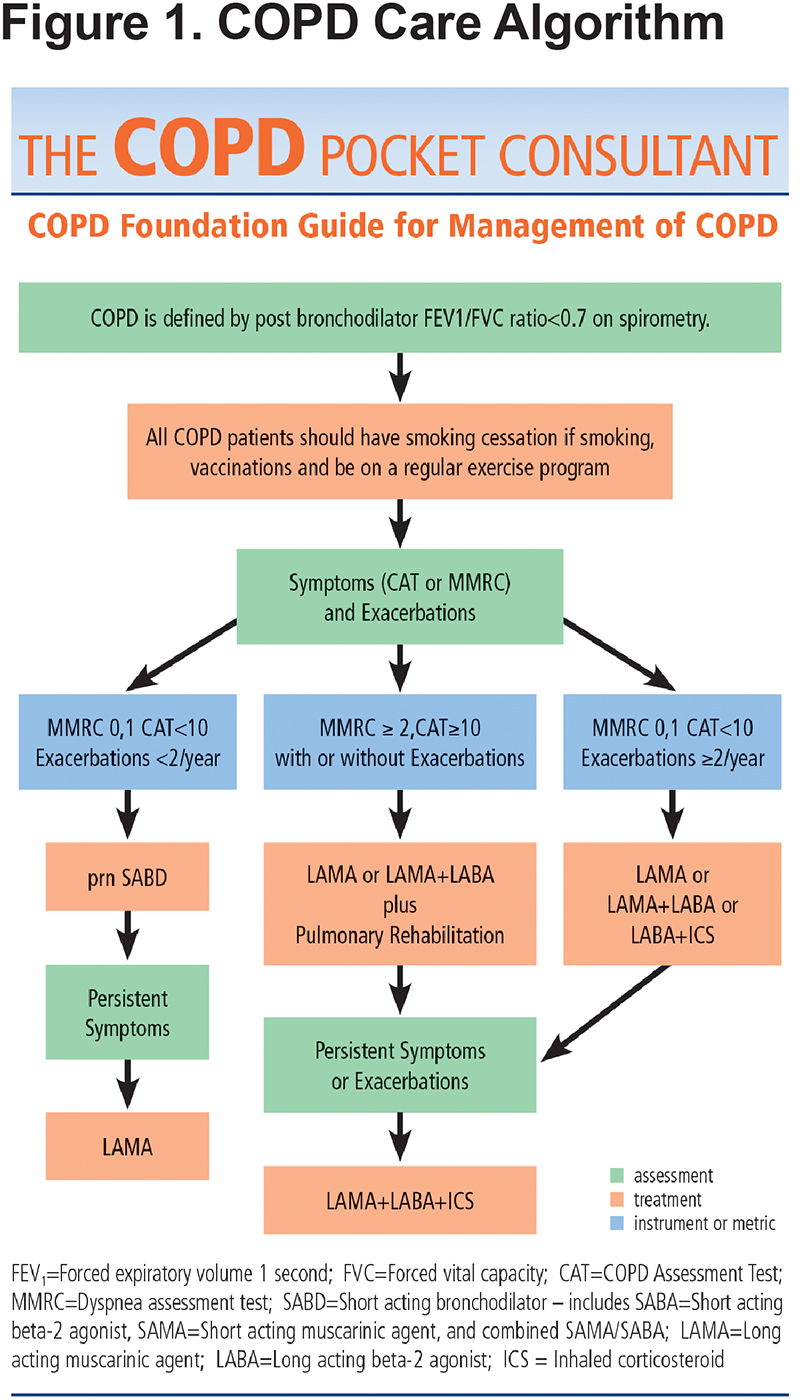
Both of the first 2 panels of the PCG note that spirometry must be accompanied with assessment of symptom, exacerbations and functional status burden plus the other domains of severity (oxygenation, emphysema, chronic bronchitis and comorbidities) to determine the best diagnostic and therapeutic approaches. While symptoms and exacerbations drive most therapeutic decisions, the presence of significant emphysema or the presence of chronic bronchitis could raise possible roles for other specialized treatments such as lung volume reduction or a phosphodiesterase 4 (PDE-4) inhibitor, respectively.
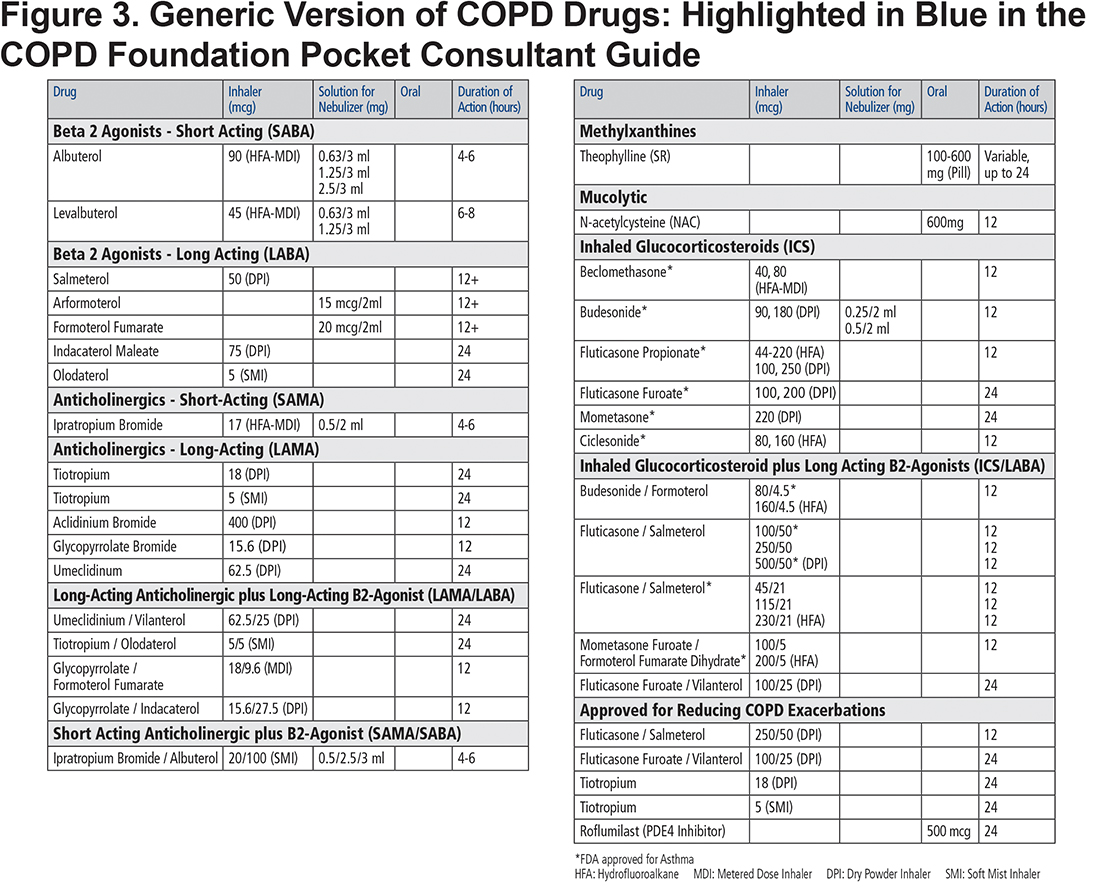
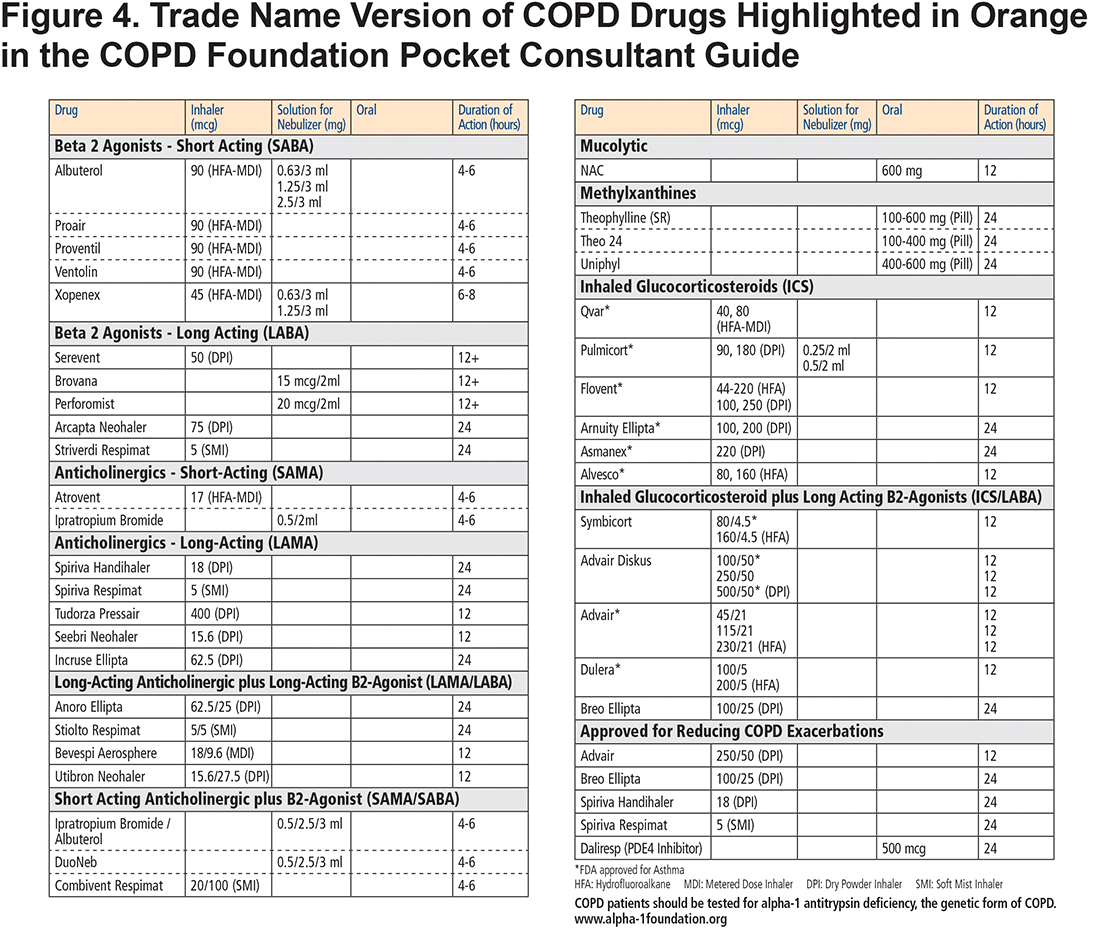
The COPD drugs available in the United States are all listed as individual drugs and combinations with doses and available routes of administration—oral, inhaler and nebulized solutions. The list includes not only the mainstays of therapy, bronchodilators and corticosteroids, but also specialized therapy such as PDE-4 inhibitors for individuals with chronic bronchitis and refractory exacerbations and less commonly used add-on therapies such as methylxanthines and mucolytics. These treatment options are included in both the blue colored generic drug classification (Figure 3) and the orange trade name versions (Figure 4) of the PCG. The drugs listed include those approved for COPD treatment as well as some approved only for asthma such as ICS inhalers since they may be combined with dual long-acting muscarinic antagonists (LAMA) plus long-acting beta2-agonists (LABA) inhalers to facilitate triple therapy (ICS+LAMA+LABA). We have not included azthromycin in the flow chart since it is not in common use in primary care and its long-term use remains unclear. 10 However, in the bullet points accompanying the flow chart, we have stressed that for those with recurrent exacerbations despite inhaled regimen, the following be considered: “Adding macrolide (if not active smoker) as immune modulator.” (Figure 2)
Currently, diagnosis and management of COPD requires consideration of asthma and COPD overlap syndrome (ACOS) for individuals with a history of previous asthma, significant atopy or seasonal allergies such as allergic rhinitis.11 For these individuals who also have significant reversibility on post-bronchodilator spirometry testing, a diagnosis of ACOS may be appropriate requiring that baseline therapy includes both ICSs and long-acting bronchodilators. These are individuals for whom triple therapy may be the initial treatment of choice. The algorithm for ACOS consideration is found on the same panel as the detailed mMRC scoring. (Figure 5.)
The final panel of the PCG includes the detailed CAT questions to facilitate quick assessment for those practices unable to embed the CAT within their electronic medical records. The panel provides all of the questions and the scoring algorithm to facilitate its use at each visit to monitor a patient’s progress. (Figure 6)
The PCG can also be linked to the mobile app for Apple iOS which is found by searching for “COPD Foundation” in the App store. This app allows health care professionals to individualize a patient’s therapy by filling in a symptoms assessment, spirometry results and exacerbation history. The app highlights further testing and/or therapy, based on the specific patient’s information.
Treatment of the COPD patient requires consideration of more than lung function and pulmonary symptoms. Much of the morbidity, mortality, and hospitalization risks experienced by those with COPD occurs due to the presence of comorbid conditions, particularly cardiovascular disease, depression, and anxiety.12 These may change the selection of therapy, as well as guide the need for adherence support and high priority of non-pharmacotherapies, such as pulmonary rehabilitation, that are effective for treating the depression and anxiety commonly accompanying COPD.
Individuals 50 years and older with a 20+ pack year history and less than 10 years of smoking abstinence are candidates for low dose CT scans to screen for lung cancer.13 (Figure 2) This U.S. Preventive Services Task Force recommendation is especially important to follow in individuals with COPD since COPD,14 and particularly the emphysematous form, is an independent risk factor for developing lung cancer.15
Comparing the PCG 2017 to GOLD 2017
The 2017 GOLD recommendations are an important desk reference and resource for in-depth review of evidence, references and the resulting care recommendations.6 At 132 pages with a 45-page executive summary, these are references that few practicing clinicians will have time to review. The PCG summarizes much of the same care recommendations in one short pocket guide that is intended to be a quick reference used at the point of care.
There are other differences also. GOLD 2017 continues to use the 4 grades of COPD based on FEV1 values from post bronchodilator spirometry to categorize mild, moderate, severe and very severe COPD (Table 1). The PCG uses 5 grades to include normal (SG0) and a category of undefined which is reduced lung function without criteria for chronic obstruction (SGU). The other 3 categories are redefined to more closely fit with the American College of Physicians, American College of Chest Physicians, American Thoracic Society, European Respiratory Society Consensus Statement recommendations16 that identified high levels of evidence to support using an FEV1 <60% of predicted (labelled moderate COPD) as the cut off for increased risk of exacerbation and another important change at an FEV1 <30% of predicted where hypoxemia is more likely to require continuous oxygen therapy. (Table 1)
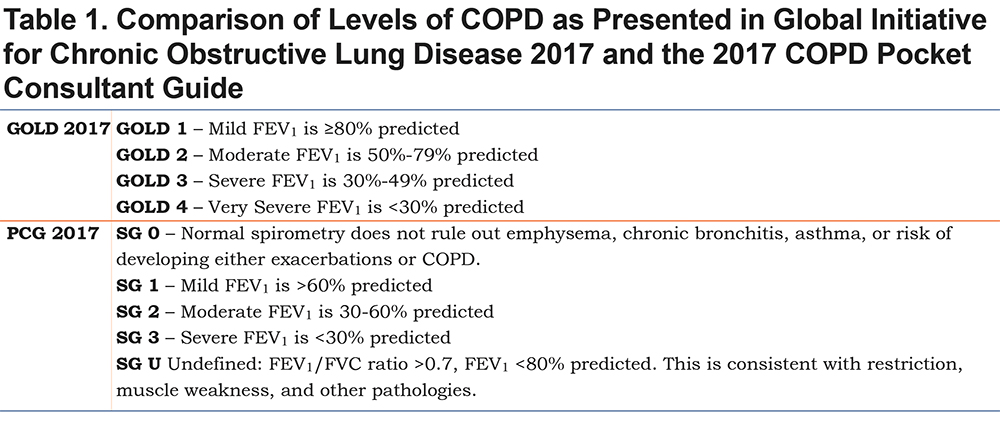
The PCG authors believe the evidence from the COPD Genetic Epidemiology study and the SPIROMICS trial17,18 warrants the reintroduction of the SG0 and SGU classifications within this pocket guide. Data from high resolution CT scans has shown that people with normal spirometry can have simple forms of chronic bronchitis and/or emphysema.17,18 Additionally, these individuals appear to experience considerable morbidity and may be at risk for disease progression. Symptomatic individuals with these spirometry grades should be evaluated for other signs of illness using other diagnostic tests and spirometry should be repeated periodically to assess possible progression. These individuals may be candidates for referral to a lung specialist.
In the PCG, we have chosen to present a summary of COPD management in a simple flow sheet defining a hierarchy of therapies that mirror practice decisions. The flow sheet, supplemented by the assessment of all of the COPD severity domains presented, as well as assessment of ACOS, should allow for a clear and uniform approach to COPD management, monitoring and repeated assessments that are so important for the care of individuals with a chronic progressive condition. The PCG suggests dual bronchodilator therapy for symptomatic patients regardless of exacerbation risk, suggesting maximal bronchodilator therapy may be required prior to significant exacerbation risk.19
The COPD Foundation Pocket Consultant Guide was designed to be a readily accessible, practical guide for the assessment and management of the COPD patient at the point of care. It represents the opinion of the authors and is presented as a statement of the Board of Directors of the COPD Foundation but not that of any corporation or government body. As the understanding of COPD evolves and therapies advance, we expect the PCG to continue to evolve.
Declaration of Interest
Barbara P. Yawn reports receiving COPD-related personal fees from Boehringer-Ingelheim, GlaxoSmithKline, AstraZeneca and Novartis and COPD-related research grant support from Boehringer-Ingelheim outside the submitted work. Scott Cerreta reports he has nothing to declare. MeiLan Han reports receiving personal fees from Sunovion, Boehringer-Ingelheim, GlaxoSmithKline, AstraZeneca and Novartis and receiving research grant support from Novartis and Sunovion outside the submitted work. David Mannino reports grants and personal fees from GlaxoSmithKline, personal fees from AstraZeneca, grants and personal fees from Novartis, personal fees from Amgen, grants and personal fees from Boehringer Ingelheim, personal fees from Merck, personal fees from Forest, personal fees from Up to Date, other from COPD Foundation, personal fees from Schlesinger Law Firm, non-financial support from Sunovion, outside the submitted work. Byron Thomashow reports receiving personal fees from Boehringer-Ingelheim, GlaxoSmithKline, AstraZeneca, and Novartis outside the submitted work. Robert Wise reports receiving personal fees from AstraZeneca, Boehringer-Ingelheim, Contrafect, GlaxoSmithKline, Merck, Novartis, Pulmonx, Roche-Genentech, Spiration, Sunovion, Teva and research grant support from AstraZeneca, Boehringer-Ingelheim, and GlaxoSmithKline outside the submitted work. Stephen Rennard is employed by AstraZeneca, Cambridge, United Kingdom. He reports receiving personal fees from Baxter, AstraZeneca, Dailchi Sankyo, Forest, Penn Technology, Novartis, Pulmirix, and Takeda as well as research grant support from Pfizer, GlaxoSmithKline, Boehringer Ingelheim, Nycomed, Astra-Zeneca, Centocor, and Almirall outside the submitted work. Ravi Kahlan reports personal fees from Forest Laboratories, Boehringer Ingelheim, Merck, Smith Medical, AstraZeneca, CVS Caremark, and Aptus Health, as well as research grant support from Boehringer Ingelheim, GlaxoSmithKline, PneumRx (BTG), Spiration, all outside the submitted work