Running Head: Steroid Dosing in AECOPD with Ventilator Support
Funding Support: This study was supported by the Colorado Clinical and Translational Sciences Institute (CCTSI) team science award (T-15-137). The CCTSI is supported in part by Colorado CTSA Grant UL1TR001082 from National Center for Advancing Translational Sciences/National Institutes of Health. Additionally, this study was also supported by R01HL129938 to R.W. Vandivier.
Date of acceptance: December 16, 2017
Abbreviations: chronic obstructive pulmonary disease, COPD; United States Critical Illness and Injury Trials Group, USCIITG; Prevention and Early Treatment of Acute Lung Injury Trials Network, PETAL; research electronic data capture, REDCap; acute exacerbation of COPD, AECOPD; intensive care unit, ICU; randomized controlled trial, RCT; methylpredniosolone, MP; length of stay, LOS
Citation: Kiser TH, Sevransky JE, Krishnan JA, et al for the DECIDE investigators. A survey of corticosteroid dosing for exacerbations of chronic obstructive pulmonary disease requiring assisted ventilation. Chronic Obstr Pulm Dis. 2017; 4(3): 186-193. doi: http://doi.org/10.15326/jcopdf.4.3.2016.0168
Introduction
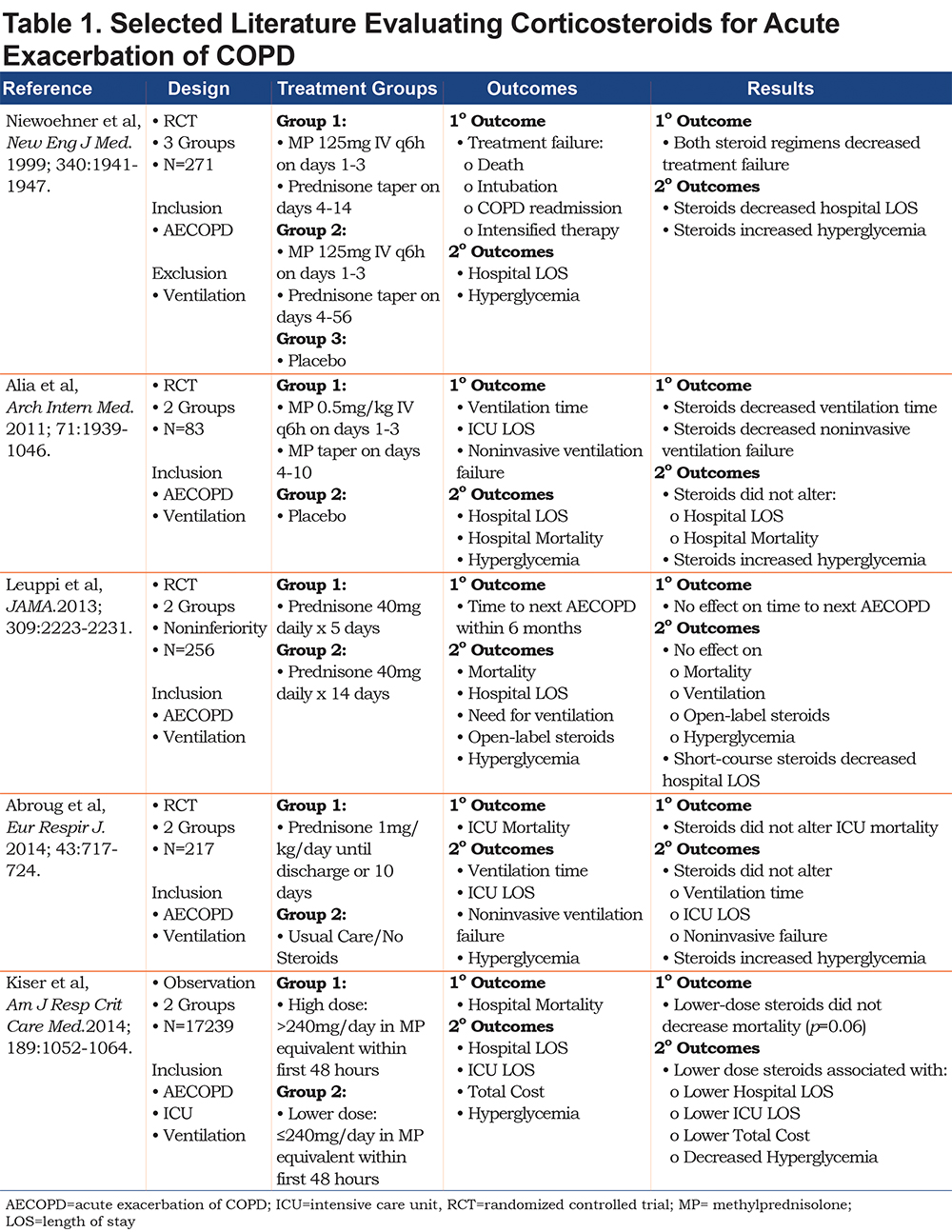
Exacerbations of chronic obstructive pulmonary disease (COPD) account for 60% of our national expenditure for COPD, herald a steep decline in quality of life, and markedly increase the risk of death.1 In patients with respiratory failure who need assisted ventilation, mortality and hospital costs soar by ~15 times compared to those without respiratory failure, with mortality rates of ~25%-30% in the hospital2,3 and 59% at 1-year.3 Patients who survive to hospital discharge are commonly transferred to long-term care facilities.4 After returning home, most patients suffer severe reductions in activities of daily living, as well as increased depression, anxiety, and social difficulties requiring assistance with at least one activity of daily living 6-months after returning home.4,5
Systemic corticosteroids are a critical therapy for COPD exacerbations, because they decrease treatment failure and prevent readmissions in patients without respiratory failure.6 In contrast, very little is known about the effect of corticosteroids in patients with respiratory failure, prompting a 2014 Cochrane Review to conclude that “there is a need for more studies in severe exacerbations of COPD in people who require assisted ventilation.” 6 This knowledge gap has occurred because the majority of large studies evaluating steroid dosing during COPD exacerbations have specifically avoided studying patients requiring assisted ventilation (e.g., those needing invasive or noninvasive mechanical ventilation).7,8 Some of these studies excluded patients with respiratory failure because the primary outcome of treatment failure was, in part, defined by the need for assisted ventilation. Simply put, investigators could not include patients who presented with the study outcome (e.g., assisted ventilation).
Given the high potential for poor outcomes and lack of definitive data, physicians likely choose higher steroid dosages in patients requiring assisted ventilation because of their severity of illness and the perception that higher intravenous doses may lead to better treatment efficacy. A potential consequence is that physicians administer steroid dosages that are up to 12 times higher than are routinely used for patients without respiratory failure,9 exposing them to a greater risk for adverse effects without clear clinical benefit.
The objective of this study was to describe corticosteroid dosing regimens used in patients hospitalized at academic institutions with a COPD exacerbation requiring assisted ventilation and to determine if there is physician support for a clinical trial to establish the optimal systemic corticosteroid dose. Additionally, we sought to determine the range of corticosteroid dosages that would be appropriate to evaluate if a clinical trial were to be conducted.
Methods
The Colorado Multiple Institution Review Board approved this study. Response to the survey was voluntary and anonymous. Completion of the survey indicated the respondent’s consent to participate in this research study. The survey was conducted via email invitations using research electronic data capture (REDCap) at the University of Colorado Anschutz Medical Campus.
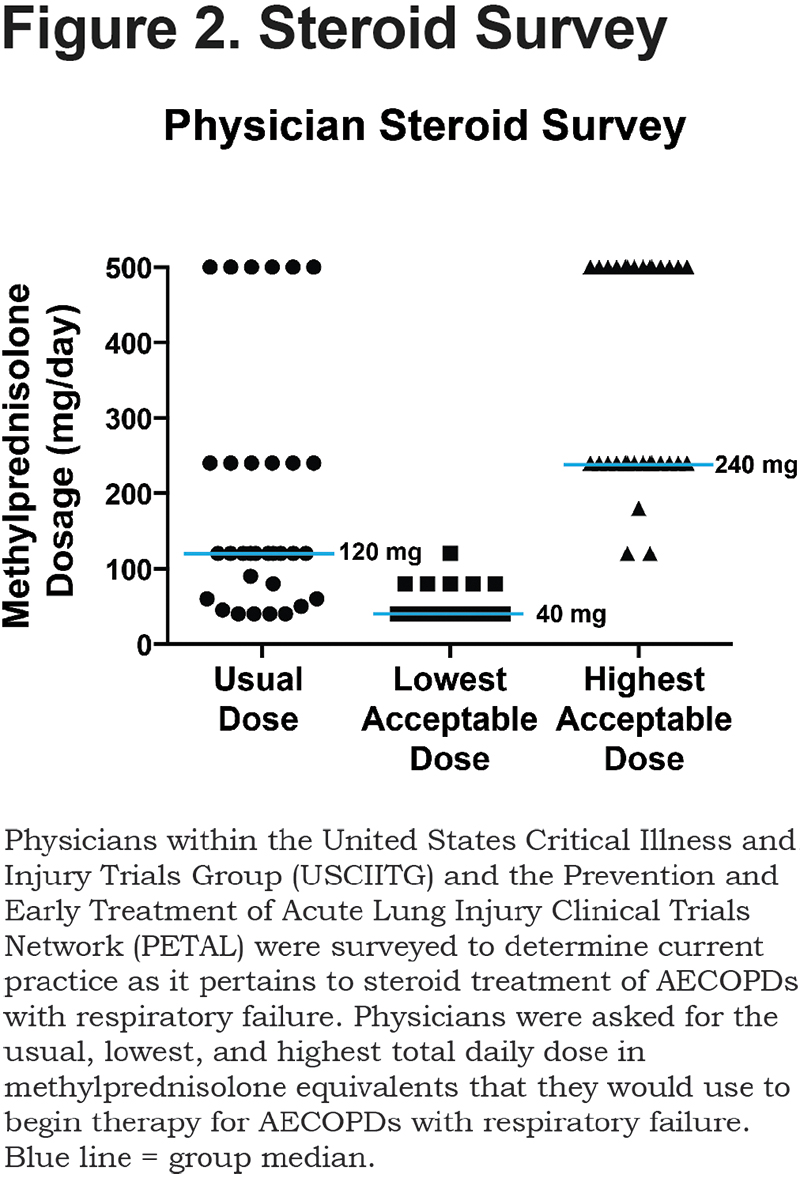
We surveyed intensive care unit physicians within 2 networks about clinical practice in patients hospitalized with a COPD exacerbation requiring assisted ventilation: Twenty-nine physicians from the United States Critical Illness and Injury Trials Group (USCIITG), and 12 pulmonary and critical care physician principal investigators from the Prevention and Early Treatment of Acute Lung Injury Trials Network (PETAL). Two physicians who were members of both groups were only invited to participate in the survey once, yielding a total eligible survey population of 39. Physicians were asked for their opinion regarding: (1) the usual daily dose of corticosteroids (in methylprednisolone equivalents) that they utilize to initially treat this patient population, (2) the need for a clinical trial to compare corticosteroid dosing strategies for COPD exacerbation patients requiring assisted ventilation, and (3) the highest and (4) the lowest daily dose of methylprednisolone that they would be comfortable using as part of a clinical trial testing different dosing regimens (Figure 1). Survey respondents were provided with a summary of recent literature7,9-12 surrounding corticosteroid dosing in hospitalized patients that they could utilize when answering survey questions (Table 1).

Data are presented as proportions or median (range). Corticosteroid dosages are presented in methylprednisolone equivalents (e.g., prednisone 5 mg = 4 mg methylprednisolone).
Results
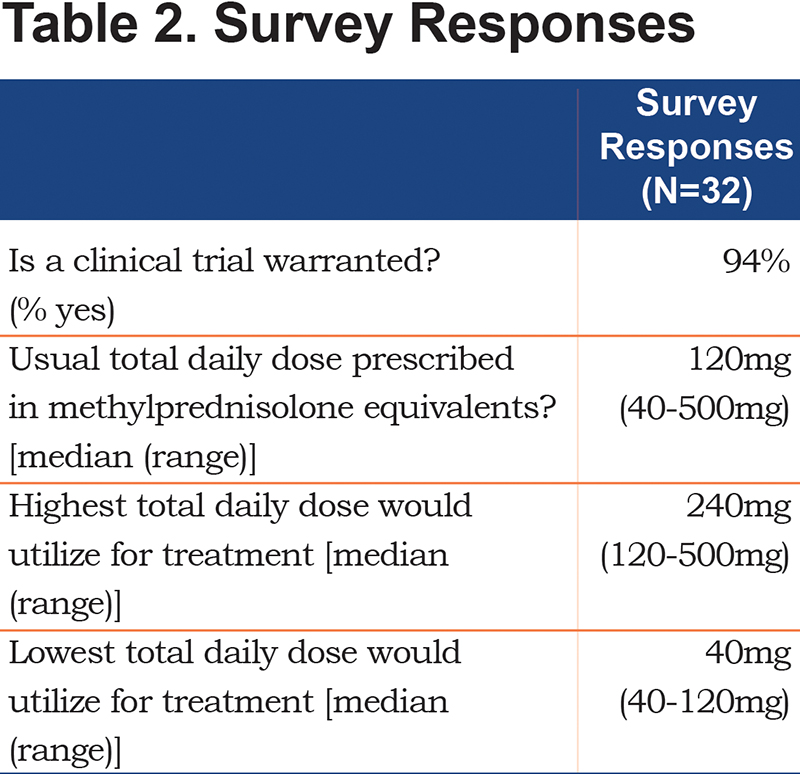
Thirty-two physicians (82%) responded to the survey (Table 2). Usual practice is to start methylprednisolone over a range of 40 to 500 mg/day, with a median of 120 mg/day (Figure 2). A total of 19% of physician respondents start corticosteroids at > 240 mg/day and 81% begin corticosteroids at ≤ 240 mg/day. Within the context of a clinical trial, 88% of physician respondents would be comfortable administering methylprednisolone at dosages as low as 40 mg/day to patients admitted with a COPD exacerbation requiring assisted ventilation (Figure 2). In contrast, 44% of physician respondents would be comfortable initiating dosages as high as 500 mg/day, 44% as high as 240 mg/day and 12% at dosages less than 240 mg/day (Figure 2). A total of 94% of respondents believed that a large randomized, controlled clinical trial is needed to determine the best corticosteroid dose for COPD exacerbations requiring assisted ventilation.
Discussion
The results of our survey indicate that corticosteroid dosing for COPD exacerbations requiring assisted ventilation varies widely from a low of 40 mg to a high of 500 mg/day of methylprednisolone equivalents, with a median of 120 mg/day. This large variability in prescribing patterns is supported by large epidemiologic studies that have evaluated corticosteroid dosing in patients hospitalized with COPD exacerbations, both with and without respiratory failure.8,9 The lack of consensus among respondents reflects the variability of dosing strategies used in clinical trials to date7,10-12 and highlights the paucity of guidance provided by clinical trials in COPD patients with respiratory failure.
Only 2 randomized, controlled clinical trials have specifically examined corticosteroid dosing during COPD exacerbations requiring assisted ventilation, and both of these studies compared treatment with corticosteroids to no treatment/placebo.10,12 Alia et al found that methylprednisolone initiated at 2 mg/kg/day (e.g., 160 mg/day for an 80 kg person) decreased noninvasive ventilator failures and the duration of ventilation compared to placebo.10 In contrast, Abroug et al found that prednisone initiated at 1 mg/kg/day (e.g., ~64 mg/day of methylprednisolone for an 80 kg person) did not improve outcomes compared to usual care without steroids.12 The reasons for these divergent findings are not known. But it is possible that the threshold for a positive corticosteroid effect is higher during COPD exacerbations with respiratory failure compared to no respiratory failure, perhaps because of increased steroid resistance. This would suggest that some commonly used corticosteroid dosages may be too low to optimally treat COPD exacerbations requiring assisted ventilation. Conducting mechanistic studies to determine if a different steroid response phenotype exists in COPD exacerbation patients with respiratory failure may be an important step to both designing steroid dosing regimens and justifying the need for a randomized controlled study in this patient population.
To determine corticosteroid prescribing patterns and to examine the effectiveness of high versus lower dose corticosteroids, we performed a pharmacoepidemiologic cohort study in 17239 patients admitted to the intensive care unit with a COPD exacerbation between 2003 and 2008.9 During this period physicians started methylprednisolone at a median dosage of ~240 mg/day. Methylprednisolone was initiated at >240 mg/day in 66% of patients, indicating that high dosages were preferred at that time. Despite this preference, methylprednisolone dosages ≤240 mg/day (median of ~100 mg/day) were associated with many positive outcomes including a nearly significant reduction in mortality (p=0.06). Our survey shows that a decade later only 19% of physicians start corticosteroids at >240 mg/day and that methylprednisolone is started at a median of 120 mg/day, suggesting that physician dosing for COPD exacerbations with assisted ventilation may be evolving toward lower doses compared with 2003-2008.9
To our knowledge, a survey has never been performed to assess physician corticosteroid prescribing patterns and the perceived need for a clinical trial of steroid dosing for COPD exacerbations requiring assisted ventilation. Our study indicates strong support by academic critical care and pulmonary physicians for such a trial. Given the variable prescribing patterns, it also suggests the presence of clinical equipoise. An efficacy trial design could be performed to determine if either of 2 corticosteroid dosages is superior or equivalent under optimal clinical trial conditions. Alternatively, an effectiveness or comparative effectiveness approach would have the advantage of answering the same question under real world conditions. Our survey supports a clinical trial comparing an initial methylprednisolone equivalent dose as low as 40 mg/day with either 240 mg/day or 500 mg/day. An alternative approach could compare an initial low methylprednisolone equivalent dosage of 40 mg/day with a high dosage of 2 mg/kg/day. The advantage of this dosing approach is that methylprednisolone initiated at 2 mg/kg/day and tapered over 10 days is the only corticosteroid dosing strategy that has yielded positive outcomes for patients with a COPD exacerbation requiring assisted ventilation.10
Our study is the first to establish current physician corticosteroid prescribing patterns and degree of equipoise by academic physicians who perform research within critical illness research networks. It is also the first to determine the highest and lowest acceptable corticosteroid dosages that these physicians would deem acceptable within the auspices of a clinical trial. Both of these elements are critical to design and implement a successful clinical study. The study is also limited because it only surveyed academic physicians from 2 clinical trial groups (USCIITG and PETAL), which may not represent physicians from all clinical settings. The number of respondents was also small and the responses do not elucidate the rationale for prescriber preferences.
Conclusion
To date there are no randomized controlled clinical trials comparing different dosages of corticosteroids in the treatment of patients with a COPD exacerbation either with or without respiratory failure.13 The absence of guidance, other than a single epidemiologic study,9 is particularly important for COPD exacerbation patients with respiratory failure because they are exposed to the highest risk and stand to lose the most.2 Our study demonstrates the variability in physician practice regarding the initial steroid dosing in these critically ill patients, and underscores the fact that in medicine disagreement is common in the absence of data. Accordingly, a prospective, randomized controlled trial is warranted and supported by physicians to determine the optimal dose of systemic steroids for patients with a COPD exacerbation and respiratory failure requiring assisted ventilation.
Acknowledgments
We sincerely thank the late John W. Walsh for his contributions to our research study team and his steadfast and significant contributions to COPD research, education, and advocacy.
Declaration of Interests
The authors have no relevant conflicts of interest to declare.