Running Head: Lung Structure in Hispanic Smokers
Funding Support: National Institutes of Health grant K01HL118714 and the Brigham and Women’s Hospital Minority Faculty Career Development Award (Diaz).
Date of Acceptance: August 4, 2017
Abbreviations: chronic obstructive pulmonary disease, COPD; non-Hispanic white, NHW; computed tomography, CT; branching generation number, BGN; odds ratio, OR; confidence interval, CI; Pittsburgh Lung Screening Study, PLuSS; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; forced expiratory flow between 25% and 75% of FVC, FEF25%-75%; percent of low attenuation areas below-950 Hounsfield units, %LAA-950; right upper lobe apical bronchus, RB1; right lower lobe basal posterior bronchus, RB10; total blood vessel volume, TBV; pulmonary volume in all vessels<10mm2 in cross section, BV10; pectoralis muscle area, PMA; subcutaneous adipose tissue, SAT; visceral adipose tissue, VAT; standard deviation, SD; body surface area, BSA
Citation: Diaz AA, Rahaghi FN, Doyle TJ, et al. Differences in respiratory symptoms and lung structure between Hispanic and non-Hispanic white smokers: A comparative study. Chronic Obstr Pulm Dis. 2017; 4(4): 297-304. doi: http://doi.org/10.15326/jcopdf.4.4.2017.0150
Introduction
In 2015, 36.5 million people in the United States were current smokers.1 Before meeting criteria for chronic obstructive pulmonary disease (COPD), smokers manifest respiratory symptoms and have emphysematous destruction of lung parenchyma and airway disease that can be measured on computed tomography (CT) similar to those with established disease.2,3 Much of what we know about these clinical and radiographic manifestations in smokers comes from large studies that have included mostly non-Hispanic white populations.2-5 Although Hispanics are the largest and fastest growing minority in the United States,6 now numbering 56.5 million people, and represent the majority population in certain areas of the United States, they are understudied. Interestingly, recent investigations suggest that despite comparable smoking habits and occupational exposures, Hispanic smokers (i.e., Hispanics from New Mexico and Mexican-Americans) have a slower rate of lung function decline and a lower risk for COPD than their non-Hispanic white (NHW) counterparts.7,8,9
These racial-ethnic differences in disease risk may be due to differential responses of the airways and lung parenchyma to tobacco injury. Detailed examination of lung structure and extra-pulmonary tissues would be necessary to show these differences. Prior studies did not find racial-ethnic differences in CT measures of emphysema and airway morphology. However, those studies were limited by their assessment only of one quantitative imaging phenotype (emphysema or airway disease) on cardiac CTs.10,11 Other CT phenotypes such as pulmonary vascular pruning and measures of extra-pulmonary tissues such as muscle and fat mass are now available, thereby expanding our ability to assess smoking-related diseases.12-15 In the present study, we aimed to explore pulmonary symptoms, lung function, and lung structure as well as muscle and fat tissue mass in Hispanic and NHW smokers. We tested the hypothesis that Hispanics might be less susceptible to smoking-induced injury compared to NHWs and that could be associated with greater airway lumens, more parallel pathways, and less emphysema and vascular pruning. To test this hypothesis, we used sex-, age-, and smoking exposure-matched Hispanic and non-Hispanic whites from the Pittsburgh Lung Screening Study (PLuSS).16
Methods
Cohort
Clinical and CT data at baseline from participants enrolled in PLuSS from January 2001 to April 2005 were used.16 Inclusion criteria were the following: age 50-79 years; body weight <400 pounds; no personal history of lung cancer; nonparticipation in concomitant lung cancer screening studies; no chest CT in the prior year; former or current cigarette smoker of at least one-half pack/day for 25 or more years; former smokers must have quit within 10 years prior to study enrollment. Symptoms were not part of the exclusion criteria.
We used all self-identified Hispanic participants and matched them with non-Hispanic whites based on same sex, age (± 2 years), and smoking exposure. Smoking exposure matching was based on the age when participants started smoking (± 2 years) and quit smoking (± 2 years) if applicable, the same current smoking status, and the same category of smoking intensity (cigarettes/day categories: 1-9; 10-19; 20-29; 30-39; and 40 or more). This approach allowed us to explore potential differences in susceptibility to smoking-induced injury based on race-ethnicity. African-American individuals were not included in this study. The institutional review board for the University of Pittsburgh approved PLuSS and its participants gave informed consent to participate in the study.16 The informed consent was given to the participants in English only.
Clinical and Functional Assessment
Demographics, smoking history, and pulmonary symptoms (non-productive cough, phlegm, wheeze, and dyspnea) were collected in standardized manner using a PLuSS designed questionnaire, which was given to the participants in English only. Participants were asked, “Have you recently experienced any of the following symptoms?” The respiratory symptoms included “dry cough”, “cough that produces phlegm”, “wheezing”, and “shortness of breath” with binary yes/no response options. The participant was considered to have a respiratory symptom if he/she answered “yes” to any of those options. Spirometry was performed and analyzed following the American Thoracic Society standards.17 We used the forced expiratory volume in one second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and forced expiratory flow between 25% and 75% (FEF25-75%) of FVC and their predicted values in our analysis. The predicted values were derived from prediction equations for the U.S. population.18
CT Protocol
PLuSS participants were imaged using a single-breath-hold, helical, low-dose CT protocol (40–60 mA; 140 kVp) to obtain axial images. The images were reconstructed with a high spatial frequency (lung) algorithm at contiguous 2.5-mm intervals.16
Emphysema and Airway Assessment
A trained analyst analyzed the CTs using the Chest Imaging Platform.19 Because PLuSS CTs are low-dose, images were first smoothed using a median filter allowing decreasing noise and increasing densitometric measurements accuracy. Emphysema-like tissue was defined as percent of low attenuation areas below -950 Hounsfield units (%LAA-950).20 Measures of airway morphology were taken using an anatomic approach at the 3rd and 4th generation level of the right upper lobe apical bronchus (RB1) and right lower lobe basal posterior bronchus (RB10). The first RB1 and RB10 segment within the lobe was considered the third generation.21,22 RB1 and R10 were chosen as they run orthogonal to the axial plane making it possible to take more accurate measurements. The analyst identified the target bronchial segment orthogonal to the axial image and placed a point at the center of the lumen to segment the airway and obtain the measurements. Two sets of bronchial measurements were obtained in the middle portion of each segment. These measurements included lumen area and wall area percentage (wall area/wall area + lumen area ‧100).23 The ratio of the area of the daughter inner lumen to parent inner lumen was also computed and used to estimate the branching generation number (BGN) to reach a <2mm-lumen-diameter airway –the site of airflow obstruction in smokers as described previously.24 The BGN is an estimation of how many branches (i.e., parallel pathways) there are between the proximal airways and a given distal airway size where greater values indicate greater number of parallel pathways. For this estimation, it is assumed that the bronchial tree is a dichotomous branching system where each generation had 2n parallel pathways (where “n” is the generation). If the projected BGN ranged from 6 to 8 (i.e., 3 to 5 generations past the 3rd generation), then a participant would theoretically have between 64 and 256 parallel pathways of similar size (for a 6th generation 2mm-airway 26= 64 parallel paths, for an 8th generation 2mm-airway 28= 256 parallel paths).
Pulmonary Vascular Assessment
We used a 3-dimensional volumetric model to assess the pulmonary vascular morphology on non-contrast CT scans.15 This methodology allows extracting the vascular tree and quantifying the total blood vessel volume (TBV). This assessment provides the aggregate pulmonary vessel volume including artery and veins in all vessels less than 10mm2 in cross section (BV10) for the whole-lung, a modified metric for low-dose CT scans from prior work.15 To account for differences in lung size across participants, the BV10 is expressed as a function of TBV. A lower BV10/TBV ratio indicates a greater vessel pruning.
Muscle and Fat Assessment
Measures of muscle and fat tissues were taken on chest CT scan. The methodology and reproducibility of these measures have been previously reported.12-14 We measured the pectoralis muscle area (PMA),12 subcutaneous adipose tissue (SAT)13 area anterior to the pectoralis muscles, and visceral adipose tissue (VAT).14 The first 2 measurements are obtained from a single-axial slice immediately above the aortic arch and the latter is taken from a single-axial slice at the inferior edge of the transverse process of the first lumbar vertebrae. The aggregate area (cm2) of the 4 pectoralis muscles and the 2 sides of SAT measurement as well as VAT total area were used for analysis.
Statistical Analysis
Data are presented as frequency (%) or mean ± standard deviation (SD). Differences between populations were tested using student t-tests or chi-squared tests for continuous and categorical variables, respectively. Logistic regression was performed to assess the relationship between a binary variable (i.e., presence of a respiratory symptom) and race-ethnicity. A P value less than 0.05 was considered of statistical significance. The analysis was performed with SAS 9.4 (SAS institute, Cary, North Carolina) software package.
Results
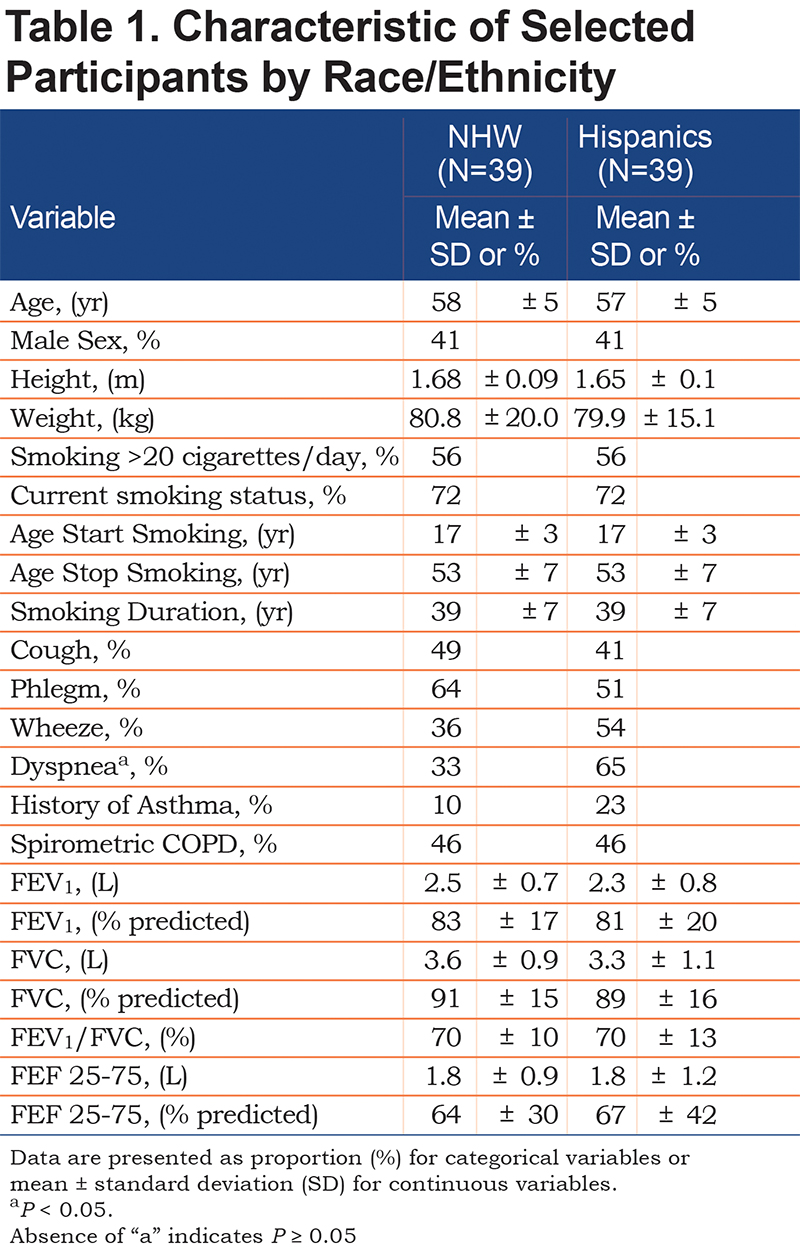
Thirty-nine out of 3642 participants who underwent CT scanning at baseline were Hispanics. The Hispanic participants were successfully matched for age, sex, and smoking history with 39 NHWs. The participants’ characteristics are shown in Table 1. Hispanic smokers had almost 2 times higher proportion of dyspnea than their NHW counterparts. The increased odds of dyspnea for Hispanics persisted after adjustment for both spirometrically defined COPD and history of asthma (odds ratio[OR] = 2.96; 95%, confidence interval [CI] 1.09-8.04). No significant differences were observed in other respiratory symptoms, FEV1, FVC, and FEV1/FVC.
CT Measurements
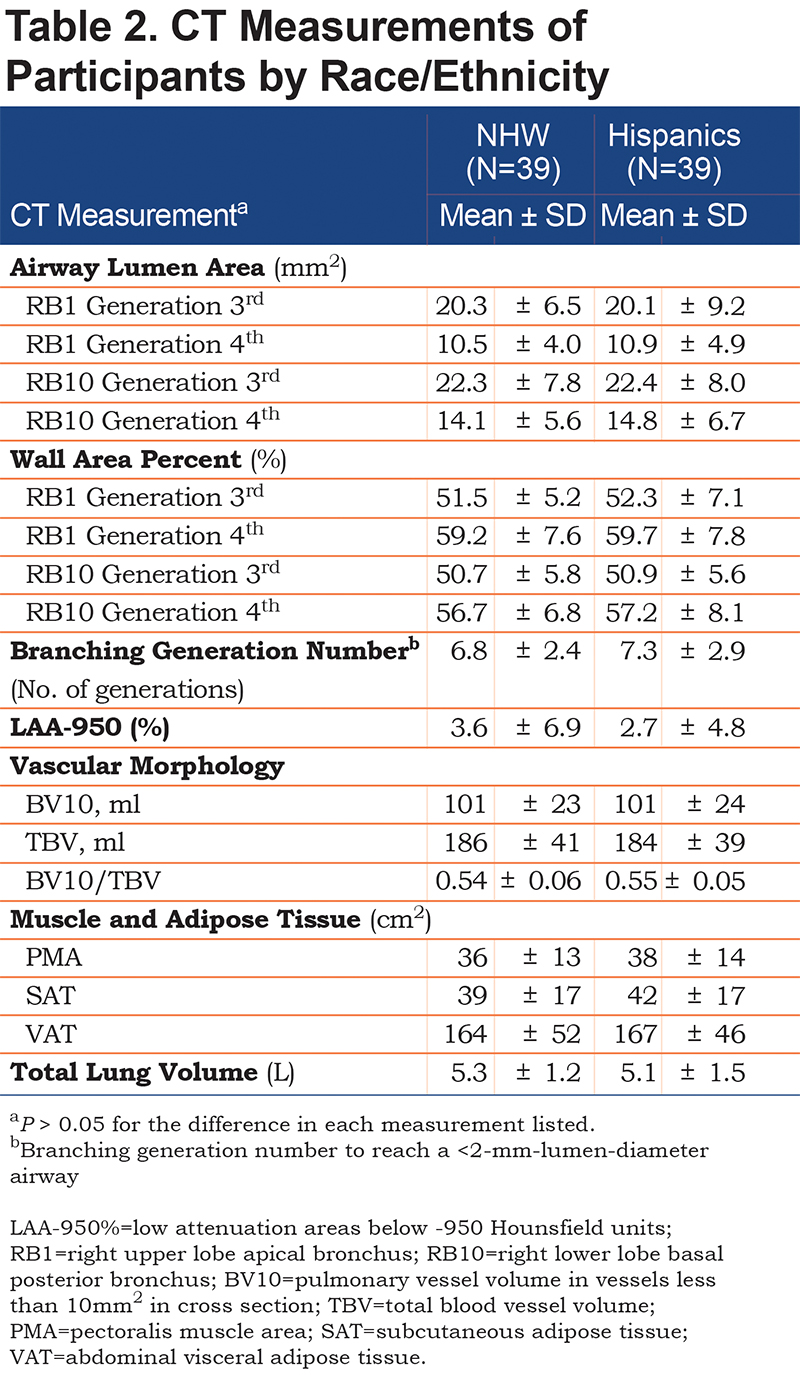
No significant differences were found in any of the CT measurements (airway morphology, BGN, %LAA-950, vascular morphology, PMA, SAT, and VAT) between Hispanics and NHWs (Table 2). The lack of racial-ethnic differences in airway lumen size was also observed when RB1 airway lumen area was adjusted for body surface area (BSA) as lumen area/BSA or height as lumen area/m2 (P >0.73 for both comparisons).
Discussion
In this study, we performed an evaluation of respiratory symptoms and lung function as well as a comprehensive analysis of CT lung measurements and extra-pulmonary tissues from Hispanic and matched-NHW smokers. We found that Hispanics reported almost two-fold more dyspnea than NHWs. However, racial-ethnic differences in lung function, lung structure, and extra-pulmonary tissues were not observed.
Smokers with and without COPD frequently report dyspnea. In our study, despite Hispanics and NHWs having similar CT-based lung structural damage and lung function, the minority group reported almost twice more often dyspnea. An explanation for this difference is that compared to NHWs with similar lung function, Hispanic smokers may have a lower perception threshold for this symptom. We have previously reported that New Mexican Hispanic smokers (versus NHWs) more often reported dyspnea in univariate analysis, and that they also experience a worse perception of their health-related quality of life as measured by the St George’s Respiratory Questionnaire that includes questions about this symptom.25 Additionally, the feeling of “breathlessness” is described with both upper airway (e.g., tight throat) and lower airway word descriptors (e.g., sore lung) by Hispanics compared to just the lower airway word descriptors used by NHWs26 supporting the racial-ethnic difference we observed. But an additional prior study in Mexican-Americans did not find differences in this symptom compared to NHWs.7 That study, however, included never smokers, which may have obscured the differences we observed in heavy smokers. A potential implication of current and prior findings is that these racial-ethnic differences in dyspnea perception might be considered when evaluating a Hispanic smoker in routine clinical visits.
We also performed a comprehensive chest CT assessment to explore potential Hispanic-NHW structural differences in response to tobacco injury. We were surprised by both the remarkable similarity in some of the imaging estimates (e.g., airway lumen area, vascular pruning) and the consistent lack of differences in all structures/tissues evaluated between the 2 populations. One possible explanation of our findings is that racial-ethnic differences in tobacco-induced injury do not translate into morphologic responses of lung structure. Other studies have failed to find racial-ethnic differences in emphysema and airway disease10,11 and here the lack of difference was expanded to vascular pruning and extra-pulmonary manifestations of COPD such as muscle and fat mass. Although some may feel that our sample size might obscure potential differences, the close estimates for some CT measurements between the populations make this less likely. However, these findings do not exclude the possibility that differences in smoking-induced changes of lung structures might exist between other Hispanic subgroups and NHWs. For example, a cohort of Hispanics from New Mexico with greater Native American ancestry than that of NHWs had lower risk of COPD.8 This suggests that lung structure might differ by ancestry rather than ethnicity as a broad category. Additionally, differences in other structures that were not measured in our study such as small airways may exist.27 Furthermore, differences in inflammatory response to smoking injury, and/or protease-anti-protease misbalance may explain racial-ethnic differences in COPD risk previously observed. Further investigations including imaging, genetics, and environmental assessments are warranted to elucidate the determinants of COPD risk and disease progression in all U.S. racial-ethnic groups.
Several limitations should be noted. First, this is a convenience, small-sized sample of heavy smokers and caution should be exercised to extrapolate these findings to the larger population of smokers or Hispanics as a whole. We do not have information on ancestry (European, Native American, African), nativity (U.S. born versus non-U.S. born), and origin (e.g., Mexican-American, Puerto Ricans) of these participants, thus those important characteristics that are associated with observed differences in lung function decline and COPD risk could not be factored into our analysis.8,28 While this lack of information limits the external validity of our findings, it might encourage further exploration of smoking-induced changes in lung structure and COPD susceptibility based on race-ethnicity using more refined methods to characterize populations such as ancestry. The assessment of respiratory symptoms was based on their presence or absence only and smoking intensity was collected as category of cigarettes/day. Finally, COPD measures on expiratory images such as air trapping were not available. Despite this limitation, we were able to expand prior imaging characterization in Hispanics by analyzing the pulmonary vasculature and extra-pulmonary tissues. Low-dose CT scans imposed challenges for lung densitometric analysis due to a reduced signal/noise ratio, but smoothing the PLuSS images allowed us to obtain emphysema-like tissue measurements that were in the range of those reported from studies using high-dose CT scans.
In summary, Hispanic heavy smokers report dyspnea 2 times more often than their age-, sex- and smoking exposure-matched NHW counterparts. It appears that there are no differences in CT measures of COPD between Hispanic and NHW smokers. Further research including imaging, genetics, and environmental exposure assessments are needed to ascertain the determinants of COPD and disease progression in all U.S. racial-ethnic groups.
Acknowledgments
Authors’ Contributions: Conception and design of this study and creation, revision, and final approval of this manuscript: Authors Diaz, Rahaghi, Doyle, Young, Maclean, Martinez, Jose Estepar, Guerra, Tesfaigzi, Rosas, Washko and Wilson; Analysis and interpretation: Diaz, Washko, Wilson; Data acquisition: Diaz, Rahaghi, Young, Wilson; Drafting the manuscript for important intellectual content: Diaz, Rahaghi, Doyle, Guerra, Tesfaigzi, Wilson; Guarantor Diaz had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of Interest
Drs. Diaz, Rahaghi, Doyle, Martinez, San Jose Estepar, Guerra, Tesfaigzi, Rosas and Wilson, and Mr. Young and Mr. Maclean have no conflicts of interest to disclose related to this manuscript. Dr. Diaz has received speaker fees unrelated to this work from Novartis, Inc.