Running Head: Supplemental Oxygen in Hospitalized COPD Patients
Funding support: Dr. Zaidi is supported by a National Institutes of Health institutional training grant (T32 HL082547).
Date of Acceptance: July 20, 2017
Abbreviations: chronic obstructive pulmonary disease, COPD; oxygen, O2; Centers for Medicare and Medicaid Services, CMS; Documentation and evaluation of Oxygen Requirements In COPD study, DORIC; electronic health records, EHR; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; Global initiative for chronic Obstructive Lung Disease, GOLD; pulse oximetry, SpO2; Long-Term Oxygen Treatment Trial, LOTT; standard deviation, SD; against medical advice, AMA
Citation: Zaidi F, Lee RS, Buchcic BA, et al. Evaluation and documentation of supplemental oxygen requirements is rarely performed in patients hospitalized with COPD. Chronic Obstr Pulm Dis. 2017; 4(4): 287-296. doi: http://doi.org/10.15326/jcopdf.4.4.2017.0148
Introduction
Chronic obstructive pulmonary disease (COPD) is a common respiratory disorder that affects 15 million individuals and is the third leading cause of death in the United States.1,2,3 Despite optimal medical therapy, patients with COPD have episodic worsening of respiratory symptoms (exacerbations). In the United States alone, COPD exacerbations account for about 750,000 hospitalizations each year.4,5 Among patients who are hospitalized with a COPD exacerbation and who survive to hospital discharge, the hospital-specific 30-day risk of death and 30-day risk of readmission varies greatly (6% to 13% and 19% to 26%, respectively).6,7 These observations led to a decision by the U.S. Centers for Medicare and Medicaid Services (CMS) to include COPD in its efforts to improve the quality and outcomes of care in hospitalized patients.8
In patients with COPD associated with severe resting room air hypoxemia, appropriate use of supplemental oxygen (O2) saves lives (~1 life saved for every 4 patients treated over 5 years).9,10 Patients with COPD who are prescribed supplemental O2 for use after hospital discharge have an increased risk of hospital readmission.11,12 As patients with more severe COPD are more likely to be prescribed supplemental O2, the association between supplemental O2 and readmission may, in part, represent confounding by disease severity. Inappropriate use of supplemental O2 after hospital discharge could impact patient safety and contribute to poor outcomes in this population.13
CMS requires specific documentation to support a prescription for supplemental O2, which includes O2 flow rate at rest, with activity, or sleep, as well as the type of O2 delivery system.14 Inadequate documentation can lead to delays in initiation of O2 therapy, as well as denial of insurance coverage with the patient becoming responsible for the costs of supplemental O2 therapy. Inadequate documentation could also lead to confusion about the appropriate use of O2 among patients and their caregivers leading to underuse or overuse of supplemental O2.15
Despite the importance of documentation to support a clinical indication for supplemental O2 in patients hospitalized with COPD, there is a surprising paucity of published information about the quality of evaluation and documentation regarding the need for supplemental O2 in this population. Addressing this information gap could help to inform the need for hospital-based quality improvement programs devoted to oxygen prescriptions prior to hospital discharge as an additional strategy to enhance patient safety and to reduce avoidable readmissions in patients with COPD.
Therefore, the objective of the Documentation and evaluation of Oxygen Requirements In COPD (DORIC) study was to determine the extent to which evaluation and documentation regarding the need for supplemental O2 occurred prior to hospital discharge in patients with COPD. Secondary objectives of the DORIC study were to examine the extent to which evaluation and documentation occurred prior to discharge in patients hospitalized with COPD with and without an exacerbation, as well as in those prescribed and not prescribed supplemental O2 at home prior to the index hospitalization.
Materials and Methods
We conducted a retrospective cohort study of adults at 2 academic health care centers in Chicago, Illinois (a university-affiliated hospital and a Veterans Affairs medical center). The study received a waiver for the requirement of informed consent and institutional review board approval was obtained at both institutions (#2015-0572; #833934-1).
Patient Selection and Baseline Characteristics
The following screening criteria were used to identify hospitalizations that were subsequently assessed for eligibility:
(1) age ≥ 40 years old;
(2) discharged from the hospital between January 1, 2013 and December 31, 2014 (study period); and
(3) an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) discharge diagnosis code used by the CMS to identify patients hospitalized with a COPD exacerbation: a) primary diagnosis of COPD (491.21, 491.22, 491.8, 491.9, 492.8, 493.20, 493.21, 493.22, and 496), or b) a primary diagnosis of respiratory failure (518.81, 518.82, 518.84, 799.1) with a secondary diagnosis of COPD exacerbation (491.21, 491.22, 493.21, 493.22).16
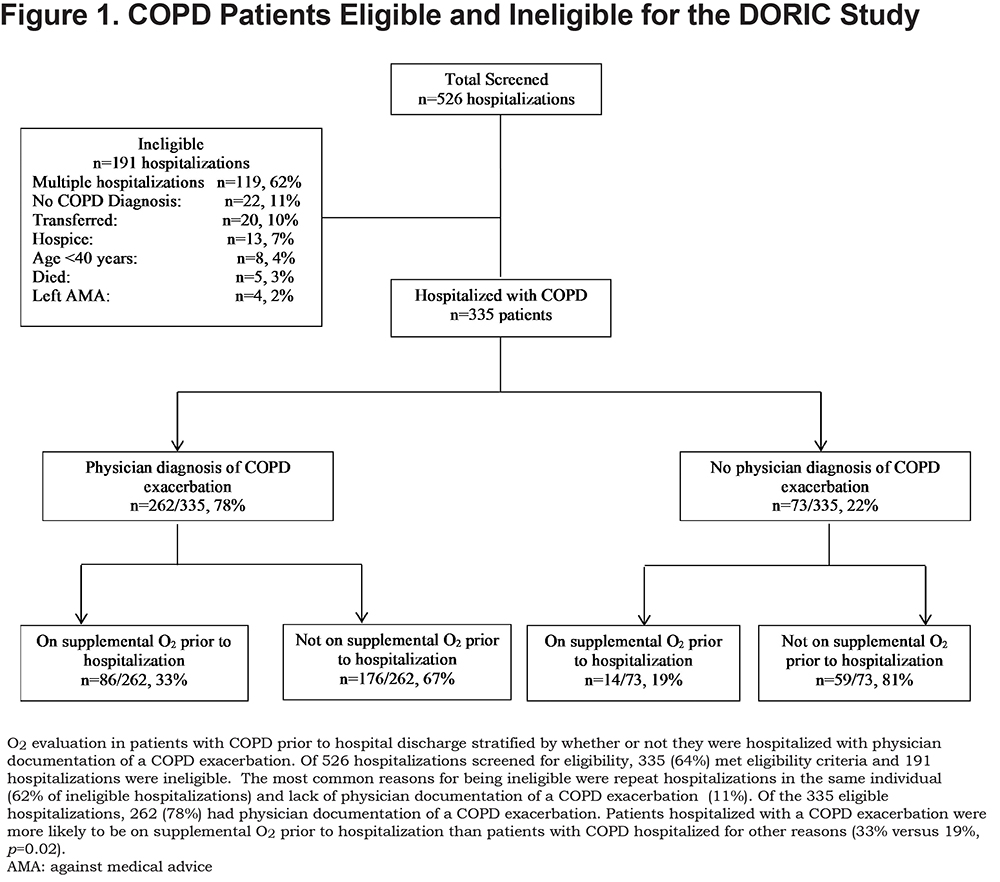
The electronic health records (EHRs) for hospitalizations that met screening criteria were reviewed by 2 clinicians (FZ or RSL) to identify hospitalizations with a physician documentation of COPD in the discharge summary (emphysema, chronic obstructive pulmonary disease, or COPD). Hospitalization records were deemed ineligible if: (1) the patient had a previous hospitalization during the study period (only the first hospitalization was eligible); or (2) the patient was transferred to another hospital, discharged to hospice, left against medical advice, or died prior to hospital discharge.
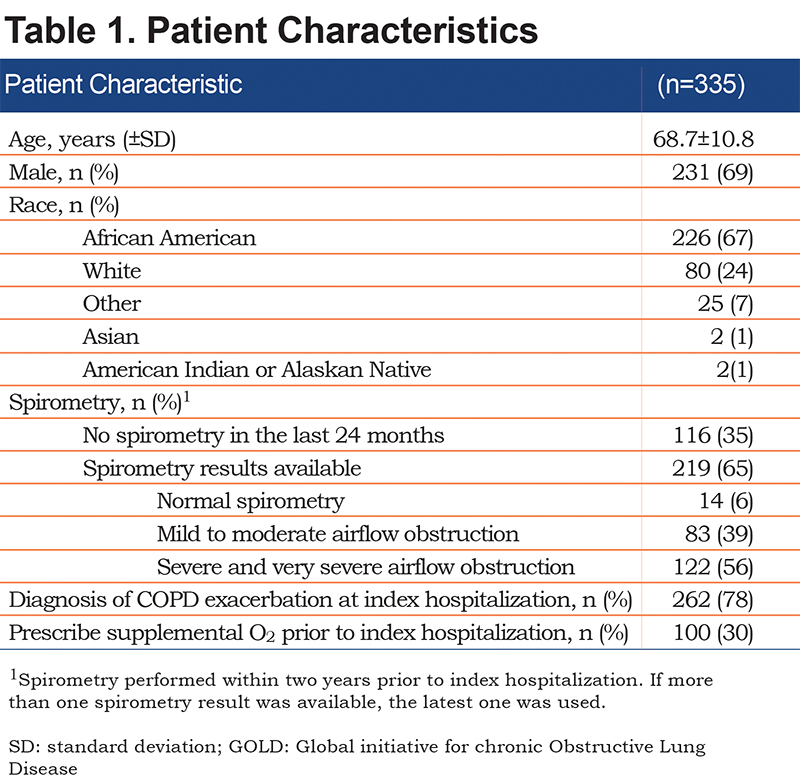
The following information was collected from the EHRs: date of birth, race, sex, physician diagnosis of a COPD exacerbation, and prescribed supplemental O2 at home prior to the index hospitalization. We also extracted information about the results of the most recent spirometry test results within a 2-year period prior to the index hospitalization. We selected this review window because we demonstrated in a previous study that spirometry test results may not be available in the EHR in a substantial proportion of patients even when reviewing a 2-year period prior to a hospitalization.17 Airflow obstruction was defined as a post-bronchodilator forced expiratory volume in 1 second (FEV1) to the forced vital capacity (FVC) ratio of <0.70. We further characterized airflow obstruction as mild or moderate (FEV1>50%), or as severe or very severe (FEV1<50%) using the 2016 Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines.1
Adequacy of Evaluation for Supplemental O2 Requirement
We reviewed the physician admission history and physical, progress notes (documented by nurses, physicians, or respiratory therapists), pulmonary function laboratory test results, and the discharge summary for the index hospitalization to determine if patients underwent an evaluation of supplemental O2 requirements beyond rest oximetry. An adequate evaluation of supplemental O2 requirements was defined as a test (documentation of a 6-minute walk test, exercise desaturation study, or bedside evaluation by a clinician) performed to assess the oxygenation as determined by pulse oximetry (SpO2) or arterial blood gas at rest and with activity within the 48-hour period prior to hospital discharge. The 48-hour window prior to hospital discharge was selected based on CMS requirements for documentation to support a prescription for supplemental O2 initiated in hospitalized patients.14
We defined an increase in O2 requirement as: (1) a new requirement in patients who were not on supplemental O2 prior to the index hospitalization; or (2) an increase in the flow of O2 at rest or with exercise. A decrease in O2 requirement was defined as: (1) no requirement in patients who were on supplemental O2 prior to the index hospitalization; or (2) a decrease in the flow of O2 at rest or with exercise.
Adequacy of Documentation of Supplemental O2 Requirements
For patients who were identified as needing supplemental O2, we reviewed the discharge summary and written instructions provided to patients at the time of the hospital discharge. Adequate documentation was defined as documentation of all the following information: the O2 flow at rest and with activity, and the type of O2 delivery device (e.g., nasal cannula).14 For example, “home O2 by nasal cannula, 2L/min at rest and 3L/min with exercise” was considered adequate documentation. “On home O2 as needed” was considered inadequate documentation. Review of data extracted from the EHR in the first 50 hospitalizations failed to identify a single instance of documentation of supplemental O2 requirements during sleep, so we did not include such documentation in the definition of “adequate documentation”. Therefore, our results could be considered a “liberal” definition of adequate documentation.
Statistical Analysis
Descriptive statistics included the mean, standard deviation, frequency, and proportions, as appropriate. Chi-square tests were used to calculate differences in proportions. Two clinicians (FZ and BB) independently abstracted information about whether an evaluation for supplemental O2 requirement occurred (yes versus. no) and whether there was adequate documentation of supplemental O2 requirements (yes versus no) in a 20% random sample of EHRs. Kappa statistics were computed to evaluate the inter-rater agreement of extracting this information from the EHRs.18 There was substantial inter-rater reliability for abstracting information about the evaluation (kappa = 0.7) and documentation (kappa = 0.8) of supplemental O2 requirements. A two-tailed p-value of less than 0.05 indicated a statistically significant difference. Analyses were performed using R, version 3.3.0
Results
Of 526 hospitalizations, 335 met eligibility criteria (Figure 1). Patients were mostly men and African American (Table 1). Two-thirds of patients (65%) had documentation of post-bronchodilator spirometry in the EHR, and within this group, about half had severe or very severe airflow obstruction. The majority (78%) of patients had a physician documentation of a COPD exacerbation. Supplemental O2 use prior to hospital admission was nearly twice as likely in patients hospitalized with a COPD exacerbation versus patients hospitalized with COPD without an exacerbation (33% versus 19%, p=0.02).
Adequacy of Evaluation of Supplemental O2 Requirements Within 48 Hours of Hospital Discharge
Overall, about 1 in 5 (22%, 73/335) patients hospitalized with COPD underwent an adequate evaluation for supplemental O2 requirement. Results were similar in the 2 hospitals that were included in the study (23% versus 21% of patients had an evaluation, p=0.58). Patients hospitalized with a COPD exacerbation were 5 times as likely to have an adequate evaluation for supplemental O2 compared to those hospitalized with COPD but without an exacerbation (26% [69/262] versus 5% [4/73], p=0.002). However, an adequate evaluation for supplemental O2 requirements was not associated with whether or not patients were prescribed supplemental O2 prior to hospitalization (Figure 2).
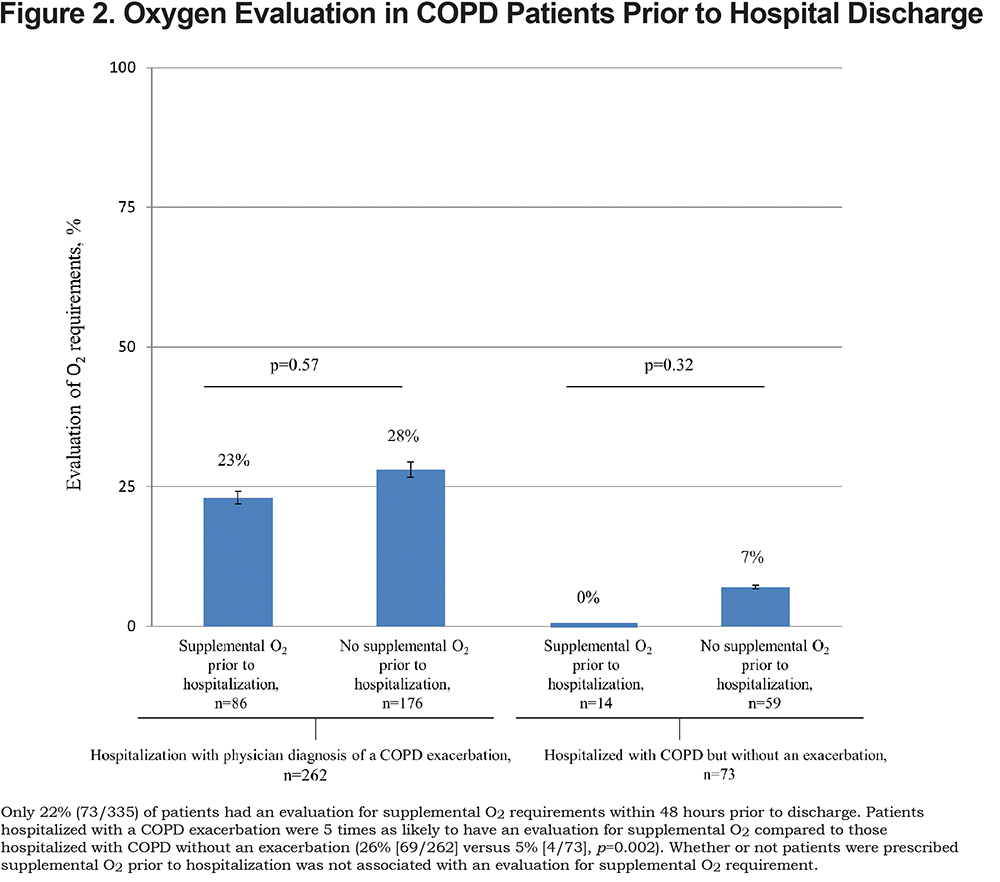
In the subgroup of 20 patients hospitalized with a COPD exacerbation, prescribed supplemental O2 prior to hospitalization, and who underwent a re-evaluation of their O2 requirement, a roughly equal number of patients had an increase (5/20, 25%), decrease (8/20, 40%), or no change (7/20, 35%) in O2 requirements. In the subgroup of 49 patients hospitalized with a COPD exacerbation, who were not prescribed supplemental O2 prior to hospitalization, and who underwent an evaluation of their O2 requirement, 20/49 (41%) were found to have a new O2 requirement. Thus, in the 69 patients hospitalized with a COPD exacerbation and who underwent an evaluation for supplemental O2 requirements, 33 (48%) had either an increase, decrease, or new O2 requirement.
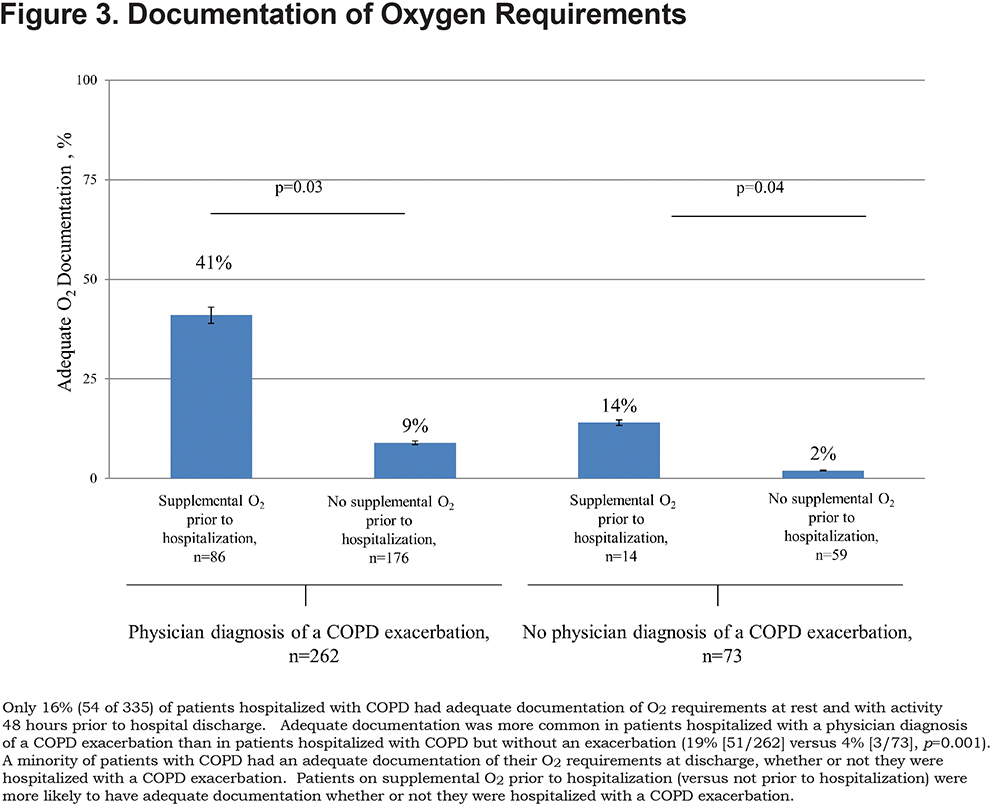
Adequacy of Documentation of Supplemental O2 Requirements Within 48 Hours of Hospital Discharge
Adequate documentation of supplemental O2 requirements at rest and with exertion, occurred in fewer than a fifth (16%, 54/335) of all hospitalized patients with COPD and was 5 times as likely in patients hospitalized with a physician diagnosis of COPD exacerbation compared to those without an exacerbation (19% versus 4%, respectively, p=0.001). Adequate documentation for supplemental O2 requirements was more likely in patients who were on versus not on supplemental O2 prior to hospitalization (patients with a COPD exacerbation: p=0.03; patients without a COPD exacerbation: p=0.04; Figure 3).
Discussion
In this two-center study of 335 patients hospitalized with COPD, the majority had gaps in the evaluation and documentation of oxygen prescriptions prior to discharge. Only 1 in 5 underwent an evaluation and fewer than 1 in 5 had adequate documentation of supplemental O2 requirements at rest and with activity prior to hospital discharge. Compared to patients hospitalized with COPD but without an exacerbation, patients hospitalized with a COPD exacerbation were 5 times as likely to have an adequate evaluation and 5 times as likely to have adequate documentation of supplemental O2 requirements prior to hospital discharge. Being prescribed supplemental O2 at home prior to the index hospitalization was not associated with a re-evaluation of supplemental O2 requirements, but was associated with an increased likelihood of having adequate documentation prior to discharge.
To our knowledge, this is the first study to report the evaluation and documentation of supplemental O2 requirements prior to discharge in patients hospitalized with COPD.1 The surprisingly poor performance on this aspect of COPD care linked to improving survival is noteworthy. Our data were collected before the publication of the Long-Term Oxygen Treatment Trial (LOTT) study in patients with COPD, which did not find a beneficial effect of supplemental oxygen in patients with moderate desaturation at rest (SpO2 89% to 93%) or with exercise-induced hypoxemia (SpO2<90% for >10 seconds, but without a SpO2<80% for >1 minute during a 6-minute walk test). 19 However, the LOTT study excluded patients who had a COPD exacerbation in the previous 30 days, so the applicability of our study among hospitalized patients to the LOTT study population is unclear. We also found that among patients hospitalized with a COPD exacerbation and who underwent an evaluation for supplemental O2 requirements, nearly half (48%) had an increase, decrease, or new O2 requirement. While clinicians may have appropriately targeted patients who required a re-evaluation, it raises the question about whether patients who did not undergo an evaluation also would have had a change in their supplemental O2 prescription. The lack of adequate documentation about supplemental O2 requirements in over 80% of participants in our study suggests that most patients discharged to home on supplemental O2 also do not receive adequate safety-related instructions, such as avoiding smoking or being near an open flame while using O2.20,21 These considerations highlight an important oportunity to improve care, and potentially safety, in patients recently hospitalized with COPD.16,22 Our retrospective study did not evaluate potential reasons for omitting an evaluation or lack of documentation about supplemental O2. Additional studies are needed to evaluate the extent to which inadequate awareness, agreement, or ability to implement guidelines contributed to gaps in the quality of oxygen prescriptions in this high-risk population.23
There is increasing interest in medication reconciliation at times of transitions in care, such as during a discharge from the hospital to home.24 In this context, medication reconciliation refers to processes to avoid inadvertent omissions, duplications, or incorrect doses of medications by reviewing the patient’s medication regimen (e.g., using self-report, prescription refills) with instructions provided to patients at the time of hospital discharge.25 A systematic review of clinical trials found that medication reconciliation reduced discrepancies, with a decrease in actual and potential adverse drug events.26 Anecdotal experience suggests that some clinicians include O2 prescriptions as part of the medication reconciliation process, however this practice is not universal and is not specifically recommended by the Agency for Healthcare Research and Quality’s Medications at Transitions and Clinical Handoffs' Toolkit for Medication Reconciliation.27 CMS does not reimburse O2 as a medication but rather as durable medical equipment.14 This could explain why appropriate documentation of O2 prescriptions is not included in hospital-based efforts to develop and implement medication reconciliation processes.
This DORIC study has a number of strengths. First, our study addresses an important aspect of care in patients with COPD – the use of supplemental O2 – one of very few treatments that has been demonstrated to increase survival.9,10 Second, we included over 300 patients and observed similarly low performance at 2 institutions, suggesting that our findings may not be an isolated problem in the quality of COPD care. Our study also has some important limitations. We assessed information in the EHR, and it is possible that evaluation and documentation of care was recorded elsewhere (e.g., notes by clinicians on paper forms submitted to durable medical equipment companies). Such information is required by durable medical equipment companies when requesting initiation of supplemental oxygen, but not when patients were previously on supplemental oxygen. In the 2 study hospitals, the EHR did not contain a scanned copy of forms submitted to durable medical equipment companies or other such documentation. It is our view that the EHR should contain information relevant to the care of patients, so that post-acute care clinicians, patients, and their caregivers have the information they need to provide appropriate care. Also, our study focused on 2 hospitals and it is not known if results are generalizable to other settings. Additional studies involving different regions, urban/rural settings, for profit versus not for profit hospitals, teaching versus nonteaching hospitals, hospital versus ambulatory settings are needed to compare performance across various care settings. We also did not assess the barriers and facilitators of evaluation and documentation of supplemental O2 requirements; additional studies devoted to this question are needed, as well as studies to identify best practices for improving the quality of care regarding the evaluation and documentation of supplemental O2 requirements.
In conclusion, our study suggests that adequate evaluation and documentation of supplemental O2 requirements is rarely performed in patients hospitalized with COPD. Further research is required to determine if our findings generalize to other settings and to assess the effectiveness of strategies that promote an evaluation and documentation of supplemental O2 requirements at rest and with activity prior to hospital discharge on patient safety, including avoidable readmissions.
Acknowledgements
The authors thank all study patients and the staff of the Breathe Chicago Center® at the University of Illinois at Chicago for their assistance in conducting this study.
Author contributions: All authors had access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis. F.Z. contributed to the study conception, hypothesis delineation, data analysis and interpretation, writing and revision of the manuscript. J.A.K. contributed to the study conception, hypothesis delineation, data acquisition, analysis and interpretation, writing and revision of the manuscript. R.S. L., V.P.C., and B.A.B. contributed to the acquisition and interpretation of the data and revision of the manuscript. A.T, H.A.J, N.E.B., and M.J. contributed to the interpretation, writing, and revision of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs, the U.S. government, or the National Institutes of Health.
Declaration of Interests
None of the authors have any real or apparent conflicts of interest including financial and consulting relationships.