Running Head: Novel Inpatient Screening for Alpha-1
Funding Support: Grifols provided free testing supplies, materials and funding for the blood analyses.
Date of Acceptance: November 8, 2017
Abbreviations: chronic obstructive pulmonary disease, COPD; alpha-1 antitrypsin deficiency, AATD; Arnot Ogden Medical Center, AOMC; American Thoracic Society, ATS; European Respiratory Society, ERS; electronic medical record, EMR; Global initiative for chronic Obstructive Lung Disease, GOLD
Citation: Tasch JJ, McLaughlan AT, Nasir AA. A novel approach to screening for alpha-1 antitrypsin deficiency: inpatient testing at a teaching institution. Chronic Obstr Pulm Dis. 2018; 5(2): 106-110. doi: http://doi.org/10.15326/jcopdf.5.2.2017.0170
Online Supplemental Material: Read Online Supplemental Material (11KB)
Introduction
Alpha-1 antitrypsin deficiency (AATD) is a genetic disorder known to be associated with early onset chronic obstructive pulmonary disease (COPD) and liver disease.1,2 AATD is relatively common in populations of European ancestry, with an estimated prevalence of 1 case per 3000 to 5000 persons in the United States.3 AATD can be difficult to diagnose as many patients commonly present with generic respiratory complaints often mistaken for other respiratory syndromes such as asthma or smoking-related COPD.4 The delay between the onset of symptoms and time of diagnosis of AATD patients exceeds 5 years.5 The 2003 clinical guidelines of the American Thoracic Society (ATS) and the European Respiratory Society (ERS) recommend AATD testing for all patients with COPD, emphysema, or asthma with irreversible airflow obstruction.3,6 Updated guidelines created by the Alpha-1 Foundation and published in the Journal of the COPD Foundation in 2016, again reinforce the recommendation to test all individuals with COPD regardless of age or ethnicity.1 Despite having clear recommendations for AATD screening, testing remains low and is almost exclusively limited to outpatient pulmonary clinics.
Since early diagnosis of the disease could prompt initiation of therapy, screening programs have been developed as a strategy to increase detection (of AATD) in the United States.2,7 Poor awareness by health care professionals is a large reason for the lack of routine screening for AATD. An example that helps facilitate awareness is the distribution of free AATD test kits. Another more recent strategy involved further educating respiratory therapists about AATD which led to an increased referral for screening.8 Yet another strategy to increase focused screening is to utilize pulmonary function laboratory personnel to test patients found to have fixed airflow obstruction while undergoing pulmonary function tests.9 A common misconception by providers is that individuals newly diagnosed with COPD who have a significant smoking history do not need to be screened for AATD. This study was designed to test the feasibility of inpatient screening for AATD utilizing resident physicians as a medium to help collect samples while simultaneously spreading awareness of this genetic disorder.
Methods
This feasibility study was approved by the institutional review board of Arnot Ogden Medical Center (AOMC) in Elmira, New York. The inclusion criteria were selected to be in conjunction with the 2003 guidelines put forth by the ATS and the ERS as well as the 2016 Alpha-1 Foundation guidelines.1,6 Individuals were required to be 18-years-old or older and admitted to AOMC with either an active COPD exacerbation or a previous diagnosis of COPD via pulmonary function testing. All physicians who admitted patients at AOMC were informed of this study via multiple modalities and were encouraged to enroll their patients. Modalities included reminder mass notifications utilizing both electronic medical record (EMR)-based messaging and health network-based emails, printed reminders which were placed at all commonly used computers, and routine verbal reminders at resident conferences. A custom nursing notification order was added into the hospital’s electronic health care software allowing for physician-friendly, time-efficient referral to the study. Through a texting service compliant with the Health Insurance Portability and Accountability Act, nursing staff notified a group of resident physicians of each new referral.
A resident physician associated with the “Alpha-1 Team” obtained informed consent utilizing a premade consent form allowing for the participation in the study and collection of a small blood sample. A brief questionnaire (online supplement), which incorporated previous pulmonary function testing results, smoking history, medical and family history, and contact information, was completed by the resident physician and the patient. An AATD brochure was given to the individual being screened in compliance with New York State policy. Utilizing free AATD testing kits, provided by Grifols pharmaceuticals, a blood sample was collected and sent to GeneAidyx LLC Alpha-1 Antitrypsin Genetics Laboratory for further analysis. Results were reported and reviewed within 2-3 weeks of screening. Any positive findings prompted a referral of the individual for an outpatient visit with a pulmonologist.
Results
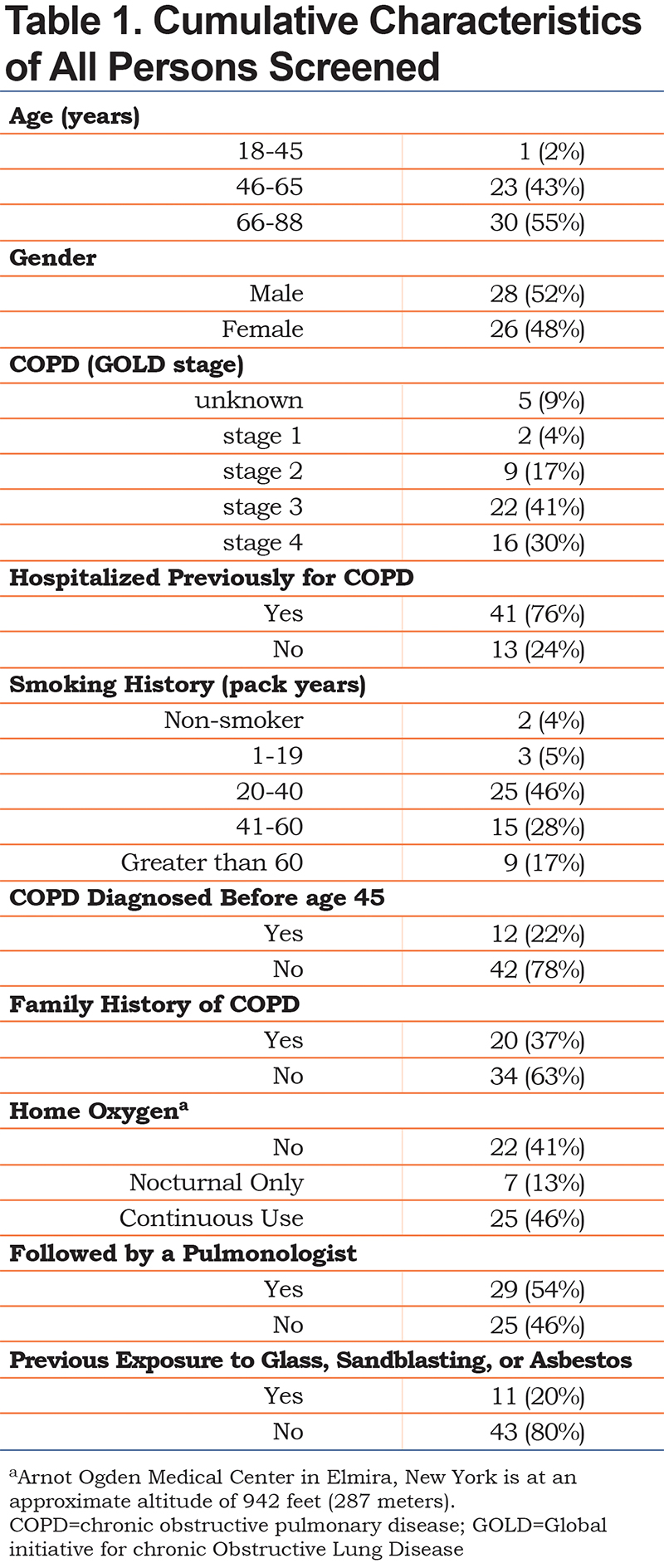
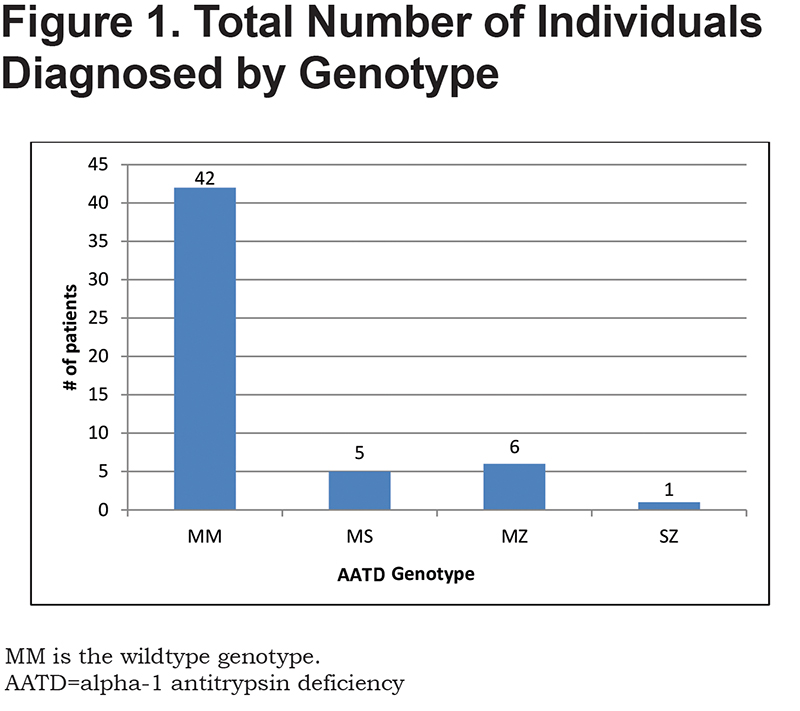
Demographics of the study population are listed in Table 1. There was a male to female ratio of nearly 1:1 with most participants older than 45 years old. A total of 71% of the individuals screened carried a diagnosis of Global initiative for chronic Obstructive Lung Disease stage 3 or 4 COPD.10 As expected, tobacco use was predominant in our patient population, with 47% being active smokers. Perhaps most importantly, only 54% of individuals evaluated were being currently followed by a pulmonologist with more than 75% of them having been hospitalized for a COPD-related issue prior to the admission in which they were screened for AATD. To date, this study has led to the discovery of 12 individuals (22.2% of the screened patients) with a variant genotype of AATD (Figure 1). There were 5 individuals with PI*MS genotype, 6 patients with PI*MZ genotype, and 1 individual with PI*SZ genotype with associated range of alpha-1 antitrypsin levels of 84.3 to 192.6 mg/dL, 74.6 to 208.4 mg/dL, and 70.6 mg/dL respectively. Additionally, 1 individual who was a newly diagnosed AATD carrier from this study, referred their child for screening who was subsequently found to have a PI*MZ genotype.
Discussion
AATD is one of the most common autosomal genetic disorders in humans, although it is still considered a rare recessive hereditary disorder and is significantly underdiagnosed.11 For many individuals, a true diagnosis may take years, even after symptoms appear, leading to significant disease progression and irreversible lung damage.1 According to the ATS/ERS recommendations, all patients with COPD and asthma (not fully reversible after bronchodilator therapy) should be tested for AATD.12 Largely, these recommendations are not being followed. Potential reasons for that include lack of awareness about the disease and the appropriate tests.5 The utilization of resident physicians to screen patients was done in part to help promote, among young physicians, the awareness of prevalence and treatment options of AATD.
There have been attempts to improve timing of diagnosis by implementing mass screening techniques in the general population and in newborns, but these are obviously limited by the high costs that can prohibit any large-scale screening program. The vast majority of modern day screening for AATD takes place in outpatient pulmonary offices. This study addressed the void of inpatient screening and targeted individuals admitted with a formal diagnosis of COPD via pulmonary function testing or had an active COPD exacerbation. There was no exclusion for comorbidities, age, medical compliance or tobacco exposure.
There were challenges throughout the implementation of this novel screening method. The first was the need for frequent reminders to all providers to refer their patients to the study due to the presumed “not important” status for screening for AATD. Another challenge was overcoming the physician assumptions that age and/or smoking history disqualified their patients for screening. Overall, through continued reinforcement of appropriate screening criteria, physicians began referring more patients. This study would have been strengthened if the patients who qualified, but were not referred by providers for screening, had been tracked. Strategies for implementing EMR-based automatic reminders to providers are being investigated for further improvements in both referral rates as well as eliminating the possibility of referring an already screened individual.
There are numerous benefits of screening for AATD. The most obvious benefit is to potentially qualify a patient for augmentation therapy with alpha-1 antitrypsin protein from donor plasma. Other benefits include: more effective smoking cessation counseling, ability to qualify family members for screening prior to developing significant disease, and the ability to provide individuals with more focused genetic counseling. More effective health care management and modification of lifestyle, such as smoking cessation, can be undertaken in individuals with AATD to help prevent the development of lung disease.13
There was an extraordinarily high percentage of individuals newly diagnosed as AATD carriers in this study as compared to the general population. This may be a selection bias based on the inpatient cohort selection. According to GeneReviews,14 North America AATD carriers are estimated to be 4.8% PI*MS, 2.1% PI*MZ, 0.1% PI*SZ. This feasibility study of 54 individuals resulted in 9.2% PI*MS, 11.1% PI*MZ, 1.9% PI*SZ. Despite finding no individuals with PI*ZZ genotype, these high percentages of newly diagnosed AATD carriers suggests the need for continued expansion of screening hospitalized patients with COPD for AATD. Further expansion may lead to the eventual utilization of ancillary hospital staff for testing of individuals.
Conclusion
The integration of inpatient genetic screening for AATD in COPD patients led to a high rate of newly diagnosed AATD carriers with a variety of AATD genotypes. This novel approach of utilizing resident physicians in the screening process for AATD led to an increased awareness among providers of AATD screening guidelines and treatment options. Furthermore, it is recommended that inpatient screening for AATD in individuals with COPD be expanded regardless of age, severity of symptoms, or smoking history.
Acknowledgments
We thank David Lester for providing assistance with publication searches. We thank Kathryn Graham, DO, Adrian Brandau, DO, Hussein Al-Mohamad, DO, Donald Foster, DO, Cliff Snellgrove, DO, Michael Kochik, DO and Joshua Turner, DO for aiding with data collection. We thank Arnot Ogden Medical Center’s institutional review board for allowing this project to continue. We thank Grifols for providing free testing supplies, materials, and the funding for the blood analyses.
Author contributions: Dr. McLaughlin contributed to the design of the study, data acquisition and analysis and revisions to the article. Dr. Nasir also contributed to the design of the study, data analysis and article revisions. Dr. Tasch is responsible for all aspects of the study and creation, revision and approval of the manuscript for publication.
Declaration of Interest
The authors have nothing to declare.