Running Head: ZZ vs. SZ Alpha-1 Antitrypsin Deficient Patients
Funding Support: This study was funded by an unrestricted research grant from AlphaNet.
Date of Acceptance: July 16, 2018
Abbreviations: alpha-1 antitrypsin deficiency, AATD; AlphaNet’s Disease Management and Prevention Program, ADMAPP; protease inhibitor, PI; negative binomial, NB; Charlson Comorbidity Index, CCI; zero-inflated negative binomial, ZINB; Big Fat Referene Guide, BFRG; Akike information criterion, AIC; incident rate ratio, IRR; confidence interval, CI; standard deviation, SD; gastroesophageal reflux disease, GERD
Citation: Choate R, Mannino DM, Holm KE, Sandhaus RA. Comparing patients with ZZ versus SZ alpha-1 antitrypsin deficiency: findings from AlphaNet’s disease management program. Chronic Obstr Pulm Dis. 2019; 6(1): 29-39. doi: http://doi.org/10.15326/jcopdf.6.1.2018.0134
Online Supplemental Material: Read Online Supplemental Material (275 KB)
Introduction
Alpha-1 antitrypsin deficiency (AATD) is an autosomal co-dominant disorder that results from mutations of the SERPINA1 gene and typically is associated with the increased risk of early onset pulmonary emphysema1 in adults, liver disease in children as well as adults and, more rarely, necrotizing panniculitis.2
SERPINA1 is considered a polymorphic gene.3 The most common mutations of the gene associated with AATD are the PiZ and PiS mutations, where Pi stands for “protease inhibitor.”4 The PiM-allele represents the normal genotype. Homozygous PiZZ is the most commonly identified severely deficient genotype while the PiS-allele leads to a milder plasma deficiency of AAT.3 Over 200 mutations of the gene have been discovered, with approximately one-third of these mutations leading to clinically significant deficiency.4 Serum levels of AAT between 85 and 215 mg/dL are considered normal,5 although normal ranges vary by laboratory. Individuals with a ZZ genotype rarely have levels above 57 mg/dL, and levels below this value are presumed to provide inadequate lung protection.4
Both Z and S mutations are believed to have originated among populations of European (non-Hispanic white) descent.6 The Z-gene is associated with the Scandinavian/Baltic region,7 and the S-gene is considered to derive from the Iberian peninsula.8 AATD can be found in all major racial subgroups in the world, although often at a very low frequency.9
Previous studies that compared clinical features of SZ and ZZ patients have found significantly fewer respiratory symptoms, less severe airflow obstruction, and fewer radiographic lung abnormalities in SZ patients.10 Similarly, a study using the Spanish AATD registry (REDAAT) determined that ZZs have greater lung function impairment than SZs.8 The results of a large study in the United Kingdom demonstrated similar disease progression between SZs and ZZs although SZs had better baseline characteristics.11 These findings were explained by the greater importance of AAT levels rather than genotype.11 Other studies have demonstrated a correlation of serum AAT levels with the severity of emphysema.12 Regardless of genotype, smoking is the major risk factor for development of lung disease in patients with AATD.13,14
It is important to know whether genotype is associated with health outcomes and health behaviors, in order to determine whether individuals with the SZ genotype have differing needs from ZZs with regard to health education and behavioral interventions such as smoking cessation. The primary aim of our study was to examine differences in demographic, health, and behavioral characteristics in individuals with ZZ and SZ genotypes among individuals who are participating in a disease management program designed for patients with AATD and lung disease.
Materials and Methods
Our study population consisted of members of AlphaNet, a not-for-profit health management organization that coordinates management and treatment of individuals with AATD and lung disease in the United States.15 Enrollment in the AlphaNet Disease Management and Prevention Program (ADMPP) is offered when an individual is prescribed plasma-derived, intravenous AAT for the treatment of lung disease due to AATD (augmentation therapy). Analyses were conducted on coded data collected by AlphaNet. The study was approved by the University of Kentucky Institutional Review Board.
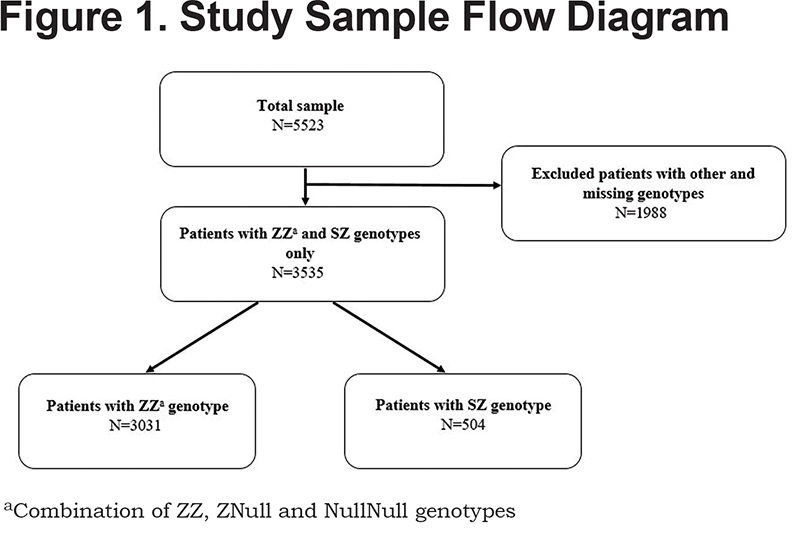
The inclusion criteria were that the participants were members of AlphaNet and had either a ZZ, ZNull, NullNull (analyzed in combination with ZZ) or SZ genotype of AATD. The final sample included 3535 respondents (Figure 1). Of these, 3031 (85.7%) were identified as ZZs. The ZZs can be broken down as follows: ZZ (n=2979, 98.3%), ZNull (n=38, 1.2%), and NullNull (n=14, 0.5%). Our analyses compared baseline characteristics of AATD patients with the ZZ genotype to those with the SZ genotype. All data were collected using questionnaires administered via a telephone interview.
Statistical Analyses
Descriptive statistics were computed for baseline characteristics for the overall sample and stratified by genotype (ZZ versus SZ). The results for continuous variables were reported as mean ± SD, and for categorical variables by frequencies and proportions. Values between the groups were compared using t-test/ANOVA, and Chi-squared test respectively. Post-hoc comparison of adjusted standardized residuals was used to determine the source of the statistically significant Chi-square for categorical variables. Negative binomial (NB) regression models were fit for frequency of exacerbations and visits to a primary care physician in the past year adjusting for age, sex, smoking status and Charlson Comorbidity Index (CCI). A zero-inflated negative binomial (ZINB) model was fit for frequency of hospitalizations adjusting for the same covariates. The significance level for all analyses was set at 0.05. False discovery rate control was used to correct for multiple univariate testing.16 SAS 9.4 and SPSS version 22 were used for analyses.
Results
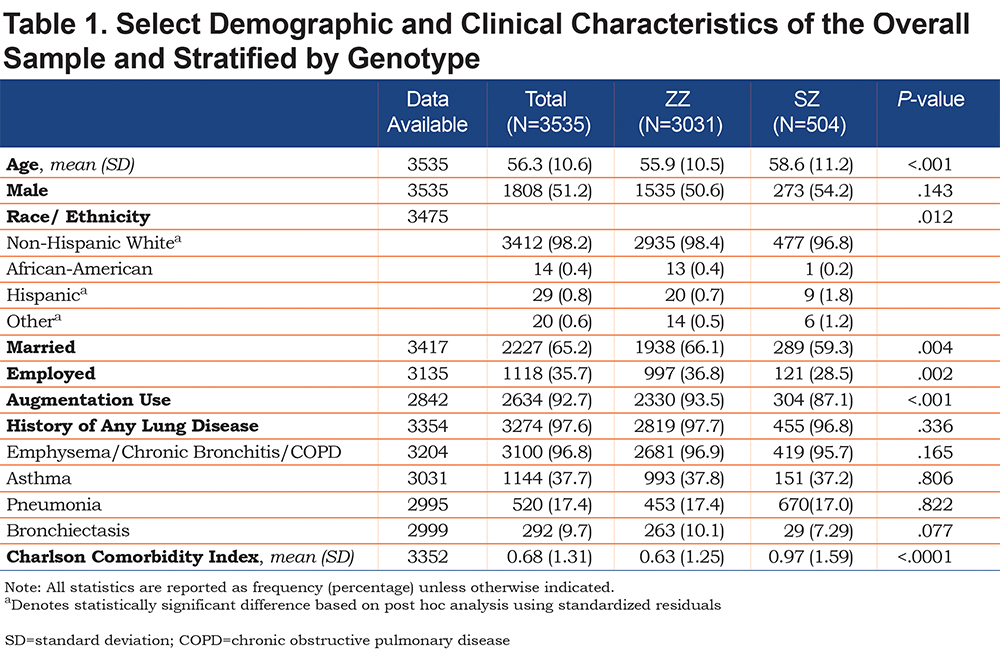
Table 1 describes the baseline demographic characteristics of the overall sample (n=3535) and stratified by genotype: ZZ (n=3031, 85.7%) and SZ (n=504, 14.3%). Average age of the study population was 56.3±10.6 years. Patients with the ZZ genotype were slightly younger than SZs (55.9 years versus 58.6 years, p<0.001), and a greater proportion of ZZs were white (98.4% versus 96.8%, p=0.012), and reported being married (66.1% versus 59.3%, p=0.004). The majority of respondents were male (51.2%) with no significant differences by genotype. Over 90% of all the respondents (92.7%) were on augmentation therapy with a greater proportion of ZZs than SZs (93.5% versus 87.1%, p<0.001). The CCI score (which accounts for number and complexity of comorbidities) was significantly higher among SZs than ZZs (p<0.0001).17
A total of 3274 (97.6%) patients reported ever having lung disease, with no significant difference between the genotypes. Emphysema/chronic bronchitis/COPD (96.8%) and asthma (37.7%) were the most frequent types of lung disease reported by the respondents.
Among those who reported ever having lung disease, significant differences were found in exacerbation frequency between ZZ and SZ patients (p<0.001, Table 2). Based on post-hoc analysis using standardized residuals, a significantly greater proportion of SZs than ZZs reported having monthly (20.2% versus 13.9%) and quarterly (21.3% versus 16.2%) exacerbations, while ZZs reported more semi-annual exacerbations (13.9% versus 9.6%). SZs did not differ from ZZs with regard to the percentage who used oxygen regularly, number of hours oxygen was used per day, or coughing up sputum regularly.
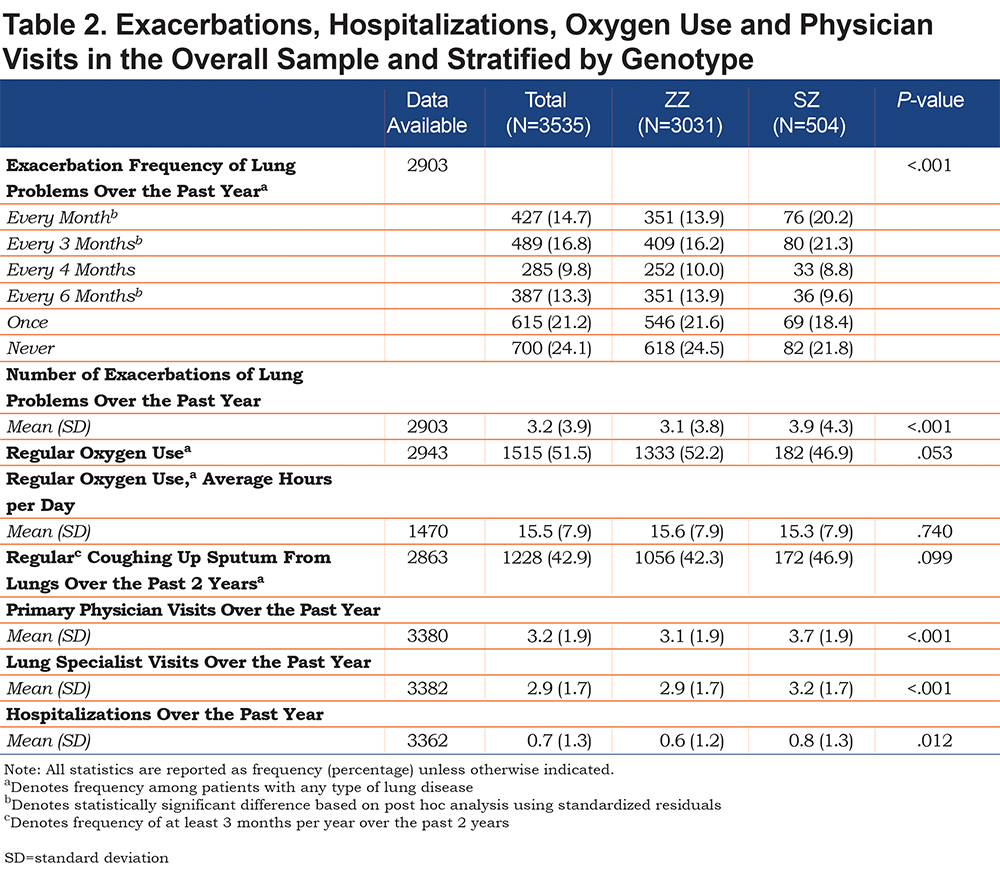
The average number of visits to a primary care physician over the past year among all the respondents was 3.2±1.9, and to a lung specialist was 2.9±1.7. The mean number of hospitalizations was 0.7±1.3. SZs reported more primary physician visits (p<0.001), lung specialists visits (p<0.001) and hospitalizations (p=0.012) than ZZs.
Table 3 demonstrates the frequencies of the 10 most prevalent comorbidities within the overall study sample and stratified by genotype. The most frequent comorbidities were high blood pressure (40.3%), gastroesophageal reflux (34.8%), sinus disease (16.1%), heart rhythm problems (12.9%) and any tumor/cancer (11.9%). Our study found that a statistically significant greater proportion of SZs in our cohort were diagnosed with the 6 most prevalent comorbidities. In addition, e-Table1 in the online supplement contains information about all comorbidities for which AlphaNet collected data, several of which were also more prevalent among SZs.
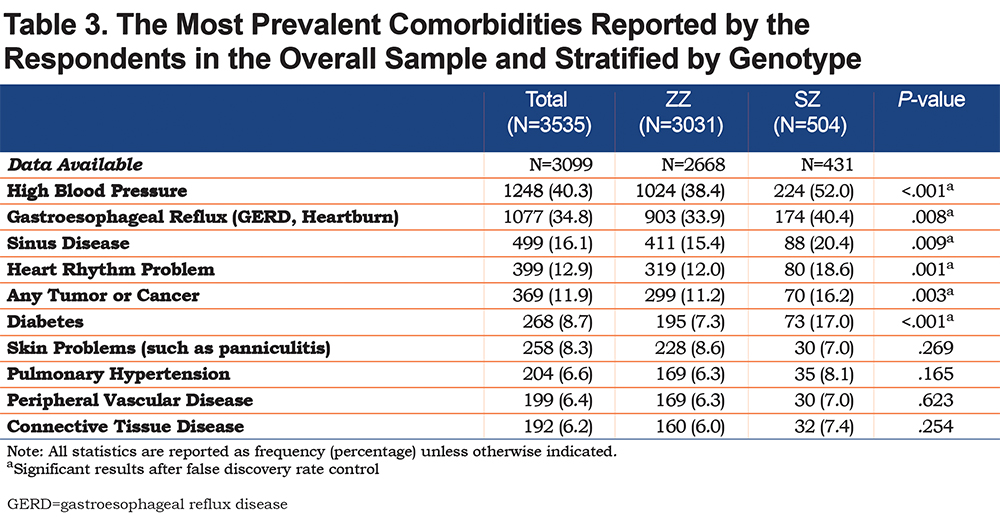
Table 4 presents the results of self-reported health behaviors and fitness characteristics of ZZs and SZs. The majority of respondents reported having ever smoked (73.1%), and a significantly greater proportion of SZs than ZZs reported having ever smoked (p<0.001). In addition, SZs are more likely to continue to smoke (p<0.001), have been smoking longer (p<0.001), and report smoking more packs per day (p<0.001). Contrary to the findings for smoking, a higher proportion of ZZs report that they consume alcohol (p=.009), and ZZs consume more drinks per week on average than SZs (p=.030).
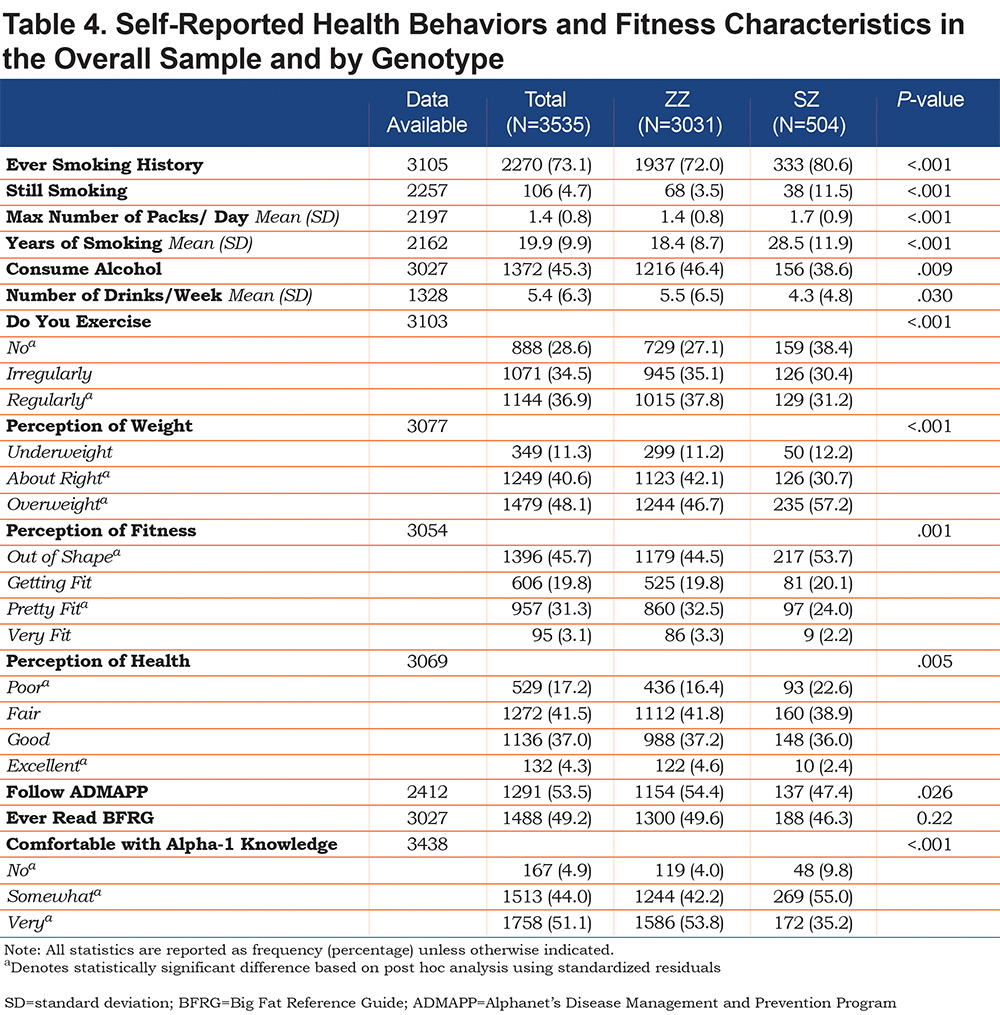
With respect to self-perceived health and fitness, a significantly greater proportion of SZs than ZZs view themselves as being overweight (p<0.001), out of shape (p=0.001) and in “poor health” (p=0.005). More ZZs report that they exercise regularly compared to SZs, and 38.4% of SZs do not exercise at all compared to 27.1% of ZZs (p<0.001).
The majority of patients reported that they follow the guidelines of ADMAPP (53.5%), and a significantly greater proportion of ZZs report following the program compared to SZs (p=0.026). Almost half of the participants (49.2%) reported ever reading the Alpha-1 Big Fat Reference Guide (BFRG)18 with no difference by genotype.
Most patients reported being very comfortable with their knowledge about AATD (51.1%). However, significant differences were found between the genotypes (p<0.001). Specifically, a greater proportion of SZs, when compared to ZZs, reported being either “not comfortable” (9.8% versus 4.0%) or “somewhat comfortable” (55.0% versus 42.2%) with knowledge about their condition.
Table 5 presents results of the adjusted NB and ZINB models. The criteria for assessing goodness of fit of each of the regression models showed adequate fit: deviance (scaled deviance) value/DF and Pearson Chi-Square (Scaled Pearson) value/DF were reasonably close to 1 (between 0.95 and 1.18) which indicates adequate fit of the models to the data. The selected regression models showed superior fit using Vuong test and Akike information criterion (AIC), corrected AIC and Baynesian information criterion when compared to other types of count models.
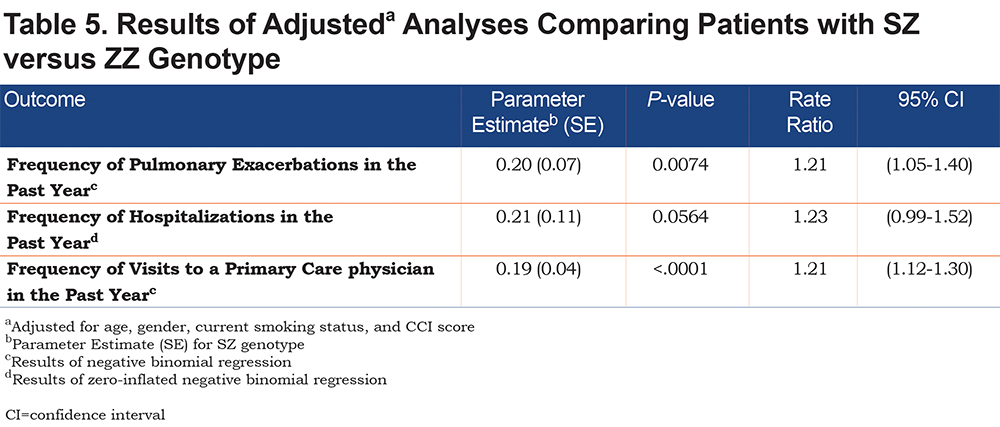
As demonstrated in Table 5, genotype was associated with the number of exacerbations and visits to a primary care physician after adjusting for age, gender, current smoking status, and CCI score. SZs had 1.21 times the rate of pulmonary exacerbations (incident rate ratio [IRR]=1.21, 95% confidence interval [CI]: 1.05-1.40) and visits to a primary care physician (IRR=1.21, 95%CI: 1.12-1.30) in the past year compared to ZZs.
Discussion
In 1996, Turino et al10 described clinical features of a relatively small number of patients with the SZ genotype of AATD and AAT concentrations above or below 11 µM (~57 mg/dL), including the effects of smoking on development of lung disease in SZs. More recent studies comparing characteristics of individuals with the ZZ and SZ genotypes were mainly carried out in Europe8,11,19 as the prevalence of S-allele is the highest in the general population of Spain and Portugal (17.3 and 13.8 per 1000, respectively).20 To our knowledge, this study is the first to focus on potential differences between SZs and ZZs in demographic, health, and behavioral characteristics among a large number of individuals (n=3535) participating in a disease management program enrolling patients with AATD and lung disease.
Although any individual with AATD can enroll in AlphaNet’s disease management program, the vast majority of individuals entered because of a prescription for augmentation therapy for lung disease due to AATD. Individuals not receiving augmentation therapy are moved to a different program within AlphaNet that focuses on risk reduction: Risk Evaluation to Ensure Continued Health. Thus, the population described here is greatly enriched for individuals with lung disease due to AATD. Since individuals with the SZ genotype are considerably less likely to develop lung disease than ZZs,10 many of the differences reported in this study may be reflective of the subpopulation of SZs with risk factor exposure sufficient to have led to clinically significant lung disease. Risk for development of lung disease is associated with the interaction between genetic factors and various environmental exposures such as smoking.13
Prior studies have noted higher mean smoking consumption by SZs compared to ZZs.21Our findings complement these observations by showing that when compared to ZZs, SZs had a significantly longer smoking history with a greater number of packs smoked per day. Further, the SZs in our sample were more likely to continue smoking after being diagnosed with lung disease. These results reflect the importance of emphasizing behavioral interventions and health education including smoking cessation, especially among SZs, as well as early diagnosis of AATD prior to the development of heavy smoking habits.21
Exacerbations commonly occur among patients with AATD-related lung disease22 and, in previous research, were demonstrated to be associated with a decline in lung function.22 In our sample, SZs reported more frequent exacerbations than ZZs, even after adjusting for age, sex, current smoking status and CCI score. Possible explanations include greater prior exposure to smoking, and lower adherence to healthy lifestyle recommendations, including adherence to AlphaNet’s disease management program. However, lack of pulmonary function data limited our ability to compare lung disease severity between genotypes.
Previous research has demonstrated an association between AATD and certain comorbidities, such as ulcerative colitis and hypothyroidism23 among ZZs. Other studies demonstrated associations between ZZ and MZ genotypes of AATD and reduced blood pressure, as well as MZ and reduced risk of ischemic cerebrovascular and ischemic heart disease.24,25 Our results show a significantly higher prevalence of cardiovascular comorbidities, including hypertension, cerebrovascular disease, congestive heart failure, and arrhythmia among SZs compared with ZZs. The reasons for this association with SZ genotype are not well understood, nor sufficiently investigated previously. It should be noted that SZs in our cohort are slightly older than ZZs; also, the diagnosis of AATD may prompt a more thorough assessment for other health problems among SZs. Additionally, our findings of unhealthy lifestyle of the majority of SZs in our study population, may have contributed to the greater prevalence of cardiovascular comorbidities among patients with this genotype.
Previous research has explored the effects of genetic information on health behaviors of patients and their families.26-30 These studies have found inconsistent results with regard to the effect of genetic information on smoking cessation and motivation to improve diet and physical activity. Our study demonstrated that ZZs and SZs significantly differ in their perception of health and fitness as well as their health behaviors. A greater proportion of SZs viewed themselves as overweight, out of shape and in poor health, and they also exercise less and report a heavier and longer history of smoking compared with ZZs in our study. These findings may be explained by the perception that the SZ genotype presents a lower risk of the disease in view of the higher AAT levels in plasma. Our findings with regard to alcohol consumption suggest that, regardless of genotype, additional education about moderation of alcohol consumption should be considered due to the increased risk of liver disease among individuals with AATD.
ADMAPP is a vital part of AlphaNet’s commitment to improve patients’ health outcomes.15,31 Our study shows that a significantly lower proportion of SZs report following the guidelines of ADMAPP compared to ZZs. This lower adherence to the program may be due to the earlier mentioned concept of the low self-perceived seriousness of their condition. It should also be noted that SZs are less comfortable with the level of their knowledge about AATD compared to ZZs.
Understanding differences and similarities between various genotypes of AATD is of great importance from the public health perspective. Early knowledge and awareness of AATD allows for timely testing, smoking prevention and cessation, and initiation of augmentation therapy when indicated.32
Strengths and Limitations
This is the first study to examine demographic, health, and behavioral factors in a large population of lung-affected individuals with AATD who are participating in a disease management program. Our results suggest that, among individuals with AATD who have developed lung disease, people with a less severe genotype who develop lung disease have worse health outcomes and health behaviors. Thus, the people who are less at risk (from a genetic standpoint) to develop lung disease may actually do worse once they have developed lung disease. While prevention efforts may need to be targeted to ZZs (since they are most at risk to develop disease) it is possible that disease management may be even more vital to SZs.
Several limitations must be acknowledged. First, a considerably larger fraction of ZZs develop lung disease compared with SZs. Since only patients with lung disease were invited to participate in ADMAPP, this may have introduced ascertainment bias into the study. Although, both SZs and ZZs were enrolled based on the presence of lung disease. This study provides no information about the comparative characteristics of SZs and ZZs without lung disease. Second, causality cannot be inferred due to the cross-sectional design of our study. Third, objective data were not available to provide more specifics on clinical phenotyping of lung disease, including CT findings and lung function measurements. Another limitation of our study is unavailability of the actual date of AATD diagnosis in most patients, which limits our ability to account for the length of time since diagnosis. The benefits of earlier age at diagnosis might be reflected in behavior modifications such as smoking cessation or improved exercise habits, which might contribute to better outcomes.
Conclusion
In summary, the results of this study document that ZZ and SZ patients in AlphaNet’s disease management program differ with regard to health outcomes and health behaviors. Individuals with the SZ genotype have more comorbid health conditions and are less likely to engage in health-promoting behaviors such as exercise and smoking cessation. It appears that individuals with the more severely deficient ZZ genotype are more adherent to ADMAPP recommendations and maintain a healthier lifestyle than individuals with the less severely deficient SZ genotype. As such, improvements in education efforts may be especially beneficial for individuals with the SZ genotype who have lung disease, even though their underlying AATD is considered to be less severe.
Acknowledgments
Author contributions: Radmila Choate conducted the data analyses and drafted the manuscript. David M. Mannino and Kristen Holm have actively contributed to the data analysis and the manuscript preparation, Robert A. Sandhaus participated in the study design and contributed substantially to preparation of the manuscript. All authors read and approved the final manuscript.
Declaration of Interest
David Mannino is a full-time employee of GlaxoSmithKline plc and owns stock of GlaxoSmithKline plc. Furthermore, he has received royalties from Up-to-Date, and has been compensated as a medical expert in legal cases. Robert Sandhaus has served on advisory boards and/or as a speaker for Grifols, CSL Behring, Shire, and AstraZeneca. He is on the board of directors of AlphaNet and the Alpha-1 Project, is on the Medical Advisory Committee of the COPD Foundation and serves as the Medical Director of AlphaNet. Kristen Holm has received consulting income from AlphaNet. Radmila Choate has received research support from AlphaNet.