Running Head: LVRS and Respiratory Muscle Strength
Funding Support: Supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services, and the Agency for Healthcare Research and Quality.
Date of Acceptance: July 16, 2018
Abbreviations: lung volume reduction surgery, LVRS; maximum inspiratory pressure, MIP; total lung capacity, TLC; residual volume, RV; functional residual capacity, FRC; National Emphysema Treatment Trial, NETT; respiratory muscle strength, RMS; body mass index, BMI; forced expiratory volume in 1 second, FEV1; confidence interval, CI
Citation: Criner RN, Yu D, Jacobs MR, Criner GJ. Effect of lung volume reduction surgery on respiratory muscle strength in advanced emphysema. Chronic Obstr Pulm Dis. 2019; 6(1): 40-50. doi: http://doi.org/10.15326/jcopdf.6.1.2018.0188
Online Supplemental Material: Read Online Supplemental Material (196KB)
Introduction
Emphysema is a progressive disease characterized by small airway destruction and reduced lung elastic recoil that precipitates air trapping during expiration.1,2 This results in hyperinflation, which is characterized by an increase in functional residual capacity and a concomitant decrease in inspiratory capacity. The overall increase in end-expiratory lung volumes causes contractile dysfunction of the inspiratory muscles, i.e., the diaphragm and external intercostal and accessory muscles, preventing adequate inspiratory muscle force generation.3
Structurally, hyperinflation flattens thediaphragm, leading to passive shortening of the diaphragm; this distorts the optimal length-tension relationship for skeletal muscle contraction and leads to weakened diaphragm contraction.3-8 Also, hyperinflation causes less rib cage area to be exposed to positive abdominal pressure generated by diaphragm contraction; this decrease in the zone of apposition prevents adequate rib cage expansion with inspiration, thus further impairing negative intrathoracic pressure generation.4,7 Biochemically, hyperinflation leads to upregulation of nuclear factor kappa beta, which leads to decreased MyoD expression, resulting in dysregulation of muscle differentiation as well as diaphragm protein degradation via the ubiquitin-proteasome pathway.9,10 When faced with increased ventilatory workloads, such as during exercise, hyperinflation and its consequences causes severely emphysematous patients to experience increased respiratory muscle fatigue and increased energy expenditure.11 An increase in respiratory muscle force generation is an important goal because hypercapneic respiratory failure secondary to inspiratory muscle fatigue is an important contributor to mortality in chronic obstructive pulmonary disease .9,12
Several studies have found lung volume reduction surgery (LVRS) to decrease residual volume and inspiratory muscle workload and to increase diaphragm length, the zone of apposition, and elastic recoil, resulting in improved inspiratory muscle mechanics; reduction in hyperinflation increases diaphragm strength, exercise capacity, maximal oxygen consumption, maximal minute ventilation, and maximum voluntary ventilation.12-15 These changes improve respiratory muscle mechanics, which increases exercise tolerance and decreases dyspnea to a greater degree than medical therapy.15 However, these studies are small in number, rarely controlled, and only report results of no more than 6 months post-LVRS.
We therefore conducted a retrospective analysis using prospectively collected data from the National Emphysema Treatment Trial (NETT), a large population of 1218 patients with severe bilateral emphysema who were followed over a 5-year period to determine the short and long-term effects of LVRS compared to optimal medical management on respiratory muscle strength (RMS).16,17 We also analyzed if age, sex, body mass index (BMI), distribution of emphysema and exercise capacity affected changes in respiratory muscle strength after LVRS compared to medical therapy.
Methods
Study Design
The National Emphysema Treatment Trial was a prospective multicenter randomized controlled clinical trial that enrolled 1218 individuals with severe emphysema between 1998-2002. The main objective was to compare the effect of optimal medical treatment plus LVRS versus optimal medical treatment alone on the co-primary outcomes of survival and maximal exercise capacity. Methods, design, and outcome for NETT were published previously.16,17
A secondary outcome of NETT was RMS evaluation via maximum static inspiratory pressures (MIP) measurements at baseline, 6 months after randomization, and then yearly for 5 years after randomization. Outcome measurements were analyzed up to 3 years post-randomization. NETT represents the largest dataset of longitudinal measurements of global RMS ever obtained in patients with severe emphysema.
Study Participants
Enrollment criteria included bilateral emphysema on high-resolution computerized tomography scan and pre-rehab forced expiratory volume in 1 second (FEV1) £ 45% predicted. Before randomization, participants underwent comprehensive medical evaluations and then participated in 16 to 20 pulmonary rehabilitation sessions over 6 to 10 weeks. Baseline measurements were obtained after rehabilitation but before randomization. Participants were then randomized to either LVRS with medical treatment or to medical treatment alone. The institutional review board for human studies approved the protocol, and all participants provided written consent.
Pulmonary Function Measurements
The forces generated by respiratory muscles, including the diaphragm and external intercostal and accessory muscles, were determined by MIP, which were measured at the airway opening with a flanged mouthpiece during a voluntary contraction against an occluded airway, using the Black and Hyatt techniques.18 MIP was measured at residual volume. Participants generated MIP 3-10 times, until 3 measurements were within 5 cm H2O of the maximum value. The average of these 3 measurements was reported in our analyses. Total lung capacity (TLC), residual volume (RV), functional residual capacity (FRC) and ratio of residual volume to total lung capacity (RV/TLC) were measured using plethysmography. TLC is the volume of air in the lungs at the end of maximal inspiration, and RV is the volume of air in the lungs after maximal exhalation. FRC is the volume of air remaining in the lungs at the end of passive expiration, and RV/TLC was obtained by dividing the volume of air in the lungs after maximal exhalation by the volume of air in the lungs at the end of maximal inspiration.
Statistical Analysis
Data are displayed as mean ± SD for continuous variables and count and percentage for categorical variables. MIP percentage changes from baseline to 6 through 36 months follow-up were analyzed and further compared between treatment arms at each follow-up interval using Wilcoxon rank-sum tests. These data analyses were repeated to determine impacts of age, sex, BMI, risk stratification, emphysema pattern, and exercise capacity on MIP percentage changes between treatment arms up to 3 years post-operatively; unadjusted p-values comparing treatment groups were obtained from this approach. Additionally, absolute changes in TLC, RV, FRC and RV/TLC from baseline to 6 through 36 months follow-up were compared between treatment arms at each follow-up interval using Wilcoxon rank-sum tests.
Participants were deemed high risk if FEV1 £ 20% of predicted value and either homogenous emphysema or a carbon monoxide diffusing capacity that was 20% or less of predicted value at baseline; those not meeting these criteria at baseline were deemed non-high risk and further categorized by emphysema lobe predominance and exercise capacity.17 The NETT characterized participants as either predominantly non-upper lobe emphysema or upper lobe emphysema based on a baseline computerized tomography scan read by each center’s radiologist.17 Predominantly non-upper lobe emphysema consisted of emphysematous patterns that were diffuse, predominantly lower lobe, or predominantly distributed in superior segments of lower lobes.17 Participants were categorized as either high exercise capacity if they had a maximum workload greater than 40% for their sex at baseline or low exercise capacity if their maximum workload was 40% or less; 40% or less corresponded to a maximum workload of less than 25 watts for females and less than 40 watts for males.17
Moreover, mixed-effects models were fitted to MIP change data, pooling all follow-up intervals together to adjust for various covariates, i.e., baseline MIP, follow-up time, sex, age, BMI, emphysema pattern, exercise capacity, and risk categorization, while also accounting for the correlation among the observations from the same participants over time when comparing treatments. Interaction terms between independent variables were also considered in the model. Adjusted p-values comparing treatment groups were derived from such models with adjustments for the aforementioned covariates at visit times 6 through 36 months. Data transformation (e.g., log or square-root) was considered when a variable on the original scale was not approximately normally distributed. P-values <0.05 were considered statistically significant. Statistical analyses were performed using SAS V9.3 software (SAS Institute Inc., Cary, North Carlolina).
Results
From January 1998 to July 2002, NETT enrolled 1218 patients with severe emphysema and recent completion of 6-10 weeks of pulmonary rehabilitation. A total of 610 individuals were randomized to medical treatment while 608 individuals were randomized to LVRS; see Table 1 for baseline demographics. Of the LVRS group, there were 603 MIP measurements with mean MIP 62.1 ± 22.7 cm H2O. For the medical group, there were 602 MIP measurements with mean baseline MIP 62.0 ± 22.3 cm H2O. Baseline MIPs for various characteristics, such as sex, age, BMI, risk stratification, and lobe predominance and exercise capacity, were similar between the groups (Table 2).
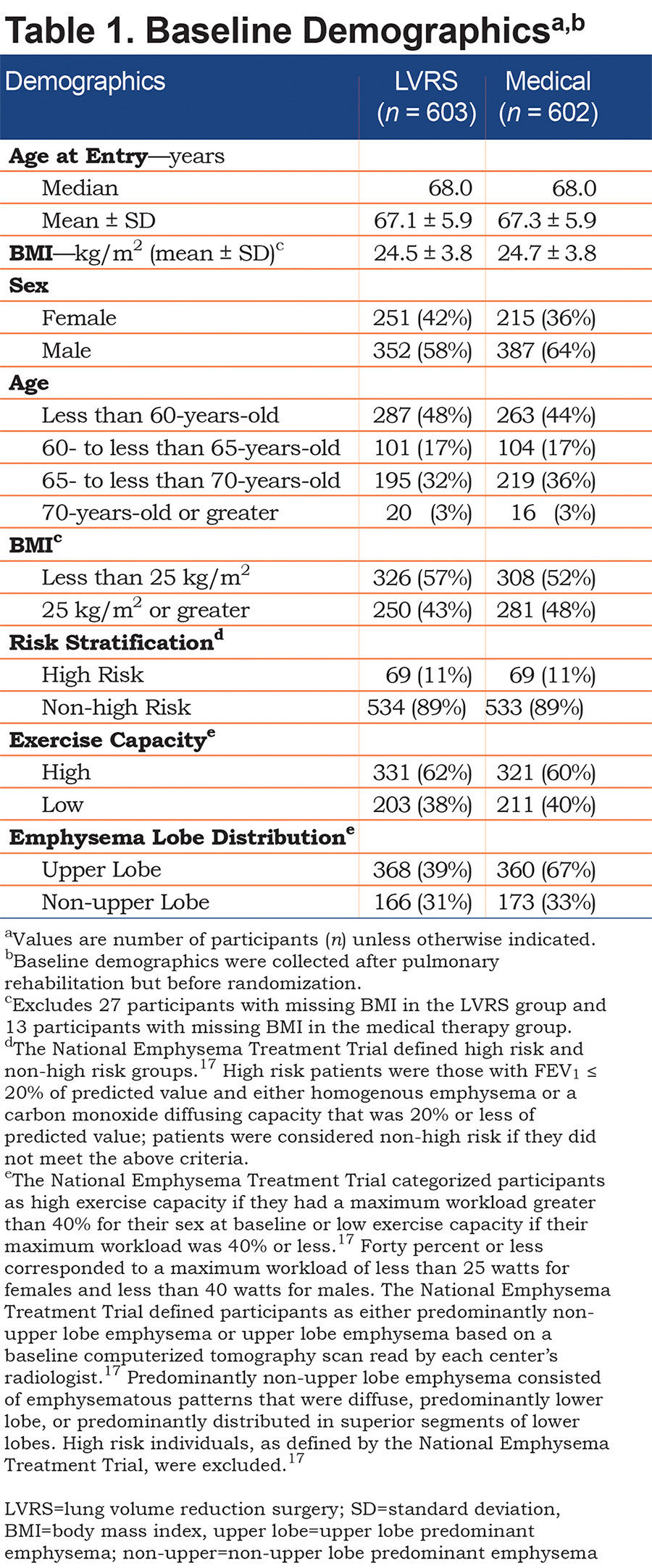
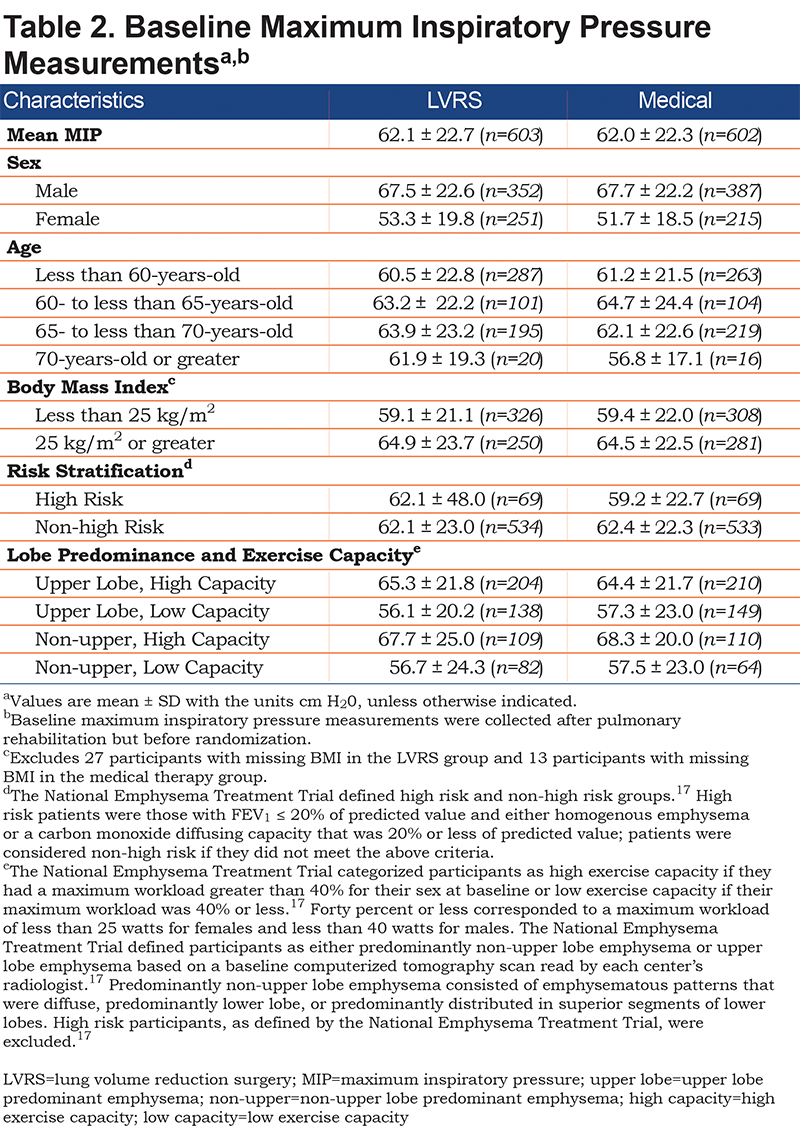
At all follow-up periods, the LVRS group had a significantly greater increase in MIP from baseline, when compared to the medical arm (19.8 ± 42.3% increase in LVRS versus 3.2 ± 29.3% increase in medical, p<0.0001 at 12 months; Table 3). The LVRS group also had significant decreases in TLC, RV, FRC and RV/TLC, when compared to the medical arm at all follow-up periods (For TLC, 0.66 ± 0.8 decrease in LVRS versus 0.09 ± 0.7 decrease in medical, p<0.0001 at 12 months; for RV, 0.83 ± 1.0 decrease in LVRS versus 0.00 ± 0.8 increase in medical, p<0.0001 at 12 months; for FRC, 0.77 ± 0.9 decrease in LVRS versus 0.03 ± 0.8 decrease in medical, p<0.0001 at 12 months; for RV/TLC, 0.06 ± 0.1 decrease in LVRS versus. 0.01 ± 0.1 increase in medical, p<0.0001; Table 3).
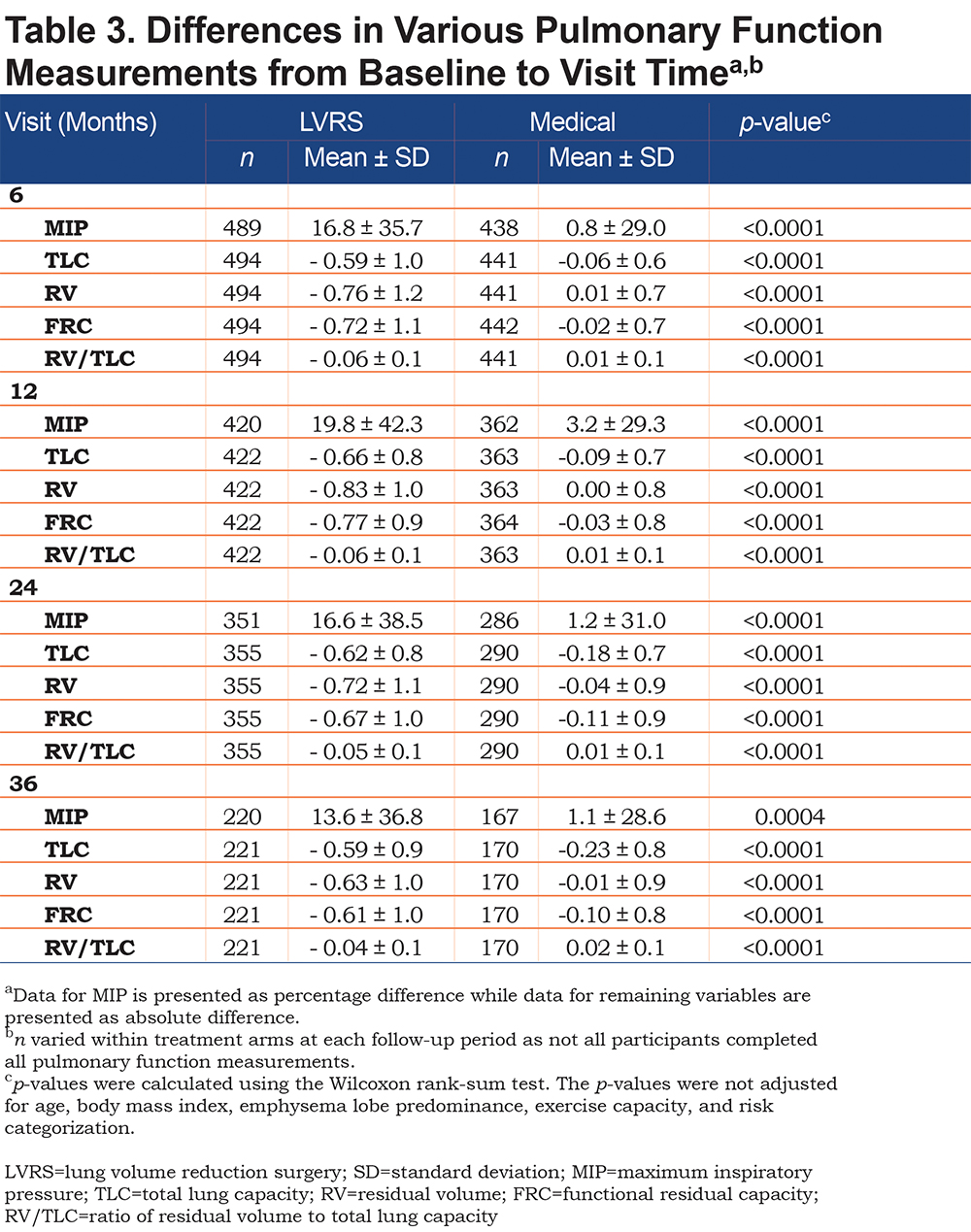
Males in the LVRS arm also had significantly greater increases in MIP, compared to those in the medical arm, at all follow-up periods (increase of 19.5 ± 3.5% at 12 months, p<0.0001, 95% confidence interval [CI] [12.7-26.3]; Table 4). Females in the LVRS group also had greater increases in MIP from baseline when compared to females in the medical arm but significance was lost at 24 and 36 months (increase of 13.6 ± 4.2% at 12 months, p=0.0017, 95% CI [5.0-21.6]).
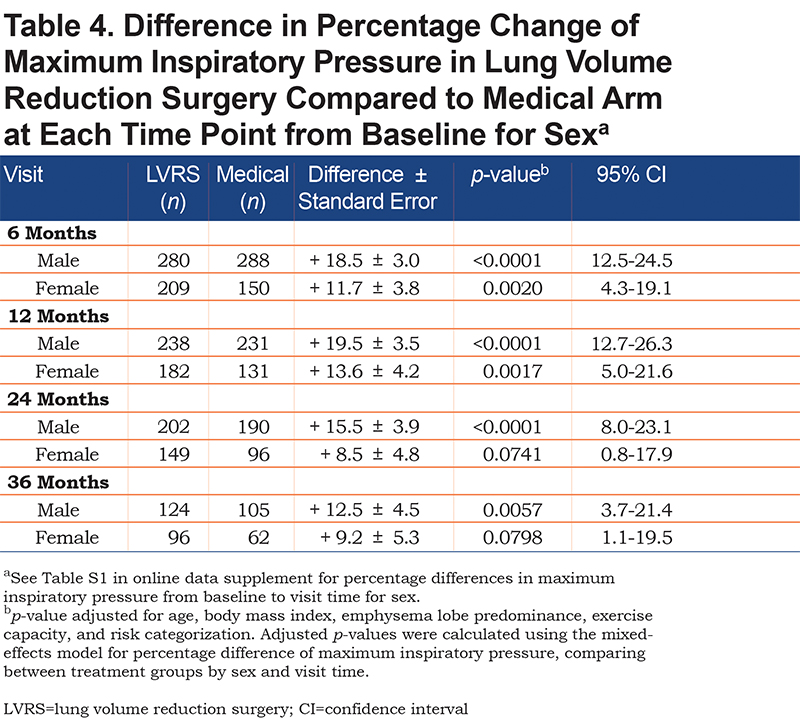
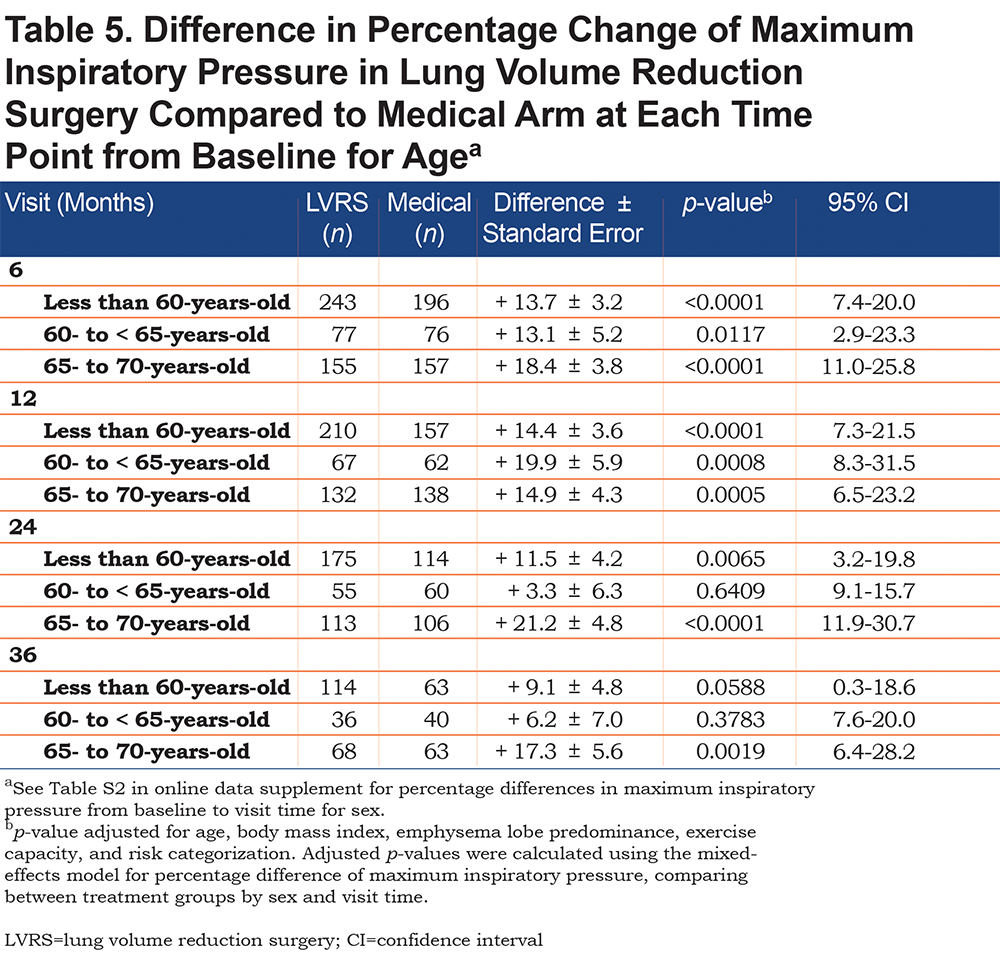
For all age groups, LVRS led to greater increases in MIP from baseline when compared to optimal medical therapy. This trend was significant at all follow-up periods for those 65- to 70-years-old (increase of 14.9 ± 4.3% at 12 months, p=0.0005, 95% CI [6.5-23.2]; Table 5). This trend was only significant at up to 24 months for those less than 60-years-old and at up to 12 months for those 60- to 65-years-old (increase of 14.4 ± 3.6%, p<0.0001, 95% CI [7.3-21.5] and increase of 19.9 ± 5.9%, p=0.0008, 95% CI [8.3-31.5], respectively). Analysis was not performed on participants older than 70-years-old because n was less than 15 at all follow-up periods for both treatment arms.
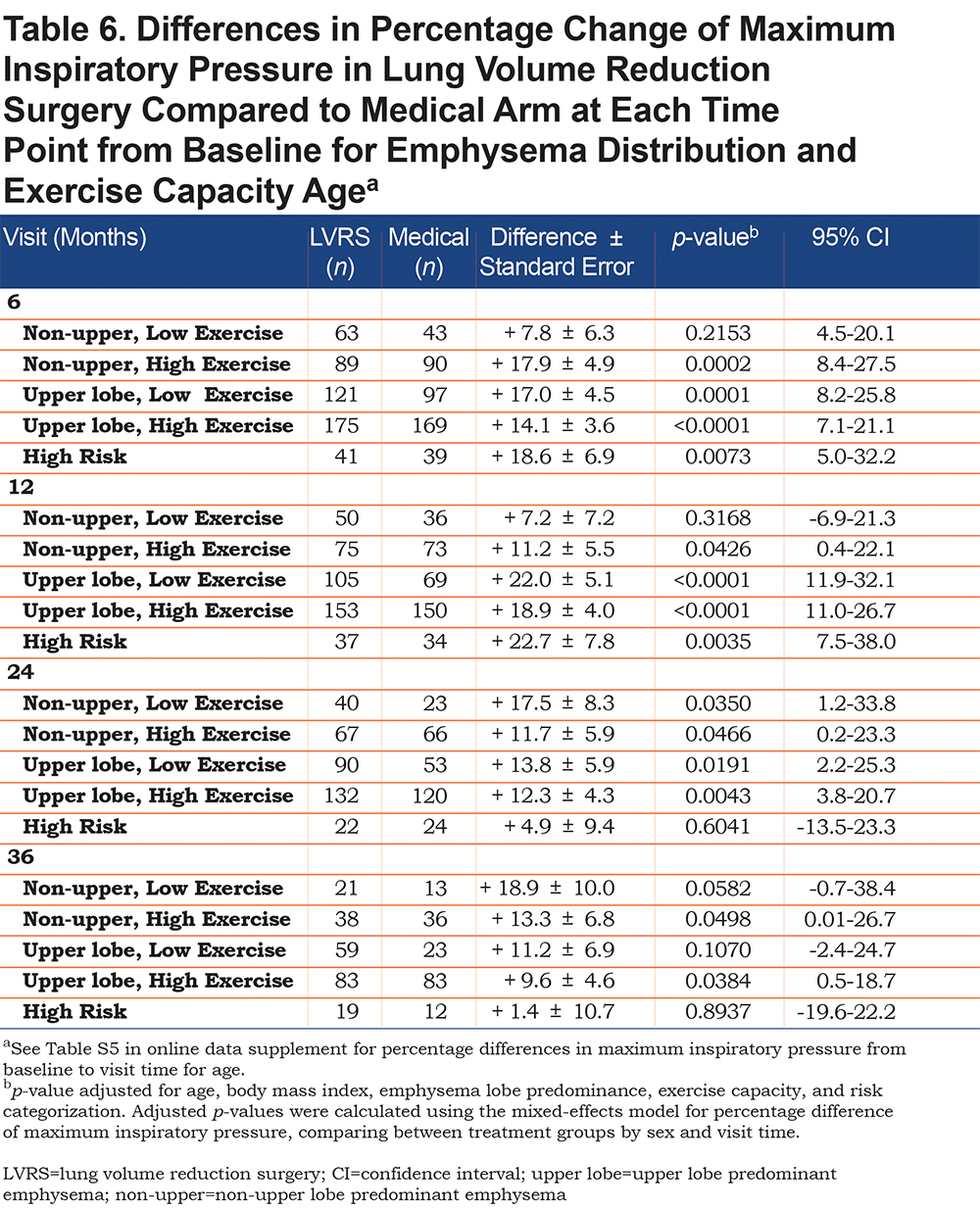
LVRS participants with upper lobe predominant emphysema and low exercise capacity had the greatest increase in MIP post procedure but was only significant at up to 24 months (increase of 22.0 ± 5.1%, p<0.0001, 95% CI [11.9-32.1]). Non-upper lobe emphysematous participants with low exercise capacity did not have a consistently significant greater increase in MIP in the LVRS arm when compared to the medical arm. Regardless of emphysema distribution, LVRS participants with high exercise capacity had significantly greater MIP increases than those in the medical arm at all follow-up periods (increase of 11.2 ± 5.5%, p=0.0426, 95% CI [0.4-22.1] in non-upper lobe emphysema and increase of 18.9 ± 4.0%, p<0.0001, 95% CI [11.0-26.7] in upper lobe predominant emphysema; Table 6). High risk participants in the LVRS arm had a significantly greater increase in MIP compared to the medical arm but only at up to 12 months follow-up (increase of 22.7 ± 7.8%, p=0.0035, 95% CI [7.5-38.0]).
BMI did not affect change in MIP for either treatment arm at any follow-up period (Tables S3 and S4 in online data supplement).
Discussion
Our data confirms findings of previous studies that found LVRS to increase inspiratory muscle strength, however with smaller study populations and follow-up durations. Martinez and colleagues found MIP to significantly increase by 22% at 3 months post-LVRS in a study of 17 patients with severe emphysema and hyperinflation with debilitating symptoms.14 Criner and colleagues found in 20 patients with severe, non-bullous and diffuse emphysema who underwent LVRS that MIP significantly increased from baseline by 48% and was significantly greater than medical management at 3 months post-operatively.11 Other indicators of inspiratory muscle strength, including transdiaphragmatic pressure during maximum sniff, electrophrenic diaphragm twitch stimulation, and combined expulsive-Mueller maneuver, were also greater at 3 months post-surgery. Teschler and colleagues also found MIP to significantly increase by 52% at 1-month post-LVRS in a study of 17 patients with severe emphysema and dyspnea on minimal exertion.19
Lammi et al previously found that LVRS leads to a significant reduction in dynamic hyperinflation up to 3 years post-operatively in a small subgroup of upper lobe-predominant emphysema patients enrolled in NETT.20 Importantly, our analysis further supports this prior finding by demonstrating significant reductions in TLC, RV, FRC and RV/TLC at all follow-up time points. We suggest that the aforementioned significant increase in MIP seen at all time points in LVRS was likely due to inspiratory muscles returning to their optimal length-tension relationship, rather than their inherent strengthening.
Men who underwent LVRS, rather than women, had overall greater and longer lasting increases in MIP compared to medical only therapy. It has been previously reported that for the same age and regardless of underlying disease state, women have about 25% to 30% lower respiratory muscle strength than men.21-23 Nonetheless, as women age, they have less decline in respiratory muscle strength than men for reasons that remain unclear.22 We may have seen greater increases in respiratory muscle strength in men because at baseline, male MIP was already greater than female MIP. Our findings suggest that LVRS may temporarily reverse the overall more rapid decline in respiratory muscle strength that has been seen in men.
Respiratory muscle strength declines with age18,21-23 ; Enright et al reported a 0.8 to 2.7 cm H2O per year decline in MIP in patients 65- to 85-years-old.21 This is likely due to natural atrophy of respiratory muscles with age, which is likely exacerbated by an underlying emphysematous state. However, we found that for all age groups, LVRS significantly reversed this decline at up to 1 year post-operatively and those who were 65- to less than 70-years-old had sustained increase at up to 3 years post-operatively. The significant improvement in respiratory muscle strength in the older participant group further supports that LVRS alters respiratory mechanics to restore the diaphragm and zone of apposition towards normal, causing an improvement in dyspnea, minute ventilation and exercise capacity.11,13-15
We found that LVRS participants with low exercise capacity and upper lobe predominant emphysema who underwent LVRS had significant increases in respiratory muscle strength at up to 24 months but those with similar exercise capacity, but non-upper lobe predominant emphysema, did not have a consistent trend in respiratory muscle strengthening at any time point. This finding agrees with prior studies that found that upper lobe emphysema leads to greater improvement in lung function after LVRS.24,25 It also correlates with the NETT trial, which found that those with low exercise capacity and upper lobe predominant emphysema had significant mortality benefit with LVRS and significant increases in exercise capacity at 24 months; they also found that those with low exercise capacity and non-upper lobe predominant emphysema had no difference in mortality and exercise capacity at 24 months.17
The NETT trial also found that those with high exercise capacity and upper lobe predominant emphysema had no mortality benefit with LVRS but did have significant improvement in exercise capacity at 24 months and those with similar exercise capacity but non-upper lobe predominant emphysema had greater risk of death with LVRS but no difference in exercise capacity at 24 months.17 Regardless of lobe predominance, we found that participants with high exercise capacity who underwent LVRS had significant increases in inspiratory muscle strength compared to the medical arm at up to 3 years follow-up. This suggests that although LVRS optimizes respiratory muscle function, this improvement in inspiratory muscle strength does not correlate to a mortality benefit. This is because low, rather than high, exercise capacity is a predictor of survival in LVRS individuals as their counterparts in the medical arm continue to functionally decline without surgery, as found by NETT.17 This finding, found in both the NETT trial and our study, is difficult to explain as it conflicts with the BODE (BMI, degree of airflow obstruction, dyspnea and exercise capacity) index, which shows that greater exercise capacity is associated with lower mortality.26
We assumed that participants with BMI < 25 kg/m2 had greater respiratory muscle atrophy than those with BMI ≥ 25 kg/m2 and thus, expected that the former would have only negligible increases in respiratory muscle strength after LVRS. However, we found that regardless of BMI, respiratory muscle strength increased at all follow-up periods. This confirms prior conclusions that inspiratory pressure also relies on the diaphragm contracting at an optimal length-tension curve, rather than solely on the inherent strength of the diaphragm muscle.10,12
A significant study limitation is that RMS measurements were obtained volitionally, making it difficult to interpret if MIP was truly low because of weakened respiratory muscles or falsely low because participants were not making maximal effort. Although the diaphragm is the dominant respiratory muscle, MIP is a global measurement of all inspiratory muscles. Therefore, from our results, we cannot imply that an increase in MIP is directly proportional to an increase in diaphragm strength, but rather may reflect an increase in external intercostal and accessory muscle strength. Additionally, although we found an association between a reduction in TLC, RV, FRC and RV/TLC and a corresponding increase in MIP at all follow-up periods, we cannot imply a direct and causal relationship that a reduction in hyperinflation led to an increase in inspiratory muscle strength.
An additional study limitation was that there was significant decrease in participants after 36 months, whether due to loss to follow-up or death. Therefore, despite having data at up to 5 years post-operatively, the lack of a large study size after 3 years makes it difficult to predict respiratory muscle strength beyond this time frame. As people continue to live longer, more adults will be living with emphysema at older ages. However, we were unable to analyze the effects of LVRS on respiratory muscle strength in those older than 70-years-old due to low sample size.
We report the effects of LVRS on inspiratory muscle strength in patients with advanced emphysema using data gathered from the largest and longest controlled trial to study this subject to date. Our analysis showed that LVRS significantly increases inspiratory muscle strength up to 3 years post-operatively, compared to optimal medical management for severely emphysematous patients. We found that an increase in inspiratory muscle strength was associated with a decrease in non-invasive markers of dynamic hyperinflation, suggesting that LVRS allows the inspiratory muscles to return to their optimal length-tension relationship. Additionally, male sex, age 65- to 70-years-old and low exercise capacity with upper lobe predominant emphysema were associated with increased inspiratory muscle strength after LVRS.
The significant and durable increase in MIP following LVRS may be an additional physiological explanation for the increase in exercise tolerance and reduction in dyspnea compared to medical therapy alone. MIP is a simple, affordable and non-invasive pulmonary measurement and thus, could be used as an indirect marker for dynamic hyperinflation in post-LVRS patients.
Acknowledgements
Author contributions: Daohai Yu performed the data analysis. Rachel N. Criner and Gerard J. Criner interpreted the data. Rachel N. Criner drafted the manuscript. Rachel N. Criner, Gerard J. Criner, and Michael R. Jacobs revised the final draft. All authors approved the final version to be published.
The authors thank Matthew R. Maisel, BS, MS, for his assistance with data transformation. Clinical Trial: NCT00000606
Declaration of Interest
Dr. Gerard J. Criner received grants from the National Institutes of Health and the Department of Defense; he consults for AstraZenaca, Boehringer Ingelheim, Holaira, Mereo, Third Pole, PneumoRx, Pulmonx, Pearl, Amirall, CSA Medical, Broncus, AVISA, Lungpacer and GlaxoSmithKline. He has also contracted clinical trials from AstraZeneca, Avisa, Mereo, Boehringer Ingelheim, Broncus, GlaxoSmithKline, Lungpacer, Pulmonx, PneumoRx/BTG and Yungjin. All other authors had nothing to declare.