Running Head: Efficacy and Safety of Nebulized GLY by CV risk
Funding Support: The GOLDEN studies were funded by Sunovion Pharmaceuticals Inc. Medical writing support was funded by Sunovion Pharmaceuticals Inc.
Date of Acceptance: September 30, 2018
Abbreviations: cardiovascular, CV; glycopyrrolate, GLY; chronic obstructive pulmonary disease, COPD; Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer, GOLDEN; tiotropium, TIO; patient-reported outcome, PRO; treatment-emergent adverse event, TEAE; cardiovascular disease, CVD; long-acting muscarinic antagonist, LAMA; Global initiative for chronic Obstructive Lung Disease, GOLD; electrocardiogram, ECG; major adverse cardiovascular event, MACE; twice daily, BID; long-acting beta2-agonist, LABA; once daily, QD; inhaled corticosteroid, ICS; forced expiratory volume in 1 second, FEV1; St George’s Respiratory Questionnaire, SGRQ; Evaluating Respiratory Symptoms in COPD, E-RS; mixed model for repeated measures, MMRM; intent to treat, ITT; myocardial infarction, MI; incidence rate, IR; total exposure rate in years, ET; QT corrected for heart rate using Friedericia’s method, QTc-F; standard error, SE; odds ratio, OR; confidence interval, CI
Citation: Ferguson GT, Tosiello R, Sanjar S, Goodin T. Efficacy and safety of nebulized glycopyrrolate/eflow® closed system in patients with moderate-to-very-severe chronic obstructive pulmonary disease with pre-existing cardiovascular risk factors. Chronic Obstr Pulm Dis. 2019; 6(1): 86-99. doi: http://doi.org/10.15326/jcopdf.6.1.2018.0146
Online Supplemental Material: Read Online Supplemental Material (278KB)
Introduction
The crude prevalence of unspecified cardiovascular disease (CVD) in patients with chronic obstructive pulmonary disease (COPD) ranges from 28% to 70%.1 The risk of CVD increases proportionally with the severity of airflow limitation, which is associated with endothelial dysfunction and results in cardiovascular morbidity.1-5 Cardiovascular (CV) events are the predominant cause of hospitalizations in patients with COPD, accounting for approximately 50% of all hospitalizations and 20% to 25% of all deaths.6
Inhaled bronchodilators are the cornerstone treatments for patients with COPD, with long-acting muscarinic antagonists (LAMA) recommended as initial therapy in the Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines.7 Due to the high proportion of COPD patients with CV comorbidities and the established relationship between some COPD treatments and increased CV risk, evaluation of the CV safety of COPD treatments, particularly in patients with existing CV risk factors, is important.8-14 Findings from recent randomized clinical trials in COPD are generally reassuring with respect to the cardiac safety of LAMAs, with multiple studies showing that treatment with tiotropium (TIO) and aclidinium bromide did not increase the risk of CV events or mortality.8,15-17 However, in patients with underlying CV risk factors, the incidence of several CV events and post-baseline electrocardiogram (ECG) abnormalities were higher with umeclidinium compared to placebo.18 Recently, GEM1, GEM2, FLIGHT1, and FLIGHT2 studies19-21 have shown that glycopyrrolate (GLY) delivered via dry powder inhaler had similar major adverse CV event (MACE) rates compared with placebo and no treatment-related CV mortality.22
The phase III Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer (GOLDEN) studies were conducted in patients with moderate-to-very-severe COPD to evaluate nebulized GLY versus placebo (GOLDEN 3 and GOLDEN 4, 12-week studies) and versus TIO (GOLDEN 5, 48-week study).23 Treatment with nebulized GLY demonstrated statistically significant and clinically important improvements in pulmonary function and patient-reported outcomes (PROs), and had no major safety signals.23 Based in part on the phase III GOLDEN studies, the U.S. Food and Drug Administration approved nebulized GLY (LONHALA® 25 mcg twice daily [BID]; Sunovion Pharmaceuticals Inc, Marlborough, Massachusetts ), delivered by the innovative eFlow® Closed System nebulizer (MAGNAIR®; PARI Pharma GmbH, Starnberg, Germany), for the long-term maintenance treatment of airflow obstruction in patients with COPD, including chronic bronchitis and/or emphysema.24
The safety and efficacy of nebulized GLY in patients in whom CV risk factors are present has not been previously reported. In addition, patients with diagnosed CVD as compared to those with hypertension without other documented CVD, and those using other concomitant inhaled medications associated with increased CV risk, such as long-acting beta2-agonists (LABAs), could have even greater risk. Given the high prevalence of CVD in patients with COPD, the phase III GOLDEN studies were prospectively designed to include patients with pre-existing CVD, CV risk factors, and concomitantly administered LABA therapies to determine the efficacy and safety of nebulized GLY. This secondary analysis is the first report of the safety and efficacy of nebulized GLY in patients with pre-existing CV risk factors.
Methods
Study Design
The phase III GOLDEN studies have been previously described.23 Briefly, in the 12-week, multicenter, randomized, placebo-controlled, double-blind studies (GOLDEN 3 and GOLDEN 4), 1294 patients were randomized (1:1:1) to receive placebo or GLY (25 mcg or 50 mcg BID) via the eFlow® CS nebulizer.23 GOLDEN 5 was a 48-week, multicenter, randomized, open-label, active-controlled, long-term safety study comparing nebulized GLY to TIO.23 In total, 1087 patients were randomized (4:3) to GLY 50 mcg BID or TIO 18 mcg daily (QD).23 Approximately 30% of patients (limited by protocol) in GOLDEN 3 and GOLDEN 4, and approximately 40% in GOLDEN 5, were permitted to continue background LABA therapy (with or without a concomitant inhaled corticosteroid [ICS]) during the treatment period. Albuterol (salbutamol) or supplemental ipratropium were permitted as rescue medication, as was supplemental ipratropium for selected patients.
Patients
Key eligibility criteria for all phase III studies included males or females ≥ 40 years of age, current or ex-smokers with a ≥ 10 pack-year smoking history, a clinical diagnosis of moderate-to-very-severe COPD (as defined by the GOLD 2014 criteria), and qualifying post-bronchodilator (ipratropium 68 mcg) spirometry (forced expiratory volume in 1 second [FEV1] ≤ 80% of predicted normal, FEV1 > 0.7L, and FEV1/forced vital capacity ratio <0.70).23 Patients with severe comorbidities and current or history of unstable cardiac or pulmonary disease were excluded.23
The phase III GOLDEN study protocols were approved by the institutional review boards prior to patient enrollment and were conducted in accordance with the protocols, International Council for Harmonization Good Clinical Practice guidelines, and Declaration of Helsinki. All patients provided written informed consent before undergoing any study procedures.23
Statistical Analysis
CV risk was assessed, and patients were stratified into high or low risk categories prior to randomization to study treatment. High CV risk was determined based on a history of one or more of the following pre-specified disorders: ischemic heart disease, cerebrovascular disease, peripheral arterial disease, clinically significant arrhythmia (defined as any arrhythmia for which the patient was receiving or had received medication, or an interventional procedure, or identified by Holter monitoring at screening), heart failure, or hypertension. Additional analyses were performed: (1) excluding patients in the high CV risk subgroup with hypertension alone (to focus on patients with documented CVD); and (2) in patients receiving background LABA during the studies (to assess the impact of LAMA with concomitant LABA usage).23 All statistical procedures were performed using SAS® v9.2 (SAS Institute Inc., Cary, North Carolina). Statistical analyses were only performed for the primary efficacy endpoints, while safety data were analyzed using descriptive statistics.
12-Week, Placebo-Controlled Studies
Data from the 12-week, placebo-controlled GOLDEN 3 and GOLDEN 4 studies were pooled for analysis. The primary efficacy endpoint was change from baseline in trough FEV1 at Week 12. Other efficacy endpoints included change from baseline in health status, measured by St George’s Respiratory Questionnaire (SGRQ)25 total score at Week 12, SGRQ responder rate (defined as a patient with a ≥ 4.0 unit reduction in SGRQ total score), change from baseline in Evaluating Respiratory Symptoms in COPD (E-RS™, Evidera Inc., Bethesda, Maryland) total score, and incidence of exacerbations.
FEV1 was analyzed using a mixed-model for repeated measures (MMRM). SGRQ total score was analyzed by analysis of covariance. SGRQ responders were analyzed with odds ratios from a logistic regression model. E-RS total scores were recorded daily by patients using an electronic diary and were analyzed using the same MMRM model as for FEV1, except that baseline E-RS total score was included as a covariate.
The intent-to-treat (ITT) and safety populations consisted of all patients receiving ≥ 1 dose of study drug and, for efficacy, 1 post-dose pulmonary function assessment. Changes from baseline in the efficacy assessments were evaluated for all patients who received the double-blind study drug, regardless of their completion status. Patients who discontinued the randomized treatment before Week 12, but continued to be followed, were analyzed using retrieved drop-out data (all collected data). Efficacy and safety analyses were performed using on-treatment and all collected data, and analyses indicated the endpoint results were similar in both datasets. On-treatment data are presented.
48-Week, Active-Controlled Study
Primary endpoints were the incidence of treatment-emergent adverse events (TEAEs), serious TEAEs, and discontinuations due to TEAEs. Other safety endpoints included the incidence of MACE, including CV death, ischemia/infarction, and stroke. Efficacy endpoints included change from baseline at 48 weeks in trough FEV1, SGRQ total score and E-RS total score, and incidence of COPD exacerbations. An analysis of covariance model and/or MMRM was utilized for the selected efficacy endpoints as per the 12-week studies.
Safety analyses were conducted using the safety population, and efficacy analyses using the ITT population, both consisting of all patients who were randomized to treatment and received ≥ 1 dose of study drug.
Safety Analyses
Safety data were analyzed using descriptive statistics. TEAEs were coded according to MedDRA v15.1. CV events of special interest were examined using Standardized Medical Dictionary for Regulatory Activities Query analysis, and included cardiac arrhythmia, arrhythmia-related events, cardiac failure, QT prolongation, and myocardial infarction (MI). Vital signs (including heart rate and blood pressure), 12-lead ECG, and clinical laboratory measures were also assessed. Holter monitoring was carried out at screening in all studies, and at Week 12 in a subpopulation of patients in GOLDEN 3.
Results
Patient Demographics and Baseline Characteristics
Baseline characteristics of the 1293 patients included in GOLDEN 3 and GOLDEN 4, and the 1086 patients in GOLDEN 5, based on CV risk status, are shown in Table 1. In total, 1526 patients had high CV risk and 853 had low CV risk. The most common CV risk factors across the studies were hypertension (58.1% to 61.2% of total population), ischemic heart disease (9.4% to 12.3%), and peripheral arterial disease (3.9% to 7.2%). High CV risk patients were older than those with low CV risk (64.3 to 65.9 versus 59.5 to 60.7 years of age) and there was a higher proportion of males in the high CV risk subgroup compared with low CV risk subgroup (57.1% to 60.7% versus 48.5% to 54.5%) in the 12-week and 48-week studies. In addition, the high CV risk subgroups in the 12-week placebo-controlled studies included a higher proportion of African Americans than the low CV risk subgroups (10.1% to 11.6% versus 7.1% to 7.6%, respectively); there was also a higher proportion of African Americans in the high CV risk subgroup receiving nebulized GLY in the 48-week, active-controlled study but not in patients receiving TIO (6.2% and 5.4%, respectively, versus 4.6% and 6.5% in the low CV risk subgroups).
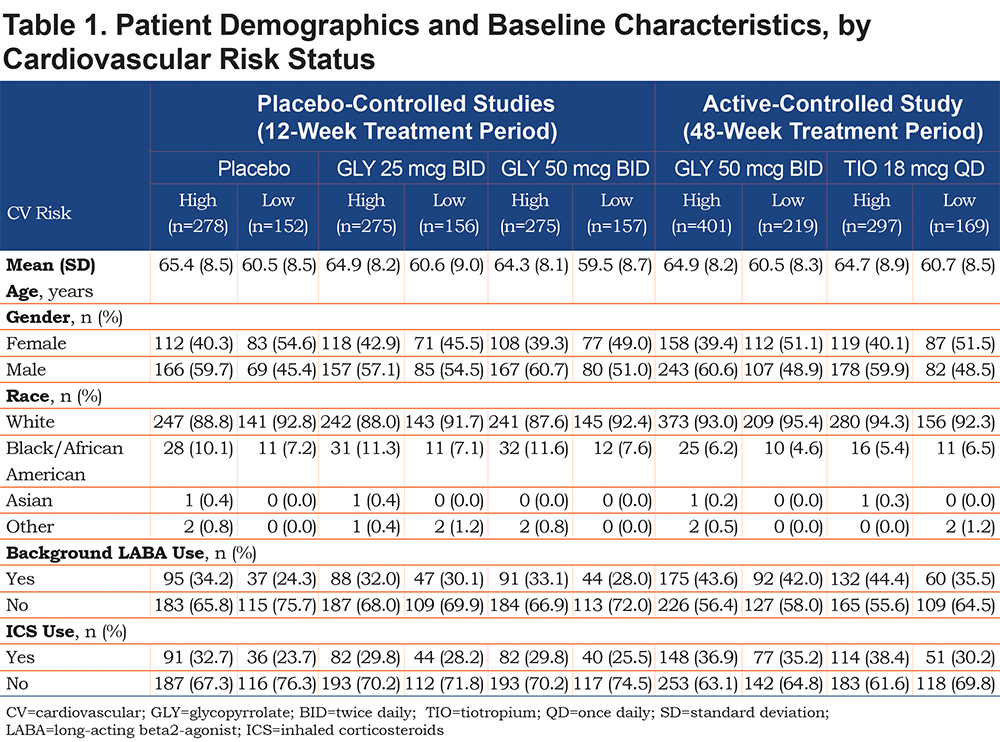
When patients with hypertension only were excluded from the high CV risk group, 495 patients (placebo: n=94; GLY: n=314; TIO: n=87) were classified as higher CV risk; demographics in this sub-population are shown in Table S1 in the online supplement. The demographics of patients in this higher CV risk population were generally consistent with the overall high CV risk population, with mostly older, male, and white patients.
Safety
Adverse Events
TEAEs occurring in ≥ 2% of patients by CV risk status are shown in Table 2. The overall incidence of TEAEs was similar between treatments and across CV risk subgroups in the 12-week studies and in the 48-week study, Table 2. In the 12-week studies and the 48-week study, the overall incidence of TEAEs was similar among patients with high CV risk when patients with hypertension only were excluded (Table S2, online supplement).
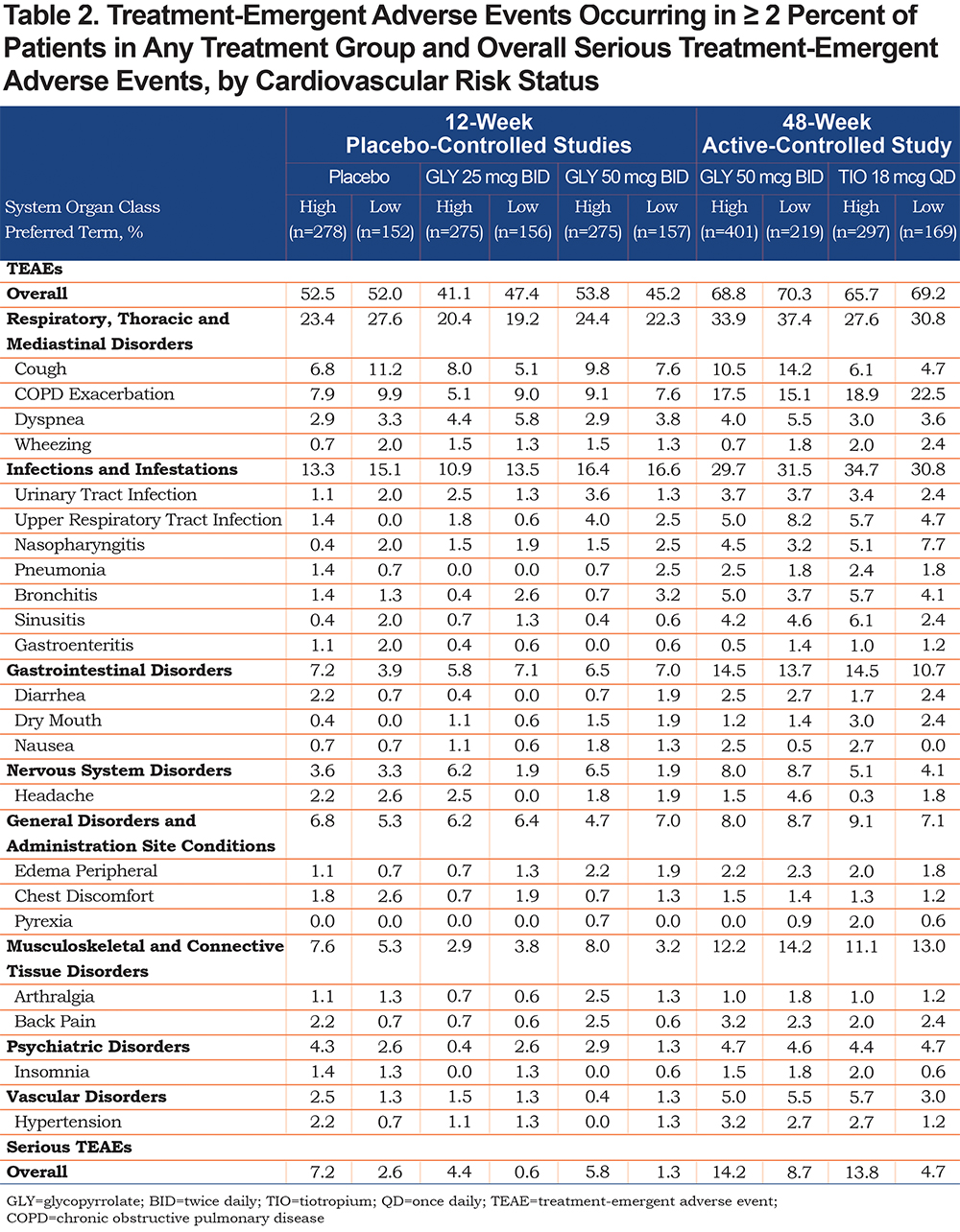
The most common TEAEs were cough and COPD exacerbation; rates were generally similar across treatments and CV subgroups except for cough in the 48-week study, which was higher with nebulized GLY compared with TIO in both CV risk subgroups (Table 2). When patients with hypertension only were excluded from the high CV risk subgroup, the most common TEAEs remained cough and COPD exacerbations, occurring to a similar extent in the high CV risk subgroup, except in the 48-week study among patients receiving GLY 50 mcg BID (Table S2, online supplement). The incidence of cough and bronchitis increased from 10.5% to 16.0% and 5.0% to 8.0%, in the high CV risk subgroup without and with hypertension, respectively. Incidences of other TEAEs in patients, with hypertension only excluded, were also modified as compared to the larger high CV risk group (Table S2, online supplement), but without any notable changes in TEAE events or patterns.
Serious Adverse Events and Adverse Events Leading to Discontinuation
The incidence of serious TEAEs was higher in the high CV risk subgroup compared with the low CV risk subgroup in the 12-week, placebo-controlled studies (Table 2); the highest incidence of serious TEAEs was among patients receiving placebo in both CV risk subgroups (Table 2). The incidence of serious TEAEs was lower among patients receiving GLY 25 mcg BID and higher among patients receiving placebo or GLY 50 mcg BID in the high CV risk subgroup when patients with hypertension only were excluded (Table S2, online supplement).
Similarly, in the 48-week, active-controlled study, the incidence of serious TEAEs was higher in the high CV risk versus low CV risk subgroup (Table 2). The rate of serious TEAEs was similar between nebulized GLY and TIO in the high CV risk subgroup, but higher with nebulized GLY versus TIO in the low CV risk subgroup (Table 2). When the subgroup of patients with hypertension only were excluded from the high CV risk group, the incidence of serious TEAEs in patients receiving GLY 50 mcg BID remained unchanged compared with the larger group comprising all high CV risk patients (Table S2, online supplement). By contrast, in patients receiving TIO, the incidence of serious TEAEs among the high-risk CV subgroup with hypertension only excluded was higher than the high-risk CV group (Table S2, online supplement).
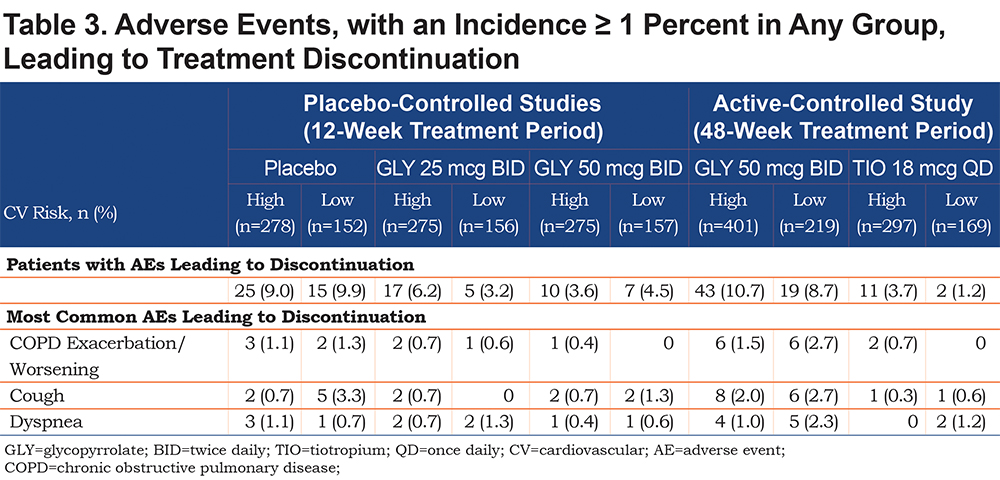
At 12 weeks, rates of AEs leading to discontinuation were similar in high and low CV risk subgroups, and numerically lower in patients receiving nebulized GLY compared with placebo (Table 3). At 48 weeks, discontinuations due to AEs were higher in the high CV risk subgroup than the low CV risk subgroup and greater for nebulized GLY versus TIO (Table 3).
CV Events of Special Interest
In the 12-week, placebo-controlled studies, the rate of CV events of special interest was similar between nebulized GLY doses, regardless of CV risk status, but was highest in patients receiving placebo in the high CV risk subgroup (Table 4). The rates of CV events of special interest were increased in the high CV risk subgroups with hypertension only excluded (3.2%, 4.9%, 4.3% with GLY 25 mcg, GLY 50 mcg BID, and placebo, respectively). However, there was no increased risk with GLY use compared with placebo in this subgroup. The incidence of MACE was low and similar among patients receiving placebo or GLY 50 mcg BID, while no MACE were identified among patients receiving GLY 25 mcg BID in either the high or low CV risk subgroups (Table 4). When the subgroup of patients with hypertension only were excluded from the high CV risk subgroup, the number of MACE in the GLY 50 mcg BID group was unchanged, whereas no events were observed in the placebo group.
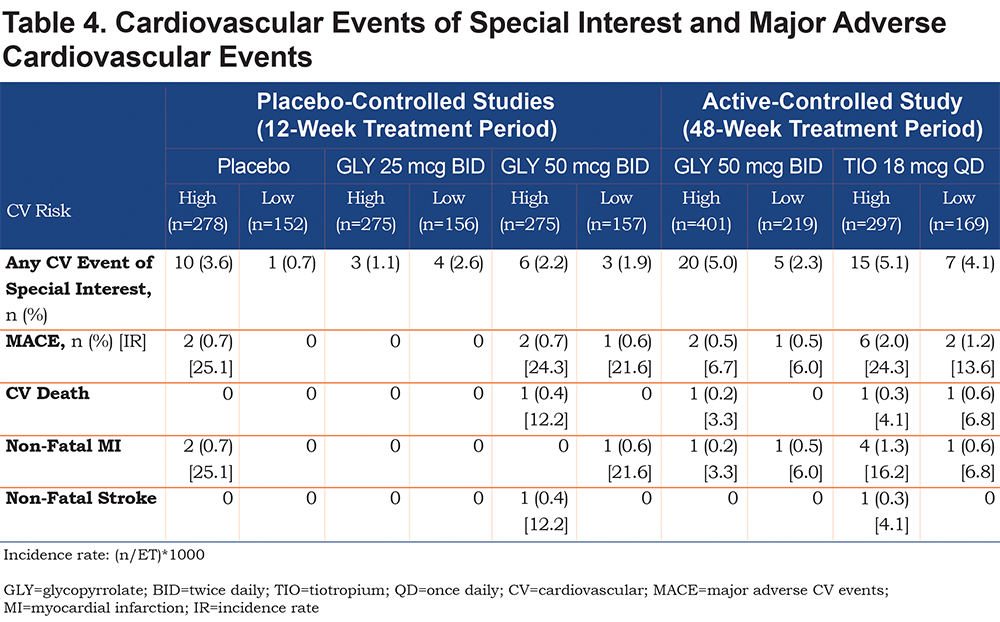
Unlike the 12-week trials, in the 48-week study, the incidence of CV events of special interest was increased in the high CV risk compared with the low CV risk group (Table 4). The rate of CV events of special interest was further increased in the high CV risk subgroup with hypertension only excluded (8.0% and 6.9%, with GLY and TIO, respectively). Incidence of MACE was lower with nebulized GLY than with TIO for both high and low CV risk subgroups (Table 4); in the subgroups of high CV risk patients with hypertension only excluded, there was 1 MACE (non-fatal stroke) in a patient receiving TIO, and none with nebulized GLY.
In addition to CV risk status, patients were further analyzed according to background LABA use (Table S3, online supplement). A subset of patients in both CV risk groups received background LABA (Table 1; patients with background LABA: high CV risk, range 32.0% to 44.4%; low CV risk, range 24.3% to 42.0%). In the 12-week, placebo-controlled studies, CV events of special interest generally occurred more frequently among patients at high CV risk not receiving background LABAs compared with those who did (Table S3, online supplement), whereas the incidence in low CV risk patients was greater among patients receiving placebo or GLY 50 mcg BID and background LABAs (Table S3, online supplement). MACE were only observed among patients in the placebo high CV risk group and GLY 50 mcg BID high and low CV risk groups who did not receive background LABAs (Table S3, online supplement). In the 48-week, active-controlled study, CV events of special interest were more common among patients not using background LABAs compared to those receiving background LABAs in both CV risk subgroups (Table S3, online supplement). MACE occurred only among patients not receiving background LABAs in the high and low CV risk subgroups treated with GLY 50 mcg BID (Table S3, online supplement). In patients receiving TIO, the incidence of MACE in the high CV risk subgroup was higher in those using background LABAs versus those who did not, whereas in the low CV risk subgroup, MACE occurred only in patients not receiving background LABAs (Table S3, online supplement).
Changes in Vital Signs and ECG Parameters
No clinically meaningful changes were observed in vital signs or ECG parameters, regardless of CV risk subgroup. In the 12-week studies, changes from baseline in QT corrected for heart rate using Fridericia's method (QTc-F; ≥ 30ms for at least 1 post-baseline measurement, but < 60 ms for all post-baseline measurements) were greater in the high CV risk versus low CV risk subgroup; within the high CV risk subgroup, change in QTc-F was greater with nebulized GLY 50 mcg BID compared with placebo (high CV risk: 9.1%, 6.2%, 4.3%; low CV risk: 3.4%, 2.1%, 3.6%, with GLY 25 mcg BID, GLY 50 mcg BID, and placebo, respectively). In the 48-week study, overall changes from baseline in QTc-F were similar among patients treated with nebulized GLY or TIO in both CV risk subgroups (high CV risk: 9.7%, 10.5%; low CV risk: 10.4%, 8.1%, with GLY and TIO respectively).
Efficacy
At 12 weeks, nebulized GLY produced statistically significant, clinically important improvements from baseline in placebo-adjusted trough FEV1 in both high and low CV risk subgroups (p<0.001 for all comparisons to placebo; Figure 1A). The overall change from baseline in trough FEV1 over 48 weeks was similar between nebulized GLY and TIO treatment groups regardless of CV risk (Figure 1B).

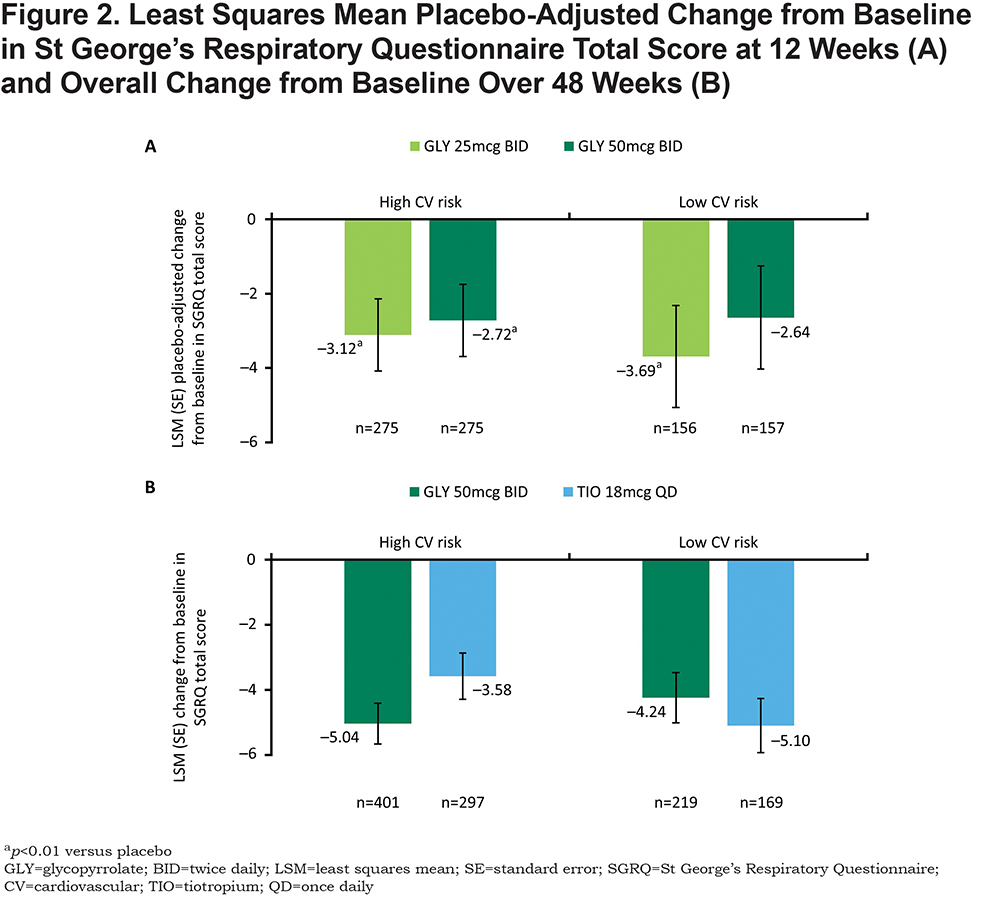
At 12 weeks, nebulized GLY produced statistically significant improvements in placebo-adjusted SGRQ total score in the high CV risk subgroup (p<0.01 for both GLY doses versus placebo in the high CV risk subgroup, p<0.01 for GLY 25 mcg BID versus placebo in the low CV risk subgroup; Figure 2A). Over 48 weeks, change from baseline in SGRQ total score was numerically higher for GLY versus TIO in the high CV risk subgroup but lower in the low CV risk subgroup (Figure 2B). SGRQ responder rates were similar between treatments and across CV risk subgroups at 12 weeks. Responder rates were higher with nebulized GLY compared with placebo (low CV risk: 48.1%, 42.8%, and 34.6%; high CV risk: 44.5%, 39.7%, and 34.4%, for GLY 25 mcg, GLY 50 mcg BID, and placebo, respectively), with significant improvement observed in the GLY 25 mcg BID high CV risk subgroup (odds ratio [OR] = 1.765; 95% confidence interval [CI]: 1.207 to 2.581). At 48 weeks, the proportion of SGRQ responders in the high CV risk subgroup was greater with nebulized GLY versus those receiving TIO but lower in the low CV risk group (high CV risk: 47.3% versus 39.1%; low CV risk: 48.5% versus 55.6%, respectively).
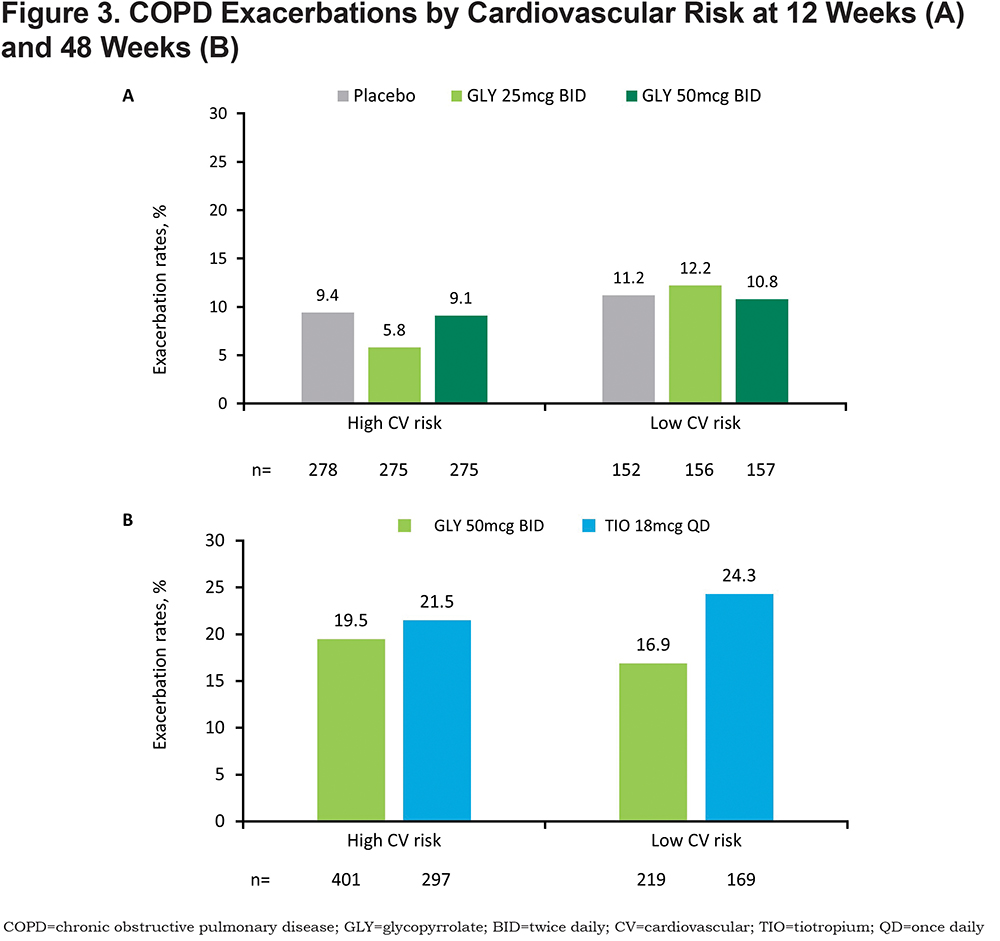
In the 12-week, placebo-controlled studies, exacerbations were reported in higher percentages of patients at low CV risk compared with those at high CV risk (Figure 3A). In the 48-week, active-controlled study, exacerbation rates were higher in patients receiving TIO compared with those receiving nebulized GLY in the low CV risk subgroup (Figure 3B). Exacerbations in all treatment groups were mostly moderate in severity.
Discussion
The CV safety of LAMAs has been extensively studied. Results from studies of TIO, umeclidinium, aclidinium, and GLY inhalation powder suggest that treatment with LAMAs does not increase the incidence of CV events or CV mortality.15-18,22,26 Our results with nebulized GLY in the GOLDEN studies, which included COPD patients with pre-existing CVD and using background LABAs, appear consistent with larger studies with handheld therapies and contribute additional evidence supporting the CV safety of LAMAs.
While there were differences in baseline characteristics between the high and low CV risk subgroups, including older age and more males in the high CV risk subgroup, these differences are consistent with the general COPD population.27,28 In addition, there was a higher proportion of African Americans in the high CV risk subgroup compared to low CV risk subgroup, which is also in agreement with previous studies.29 Excluding patients with hypertension only from the high risk CV group identified a subgroup of patients with known CVD who may be at even greater CV risk than the overall high CV risk group. This distinction is important because hypertension alone is not a definitive CV risk factor,30 with patients having “mild hypertension” not considered to be at high CV risk, unless other confounding risk factors are present. The “higher CV risk” subgroup had a similar mean age to the overall high CV risk group but included an even greater proportion of males, consistent with the higher incidence of CV risk factors in males.23,28 Differences between the high CV risk population and this “higher CV risk” sub-population are consistent with the increasing incidence of CVD with age. Importantly, the safety profile of nebulized GLY was similar regardless of patient CV risk status at baseline.
In patients with and without pre-existing CV risk factors in the phase III GOLDEN studies, the incidence of TEAEs was similar across treatments and CV risk subgroups. The overall incidence of TEAEs in the “higher CV risk” subgroup was similar to the high CV risk group. The most common TEAEs, occurring in ≥ 2% of patients, were cough and COPD exacerbations. In general, the incidence of serious TEAEs was higher in patients with high versus low CV risk, irrespective of treatment, with no increase in TEAEs in those receiving nebulized GLY compared to placebo over 12 weeks or compared to TIO over 48 weeks. In the higher CV risk subgroup excluding patients with hypertension only, no additional risk for serious TEAEs was seen with nebulized GLY, whereas patients treated with TIO had a higher incidence of serious TEAEs in the 48-week trial. Importantly, in patients with high CV risk, concomitant use of a LABA with nebulized GLY did not add to CV risk.
The rates of discontinuations due to TEAEs with nebulized GLY were similar between the high and low CV risk groups in the 12-week, placebo-controlled studies, but increased in the high CV risk group in the 48-week, active-controlled study. The higher rate of discontinuations due to TEAEs with nebulized GLY compared with TIO in the 48-week study may have been due in part to the open-label study design and prior use of COPD medications, particularly TIO; the increased incidence of cough may be attributable to incorrect nebulizer inhalation technique as > 90% of patients were naïve to nebulizer therapy while approximately 30% received TIO for ≥ 2 years prior to randomization, both of which may have introduced bias contributing to TEAE-related discontinuations.23
CV risk status did not impact the incidence of CV events of special interest or MACE. In addition, lung function parameters and PROs improved in patients receiving nebulized GLY, regardless of CV risk status. These improvements in lung function and health-related quality of life measurements were clinically important and significantly greater than with placebo (except for changes from baseline in SGRQ for patients with low CV risk receiving GLY 50 mcg BID in the 12-week, placebo-controlled studies), while changes from baseline with GLY were comparable to TIO in the 48-week, active-controlled study.
A recent report suggests that LABA/LAMA and LABA/LAMA/ICS combinations may have CV safety outcomes similar to placebo or monotherapies, respectively.10 However, the results of our additional analysis by background LABA use suggested that the rate of CV events of special interest and MACE was higher among patients not receiving background LABAs. In the 48-week, active-controlled study, MACE incidence was higher among TIO patients with high CV risk receiving background LABA therapy. These data suggest the need for additional analyses to assess the impact of combination therapies on CV events in patients with COPD.
The GOLDEN studies were prospectively designed to include patients with CV risk factors; however, patients with unstable CVD and/or long QT syndrome were excluded. Clinical trials and real-world studies of handheld and nebulized GLY treatment in patients with severe CV risk would extend our understanding of the CV safety of LAMAs in patients who are currently under-represented in the clinical trial setting.
Conclusions
Nebulized GLY is well tolerated, has no major safety signals, and improves lung function and health status (SGRQ) in moderate-to-very-severe COPD patients with and without pre-existing CV risk factors and background LABA therapy. These data provide important information to clinicians who manage patients with COPD and CV comorbidities.
Acknowledgements
Medical writing support was provided by Hashem Dbouk, PhD, of FireKite, an Ashfield company, part of UDG Healthcare plc and funded by Sunovion Pharmaceuticals Inc.
Author Contributions: GTF and RT contributed to protocol development and design. GTF was involved in data acquisition; GTF, RT, SS, and TG were involved in data analysis and interpretation. GTF, RT, SS, and TG were involved at all stages of manuscript development, writing and revision.
Data sharing statement: Sunovion Pharmaceuticals Inc. is part of a clinical trial data sharing consortium that facilitates access for qualified researchers to selected anonymized clinical trial data. For up-to-date information on data availability please visit https://www.clinicalstudydatarequest.com/Study-Sponsors.aspx and click on Sunovion.
Declaration of Interest
Gary T. Ferguson reports grants, personal fees, and non-financial support from Boehringer Ingelheim, AstraZeneca, and Sunovion Pharmaceuticals Inc., as well as grants and personal fees from Novartis, Pearl Therapeutics, and Theravance, grants from Forest, and personal fees from Meda, Verona, Mylan, Innoviva, and GlaxoSmithKline. Robert Tosiello, Shahin Sanjar, and Thomas Goodin are employees of Sunovion Pharmaceuticals Inc.