Running Head: Roflumilast Effect on COPD Rehospitalization
Funding Support: Investigator initiated grant funded by Forest Pharmaceuticals and Astra Zeneca. ClinicalTrials.gov Identifier: NCT01973998
Date of Acceptance: September 18, 2018
Abbreviations: chronic obstructive pulmonary disease, COPD; St George’s Respiratory Questionnaire, SGRQ; EuroQuality of Life Five Dimension scale, EQ5D; acute exacerbation of COPD, AECOPD; phosphodiesterase-4, PDE-4; cyclic adenosinemonphosphate, c-AMP; Global initiative for chronic Obstructive Lung Disease, GOLD; body mass index, BMI; forced vital capacity, FVC; short-acting beta-agonist, SABA; long-acting beta2-agonist, LABA; short-acting muscarinic antagonist, SAMA; long-acting muscarinic antagonist, LAMA; modified Medical Research Council, mMRC; white blood count, WBC; glycosylated hemoglobin, HgbA1C
Citation: Criner GJ, Jacobs MR, Zhao H, Marchetti N.Effects ofroflumilast on rehospitalization and mortality in patients hospitalized with a COPD exacerbation. Chronic Obstr Pulm Dis. 2019; 6(1): 74-85. doi: http://doi.org/10.15326/jcopdf.6.1.2018.0139
Introduction
Chronic obstructive pulmonary disease (COPD) afflicts 24 million U.S. residents and is the 4th leading cause of death.1,2 COPD exacerbations add considerably to that burden because they cause frequent hospitalizations and readmissions, contribute directly to the death of many patients, dramatically reduce quality of life, consume the majority of resources used to manage COPD, and may hasten the progressive loss of lung function.3,4 Acute exacerbations of COPD (AECOPD) account for 31% to 68% of the total costs of COPD care in the United States.5,6 Treatment that prevents or ameliorates frequent or severe AECOPD could significantly lessen COPD morbidity and mortality as well as costs.
Hospitalized exacerbations are particularly important in COPD patients because they profoundly impact patient survival, function, symptoms and health status as well as account for a significant component of COPD-related costs. A review of patients from the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study7 showed that a prior history of being hospitalized for an acute exacerbation was associated with the greatest risk of readmission (47%), and that 15% of patients reported multiple readmissions. Importantly, those with repeat hospitalizations had significantly increased mortality at 1 year.
The heightened inflammation that occurs during an acute exacerbation, especially a hospitalized exacerbation, may contribute to the higher rates of morbidity and mortality. COPD exacerbations are linked to increased airway inflammation driven by neutrophils within the airway lumen, and elevated levels of pro-inflammatory cytokines and mediators of oxidative stress (e.g., higher lipid peroxidation byproducts); up-regulated CD11/CD18 neutrophil adhesion molecules; and increased cytochrome oxidase activity that are also found in the systemic circulation.8-11 Increases in systemic inflammation may contribute to the increased incidence of major cardiac events associated with acute exacerbations of COPD.12
Roflumilast is a potent inhibitor of the phosphodiesterase-4 (PDE-4) pathway and is reported to have protean anti-inflammatory properties such as inhibiting hydrolysis of cyclic adenosine monophosphate (c-AMP) in inflammatory cells and decreasing neutrophilic release of inflammatory mediators and cytokines while decreasing apoptosis and expression of cell surface markers.13,14 Studies in patients with moderate to severe COPD who were given roflumilast have reported significant improvements in forced expiratory volume in 1 second (FEV1) measurements and a moderate reduction in exacerbation rates.15,16 Post hoc analyses of the REACT and RE2SPOND studies suggest that roflumilast is most effective in reducing the rates of moderate or severe exacerbations in a subgroup of patients who have been hospitalized with an exacerbation of COPD within the past year.17-19 These findings highlight the potential importance that roflumilast may have on decreasing the intensity of respiratory symptoms around the time of an acute severe exacerbation that requires hospitalization, and its potential benefit on reducing mortality and the need for readmission. However, despite data suggesting that patients hospitalized with a COPD exacerbation may benefit the most from roflumilast to decrease future events, there is no data that demonstrates the safety and efficacy of administering roflumilast to patients with moderate to very severe COPD while clinically unstable during the index hospitalization, or shortly thereafter.
In this pilot study, we assessed the safety and efficacy of roflumilast initiated in patients with moderate to very severe COPD while hospitalized with an acute exacerbation, with and without a history of chronic bronchitis, on time to all cause rehospitalization or death during the 180 days post initiation of treatment.
Methods
Study Design
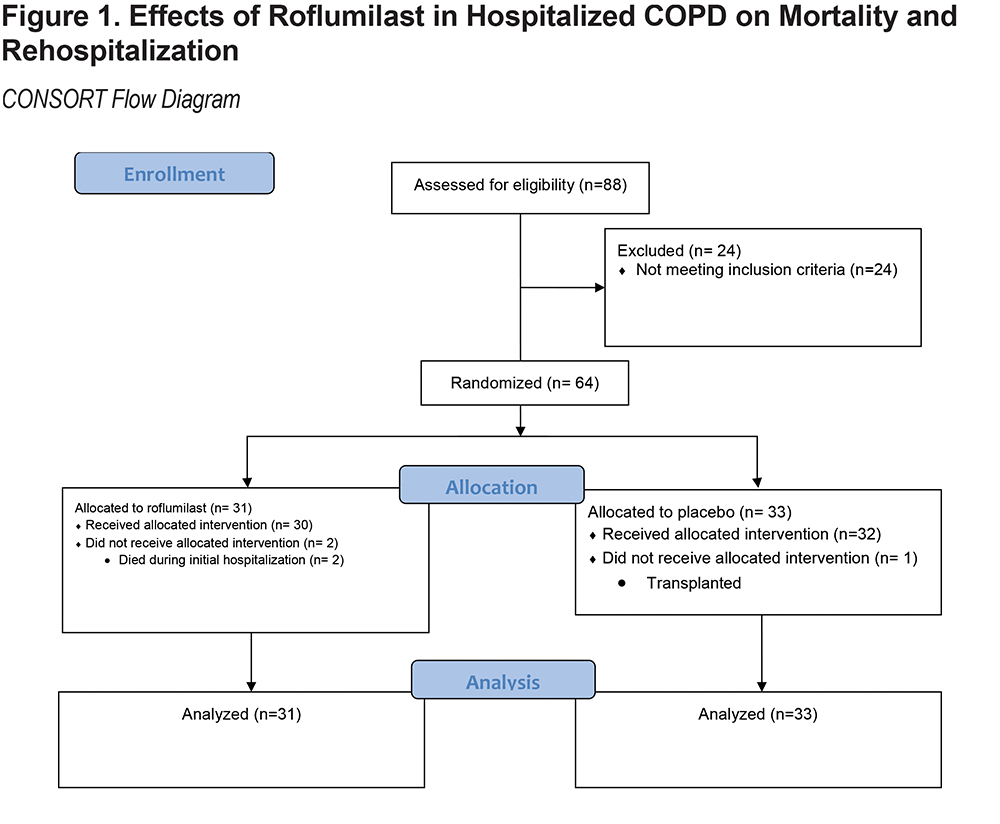
We conducted a parallel-group, prospective, randomized, double blind, placebo-controlled trial of roflumilast 500 ug daily versus placebo in patients following hospitalization for a COPD exacerbation at a single center. This was done to detect a treatment effect as the initial step to complete a power analysis in preparation to conduct a larger, multicenter, prospective, randomized and controlled trial. (Figure 1). The study was approved by our Institutional Review Board for Human Research at Temple University (IRB# 21474).
Outcomes
Outcomes included: (1) Primary: Time to all-cause mortality or rehospitalization during the 180 days post-randomization to treatment; (2) Secondary: Respiratory death or respiratory rehospitalization at 180 days post-randomization; rate of death or readmission during the 30 days post-discharge; change in FEV1, and dyspnea from baseline to 180 days post-randomization; (3) Other: tolerance of roflumilast versus placebo in patients hospitalized due to AECOPD.
Study Population
The study population consisted of 68 patients hospitalized with AECOPD at Temple University Hospital.
Inclusion Criteria
Inclusion criteria consisted of a primary diagnosis of AECOPD defined as acute increase in dyspnea, sputum volume, and/or sputum purulence without other identified cause; hospitalization; patient age greater than 40 and less than 80 years old; cigarette smoking ≥ 10 pack years; informed written consent.
Exclusion Criteria
Exclusion criteria included a prior diagnosis or high suspicion for asthma based on investigator judgment; pulmonary edema, pneumonia, interstitial lung disease or significant bronchiectasis based on admission chest x-ray; intubated and mechanically ventilated at the time of evaluation; active liver disease, or transaminase elevations (≥ 3xULN); history of alcoholism or heavy ethanol use; history of suicidal behavior ≤ 2 years or suicidal ideation ≤ 6 months prior to enrollment; pregnant or lactating females. Those taking excluded medications: P450 inducers (e.g., rifampicin, phenobarbital, carbamazepine, and phenytoin) and CYP3A4 inhibitors or dual inhibitors that inhibit both CYP3A4 and CYP1A2 simultaneously (e.g., erythromycin, ketoconazole, fluvoxamine, enoxacin, cimetidine) were also excluded from the study.
Study Design and Synopsis
Baseline
Patients were enrolled after admission to the hospital. Both groups received Global initiative for Obstructive Lung Disease (GOLD) guideline-recommended care.20 At baseline, all patients had a medical history and physical examination with spirometry performed. Women with the potential to become pregnant were given a pregnancy test. Dyspnea scales, Deyo-Charlson index, and GOLD classification were performed. Patients completed a Columbia Suicide Severity Rating Scale to exclude patients with a history of suicidal behavior ≤ 2 years or suicidal ideation ≤ 6 months prior to enrollment.
Randomization
Patients were randomized to 1 of 2 treatment groups using a randomized block design. One group received roflumilast 500 mcg (Daliresp®) daily and the other received a matched placebo tablet. Patients were allocated to one of these treatment arms prior to hospital discharge for a total period of 180 days post enrollment.
Day of Hospital Discharge
On the day of discharge, spirometry was performed and a questionnaire assessing any adverse events during the hospitalization was completed.
Measurements
Demographics and Medical History
Age, gender, body mass index (BMI), presence of comorbidities, current medical therapy, history of pulmonary rehabilitation, influenza and pneumococcal vaccinations, emergency department visits and hospitalizations during the last year, number of exacerbations in prior year, history of coronary artery disease, stroke, transient ischemic attacks, and peripheral vascular disease were documented. The Deyo-Charlson index21 was used to assess the impact of other chronic illnesses on outcome.
Spirometry
Spirometry was performed (post bronchodilator administration) at the time of enrollment (baseline) or as soon as the participant was able to perform spirometry while hospitalized, at the day of discharge and then 180 days post randomization.22 Airflow obstruction was defined by postbronchodilator measured FEV1 to forced vital capacity (FVC) ratio < 70% and FEV1 < 70% predicted at time of inclusion and was used to define GOLD Stages.
Quality of Life and Functional Status
Patients completed general and disease-specific, self-administered quality of life and functional questionnaires: EuroQol Five Dimension scale (EQ5D)23 and the St George’s Respiratory Questionnaire (SGRQ).24 The Columbia–Suicide Severity Rating Scale (C-SSRS)25 was used to prospectively assess suicidal ideation and behavior using a structured interview face to face for patient responses.
Measurements of Dyspnea
Dyspnea was measured by the modified Medical Research Council (mMRC) Dyspnea score.
Description of Optimized Standard Care for COPD Exacerbations
All patients received standardized, optimized care for AECOPD. Noninvasive positive pressure ventilation was utilized at the discretion of the treating physicians but followed accepted guidelines.26
Drug/Placebo Supply
Roflumilast and matching placebo were provided by Forest Laboratories and subsequently Astra Zeneca and stored by the Investigational Pharmacy Unit at Temple University Hospital.
Statistical Methods
This was a pilot study and the intent was to determine if there is a signal that would justify a larger clinical trial. Therefore, the significance level was set to 0.1 and the power was set at 0.7. A total of 100 patients is required in a 2 treatment parallel-design study. There is a 70% probability that the study will detect a treatment difference at a 2-sided 10% significance level, if the true hazard ratio is 1.654. This is based on the assumption that the accrual period will be 36 months and the follow up period will be 6 months and the median time to event is 8 months. The total number of events will be 73.
Vital status was determined for all randomized patients for the intention to treat analysis. Data are presented as the mean (standard deviation) for continuous variables. Statistical comparisons were performed using the Student’s t-test for continuous data and χ2 test for categorical data. Categorical and continuous data were analyzed using JMP® Pro 13.0.0© 2016 SAS Institute. Event-free survival curves were determined by Kaplan-Meier analysis, and differences between survival curves were compared using the log-rank test. Event was defined as the first readmission or death. Univariate and multivariable Cox regression was done using Stata® release 15. P values less than 0.05 are considered statistically significant.
Results
Patient Population
Over 500 patients were prescreened to determine eligibility for the trial. Most were initially excluded because they were identified outside of a 12-hour time enrollment window post hospitalization; because of this, the enrollment window was increased to begin the investigational drug while hospitalized. Ultimately, 88 patients were screened for study enrollment and 64 were enrolled. The consort diagram for the study is provided in Figure 1.
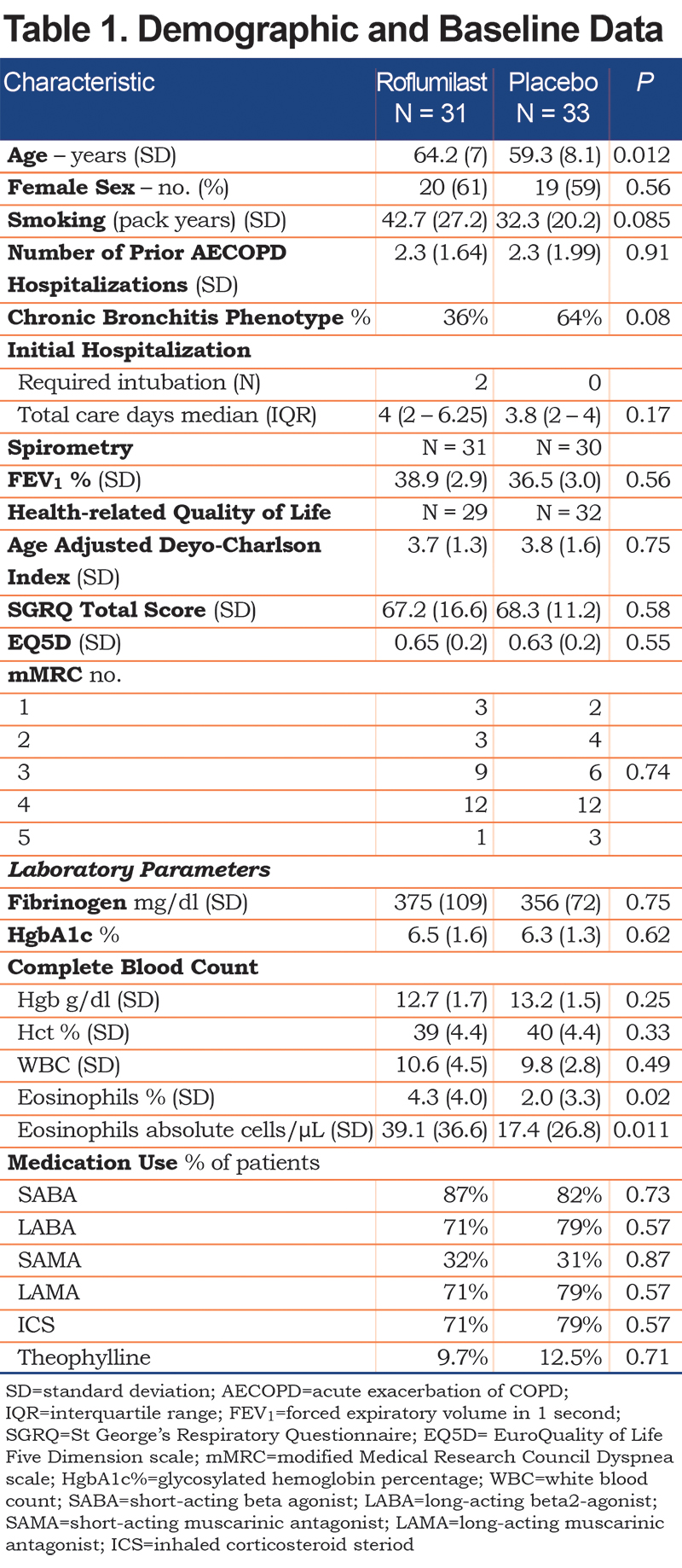
Patient Demographics
Baseline characteristics are provided in Table 1. The roflumilast and placebo groups were well matched on most clinical characteristics including sex, smoking history, number of COPD exacerbations in the year prior to enrollment, level of airflow obstruction, fibrinogen levels and total white blood count (WBC), SGRQ and EQ5D scores, distribution of mMRC scores and baseline respiratory medication use. The group assigned to roflumilast were slightly older than the placebo group and had eosinophil levels that were statistically significantly higher at the time of enrollment.
Chronic Bronchitic Phenotype
Chronic bronchitis was identified using the first 2 responses on SGRQ. Individuals were classified as having SGRQ chronic bronchitic phenotype if they answered “almost every day” or “most days a week” to the following questions: “Over the last 4 weeks, I have coughed:” and “Over the last 4 weeks, I have brought up phlegm (sputum).27 Twenty-seven patients were found to have a chronic bronchitic phenotype based on this methodology. The distribution between the 2 groups was uneven (33% roflumilast versus 67% placebo) although this did not quite reach statistical significance.
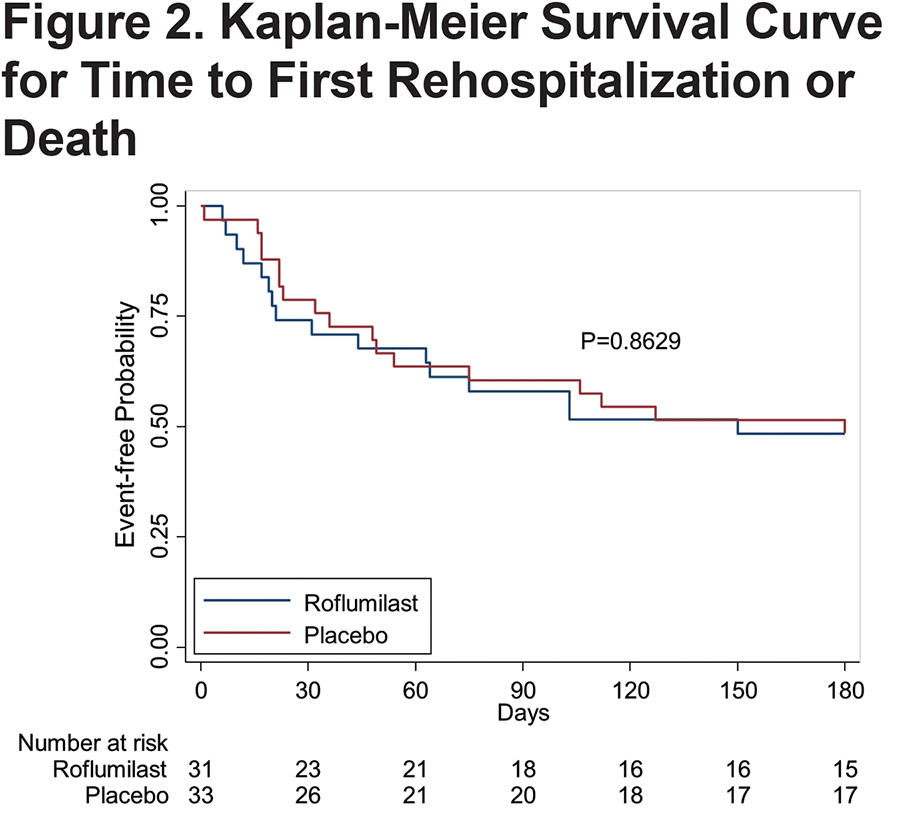
Primary Outcome Parameter:Time to COPD Readmission or Death
The primary outcome for the study was the difference between placebo and roflumilast on the time to first all cause rehospitalization or death. Although 2 patients died (both assigned to the active treatment group during the initial hospitalization) there were no deaths that occurred in the follow-up period. There was no difference in the time to first readmission between the roflumilast and placebo groups (54 days versus 55 days respectively [p=0.93]). (Figure 2)
Secondary Outcome Parameters
There was a total of 31 and 35 all-cause readmissions in the roflumilast and placebo groups respectively (p=0.93). (Table 2)

Change in Quality of Life Scores
SGRQ scores for both the roflumilast and placebo groups improved from the point of hospital discharge to the 180-day study visit. SGRQ decreased an average of 10.8 points and 7.8 points in the roflumilast and placebo groups, respectively. No significant differences were detected between groups. (Table 2)
The EQ5D scores improved slightly, but not significantly in both the roflumilast and placebo groups. No difference between groups was detected.
Change in Glycosylated Hemoglobin Percentage
The glycosylated hemoglobin percentage (HgbA1C%) dropped slightly in both groups. No significant difference between groups. (Table 2)
Univariate and Multivariable Analyses
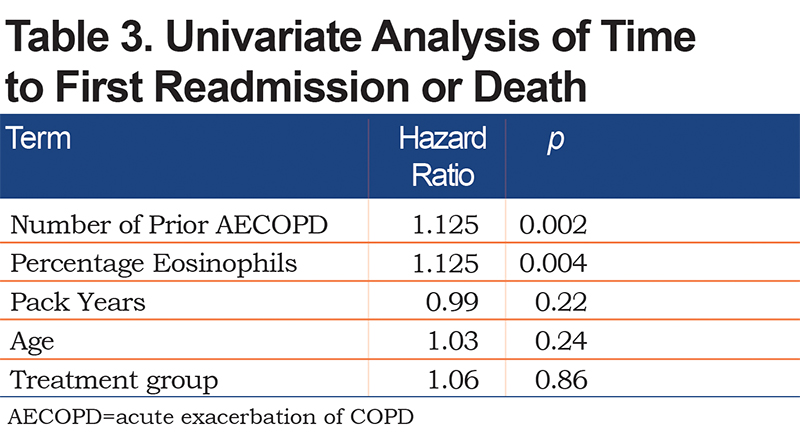
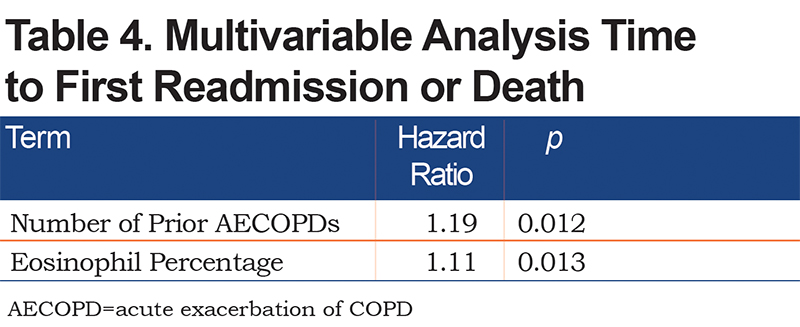
Two characteristics were found to be significantly different between the roflumilast and placebo groups at baseline, age and eosinophil levels whether reported as a percentage or as an absolute number. To determine the effect of underlying clinical characteristics on the time to first exacerbation, a standard least squares regression model was developed that included the patient’s age and percentage of eosinophils as these were statistically significantly different between groups at baseline. Also included in the model were the number of prior exacerbations, which is known to affect the rate of future exacerbations and the number of pack years of smoking. The results of the univariate analysis are found in Table 3. As shown, the time to admission was related to the number of prior acute exacerbations and a higher percentage of eosinophils in the peripheral blood. Pack years, age, and treatment group assignment did not influence time to readmission. Percentage of eosinophils and number of prior exacerbations remained significant in the multivariable analysis (Table 4).
Discussion
In this single center, prospective, randomized and placebo-controlled pilot study conducted in patients hospitalized with an exacerbation of COPD, roflumilast had no effect time on all-cause mortality or rehospitalization during the 180 days post-randomization to treatment, on the number of respiratory deaths or respiratory rehospitalizations during the 180 days post-randomization; on rates of death or readmission during the 30 days post-discharge; and changes in dyspnea during the 180 days post-randomization. We found no effect of roflumilast on any of these parameters in the subgroup of patients with the chronic bronchitic phenotype. Tolerance of roflumilast in patients during the period surrounding the hospitalized COPD exacerbation was limited due to nausea and loose stools, fatigue, weight loss and headache with a frequency and severity that was similar to prior reports (Table 5). Two large, multicentered trials have shown that roflumilast, as an add-on therapy to inhaled bronchodilators in patients with moderate to severe COPD and a history of chronic bronchitis and prior exacerbations, had a 48 ml increase in FEV1 and 17% reduction in the rate of moderate and severe exacerbations of COPD compared to placebo at 52 weeks.28

Another post hoc analysis of the same dataset pooling patients from the 2 studies and classifying them as frequent (≥ 2 events in prior year) or infrequent exacerbators (<2 events) showed that treatment with roflumilast shifted patients from the frequent to the more stable infrequent exacerbator state.29 The REACT study enrolled patients with moderate to severe COPD with a history of chronic bronchitis and at least 2 exacerbations in the previous year to receive roflumilast 500 μg or placebo orally once daily together with a fixed inhaled corticosteroid and long-acting beta2-agonist combination. The rate of moderate-to-severe exacerbations was 13.2% lower in the roflumilast group than in the placebo group.17 Another recent study (RE2SPOND) was conducted in patients with moderate and very severe COPD, chronic bronchitis and 2 or more moderate exacerbations or hospitalizations in the previous year who were receiving inhaled beta-agonists and corticosteroids either with, or without, a long- acting anticholinergic agent to once daily roflumilast or placebo for 1 year.18 Roflumilast failed to reduce moderate or severe exacerbations in the overall population, but was reported to improve lung function and reduce exacerbations in the subgroup of patients with a history of more frequent exacerbations or hospitalization.
Another post hoc pooled analysis of the REACT and RE2SPOND trials suggests that patients with prior hospitalization for COPD exacerbations had the greatest benefit with roflumilast in terms of reduction of future exacerbations or rehospitalizations.19 In REACT, patients with prior hospitalization had a significant reduction in the combination of moderate and severe exacerbations and prolongation in the time to rehospitalization. Post hoc analysis of RE2SPOND showed similar benefits with roflumilast, those with prior history of hospitalization had a 25% greater reduction in rehospitalization. An analysis of summary data released by the U.S. Food and Drug Administration found benefit with roflumilast reducing future exacerbations if the risk of 1 severe exacerbation per year exceeded 22%.30 Together these results suggest that roflumilast is potentially most effective in reducing moderate to severe exacerbations in the subgroup of patients who required hospitalization in the prior year.
Why would a history of prior hospitalizations for COPD exacerbations indicate a subgroup that may have an enhanced beneficial response to roflumilast? Prior hospitalization may be an epimarker of a sicker patient group— one with more airflow obstruction, poorer health status, older age, more radiologic evidence of emphysema and leukocytosis, factors that also increase the risk for repeated hospitalization.31 Prior hospitalizations may also indicate a patient group that is more unstable and have more active disease that is not maximally controlled by background anti-inflammatory therapy. Besides the previously described anti-inflammatory effects of roflumilast mediated by an increase in intracellular c-AMP in inflammatory cells, bronchial and smooth muscle cells and reduction in leukotrienes, reactive oxygen species and tumor necrosis factor, roflumilast may also attenuate inflammation by interrupting the proline-glycine-proline and its actelyated form breakdown of extracellular matrix generated proteins that act as neutrophilic chemoattractants.32 These anti-inflammatory effects may be most important at the small airway level where roflumilast treatment has been reported to improve lobar ventilation in patients also treated with triple inhaled therapy assessed by functional respiratory imaging.33 Thus, prior hospitalization may indicate a patient group that predominately suffers from small airway dysfunction that benefits from roflumilast decreasing airway resistance and enhancing the delivery of inhaled bronchodilators and steroids at the lobar level.
We did not enroll patients based on the presence of a chronic bronchitis phenotype but sought to evaluate the effects of roflumilast based on a history of 1 or more prior hospitalizations in the previous year. Although the publication was not available to us at the start of our study, this approach is partially supported by the retrospective analysis of Rabe and colleagues who found that a history of COPD hospitalization was also a predictor of a benefit to roflumilast use.19
Despite these potential benefits of roflumilast, we found no benefit of using roflumilast compared to matched placebo in our patient group that began roflumilast during a hospitalization for a COPD exacerbation and was followed for 180 days. The reasons for our failure to detect a treatment effect are not known but could be due to several factors. It is possible that our patient population was more impaired by airflow obstruction, hyperinflation, a greater degree of emphysema compared to prior studies or that the treatment effects of roflumilast take longer to manifest their benefits than 6 months in patients with an active ongoing exacerbation.
Other studies have also failed to detect a significant clinically meaningful short term benefit with roflumilast therapy. A prospective controlled trial showed that 12 weeks of roflumilast therapy was associated with small increases in FEV1 and FVC and small decreases in specific airways resistance and no change in any measurement of lung hyperinflation.34 Another prospective, randomized controlled trial in 81 patients (TREAT) who were treated at outpatient exacerbation presentation (those who required hospitalization were not included) were randomized to roflumilast or placebo for 4 weeks with a change in sputum neutrophil count being the primary endpoint.35 Although patients treated with roflumilast had a significant reduction in percentage of sputum neutrophils and sputum myeloperoxidase, the primary endpoint, a reduction in sputum neutrophils at 2 weeks, was not different, nor was a change in lung function at 4 weeks. Additionally, adverse events and drug withdrawal were more common in the roflumilast than placebo group with a 2kg weight loss being observed in the roflumilast group. These data suggest that the acute effects of roflumilast on attenuating airway inflammation may not be immediate, or of the magnitude of the effect that is needed to induce a meaningful improvement in clinical outcome and may account in part for some of our trial’s negative results.
Safety is always a concern when a drug that has been reported to be efficacious in a restricted patient population during a stable state in a controlled outpatient trial is utilized in a more severely ill and potentially less stable inpatient population. Most random controlled trials have shown rates of adverse effects to be about 9.5% when compared to placebo, but real-life use analyses have reported much higher adverse events rates of 69%-72%.36 Our rates and types of adverse events reassuringly are in line with prior random controlled trial data and suggest that the acutely hospitalized group of patients tolerated roflumilast as well as the more stable outpatient group of patients with moderate to severe COPD.
The influence of eosinophils on time to first readmission was unexpected since the absolute eosinophil number (median 15.8 cells per mL; interquartile range [IQR] 0.69 – 42.5) was well below the that reported as a COPD eosinophilic phenotype of > 150 or > 300 cells per mL.37 Kim et al, in an analysis of the AERIS cohort, found that blood eosinophil levels ≥ 2% placed individuals at risk of eosinophilic inflammation and exacerbation.38 In our study the median eosinophil percentage for the overall study population was 1.4% (IQR 0.1% - 4.5%). As reported by Pavord etal, eosinophil numbers less than 150 cells per mL may be predictive of response to mepolizumab (and therefore related to eosinophilic inflammation) in those patients who have a historical eosinophil count ≥ 300 cells per mL.37 Unfortunately, we do not have historical eosinophil counts collected for the patients in this study.
Our study had several important limitations that may affect our results, notably its small sample size, shorter duration of exposure (6 months), single center nature, absence of chronic bronchitic symptoms in all participants and lack of mortality events as a measurable endpoint. The trial was to be conducted at 3 sites, however 2 of these were never activated. Treatments administered prior to hospitalization were not collected as the purpose of the study was to determine the effect of roflumilast on subsequent hospitalizations. The differences we found in baseline characteristics between the intervention and control groups were unexpected. The randomization scheme was prepared by the sponsor prior to enrolling any patient into the study. We recognize that differences in prehospitalization treatments may have influenced the disparities we found in baseline eosinophil counts. The limitations of a single center design, small numbers and short duration of drug exposure were preplanned as this was a pilot study to determine the feasibility and safety and potential effect size of the intervention to design a prospective larger and longer multicenter trial in this population. The low mortality in our trial is a reflection of the small numbers and short duration of our study. We found no significant treatment effect of roflumilast in patients with symptoms of chronic bronchitis (as defined by the first 2 questions of the SGRQ) in the sub-analyses. We acknowledge the limitations of this approach in identifying chronic bronchitis in all enrolled participants, which may have influenced our negative results.
In summary, in this small single center prospective and controlled pilot efficacy study, we found no effect of roflumilast initiated during hospitalization on prolonging the time to readmission, or treatment effect on any other measured outcome in patients with, and without chronic bronchitis and severe COPD. Hopefully, a soon to be initiated comparative effectiveness trial that compares roflumilast to azithromycin on time to next readmission (RELIANCE) will provide important knowledge on the utility of roflumilast to decrease rehospitalization in patients with moderate to severe COPD.
Acknowledgements
Author contributions: Huaqing Zhao performed the data analysis. Michael R. Jacobs and Gerard J. Criner interpreted the data. Gerard J. Criner drafted the manuscript. Gerard J. Criner, Nathaniel Marchetti and Michael R. Jacobs revised the final draft. All authors approved the final version to be published. Clinical Trial: NCT01973998
Declaration of Interest
Dr. Gerard J. Criner received grants from the National Institutes of Health and the Department of Defense. He consults for AstraZeneca, Boehringer Ingelheim, Holaira, Mereo BioPharma, Third Pole, PneumRx, Pulmonx, Pearl Therapeutics, Almirall, CSA Medical, Broncus, AVISA, Lungpacer, and GlaxoSmithKline. He has also contracted clinical trials from AstraZeneca, Avisa, Mereo BioPharma, Boehringer Ingelheim, Broncus Medical, GlaxoSmithKline, Lungpacer Medical, Pulmonx, PneumRx/BTG and Yungjin. All other authors have nothing to declare.