Running Head: Health and Cost Outcomes of Arformoterol Therapy
Funding Support: Sunovion Pharmaceuticals Inc.
Date of Acceptance: June 14, 2019
Abbreviations: long-acting beta2-agonist, LABA; inhaled corticosteroid, ICS; chronic obstructive pulmonary disease, COPD; health resource utilization, HRU; arformoterol, ARF; international classification of disease, 9th revision, clinical modification, ICD-9-CM; emergency department, ED; durable medical equipment, DME; long-acting muscarinic antagonist, LAMA; Centers for Medicare and Medicaid Services, CMS; chronic illness and disability payment system, CDPS; short-acting muscarinic antagonist, SAMA; short-acting beta2-agonist, SABA; systemic corticosteroids, CS; phosphodiesterase inhibitors, PDE4; difference-in-difference, DID; short-acting bronchodilators, SABDs; hospital readmission reduction program, HRRP
Citation: Navaie M, Celli BR, Xu Z, Cho-Reyes S, Dembek C, Gilmer TP. Exacerbations, health resource utilization, and costs among Medicare beneficiaries with chronic obstructive pulmonary disease treated with nebulized arformoterol following a respiratory event.
Chronic Obstr Pulm Dis. 2019; 6(4): 297-307. doi:
http://doi.org/10.15326/jcopdf.6.4.2019.0127
Note: An earlier version of this study was presented, in part, at the American Thoracic Society International Conference May 18-23, 2018 in San Diego, California.
Introduction
Chronic obstructive pulmonary disease (COPD) remains a public health challenge in the United States.1,2 The condition affects an estimated 16 million U.S. adults3 and is a leading cause of mortality.1,3 The natural progression of COPD is often aggravated by exacerbations which lead to an increase in health resource utilization (HRU), a reduction in health-related quality of life, a higher risk of mortality, and a greater cost burden.1,4,5 Thus, reducing the risk and severity of exacerbations is a key goal in the clinical management of COPD.6
Long-acting bronchodilators including long-acting beta2- agonists (LABAs), long-acting muscarinic antagonists (LAMAs), or LABA/LAMA combinations with or without inhaled corticosteroids (ICSs) are recommended as maintenance treatment for COPD.6These therapeutic options can be administered by various devices including handheld inhalers and nebulizers. Across all patients with COPD in the United States, handheld ICS+LABA is the most commonly prescribed maintenance therapy.7-10 Among Medicare beneficiaries with COPD, nearly half are treated with ICS+LABA combination therapy.11
Individualized treatment approaches in COPD include assessing patient preferences and abilities to correctly use the inhalation device.12-15 Handheld inhalers are the most frequently used inhalation devices to administer COPD pharmacotherapies.16 However, for patients on handheld devices with poorly controlled COPD symptoms, nebulized maintenance treatment may be a more suitable choice.6 Indeed, past research has shown that some patients prefer using nebulizers.15
Whether patients with COPD on handheld ICS+LABA inhalers with a history of exacerbations can benefit from switching to a nebulized maintenance treatment remains unclear. Addressing this dearth of evidence could lead to valuable insights to potentially improve care delivery and yield cost savings, especially for the Medicare program given that 12% of Medicare beneficiaries are diagnosed with COPD17 and 69% of all hospitalized beneficiaries have COPD.18 The purpose of this retrospective study was to evaluate exacerbations, HRU, and costs among Medicare beneficiaries with COPD on handheld ICS+LABA therapy who switched to nebulized arformoterol (ARF) or continued to use handheld ICS+LABA following a respiratory event.
Methods
Data Source
This retrospective study used 100% fee-for-service Medicare administrative data from the Centers for Medicare and Medicaid Services’ (CMS) Chronic Condition Warehouse. The data included inpatient, outpatient, prescription (Part D), and long-term care claims from 2010 to 2014. The study was approved by the Institutional Review Board of the University of California San Diego and was subject to a CMS data use agreement.
Cohort Selection and Study Design
Medicare beneficiaries diagnosed with COPD (i.e., International Classification of Disease, 9th Revision, Clinical Modification [ICD-9-CM] codes 490.xx, 492.xx, 494.xx, 496.xx) who had continuous enrollment in Parts A, B and D for a full year were identified (N=676,924). A total of 2412 beneficiaries met additional inclusion and exclusion criteria (Figure 1). Among these beneficiaries, 448 initiated nebulized ARF (cases) and 1964 received a fixed-dose ICS+LABA (controls) including fluticasone/salmeterol (Advair), budesonide/formoterol (Symbicort), or mometasone/formoterol (Dulera) using a handheld inhaler. After performing 1:1 propensity score matching using the nearest neighbor without replacement, 423 beneficiaries were identified in each group. Matching covariates included sociodemographic characteristics, comorbidities, COPD treatment, and HRU in the pre-index period.
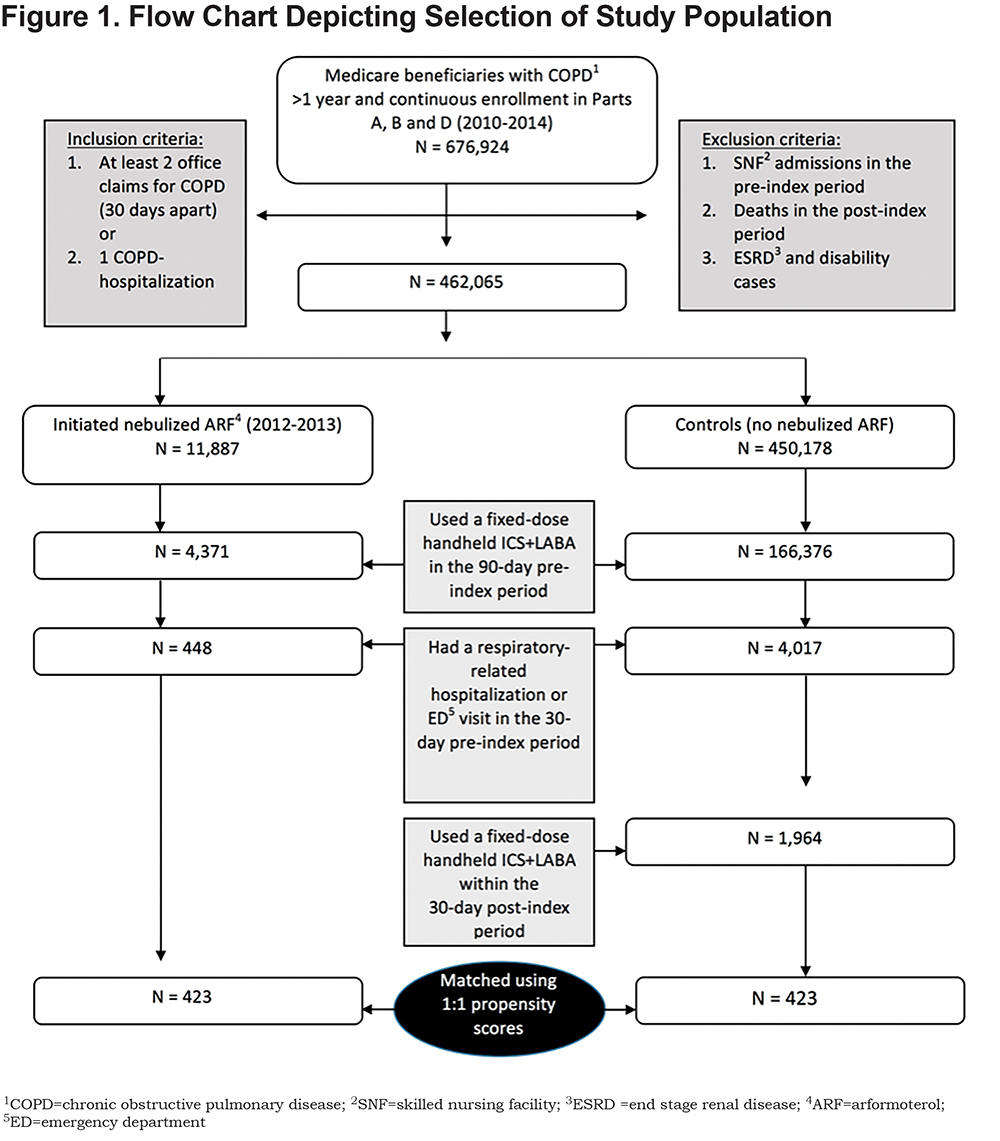
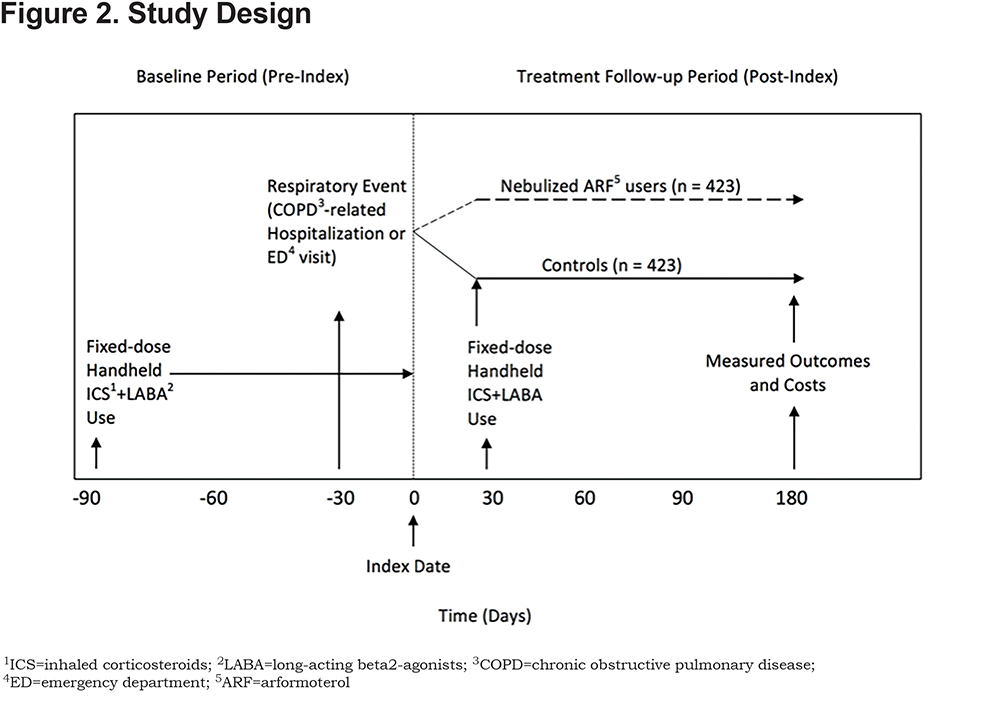
To evaluate the effects of switching from a fixed-dose handheld ICS+LABA to nebulized ARF, beneficiaries were retrospectively assessed for a 9-month period that included 2 segments: (a) a 90-day pre-index (baseline) period, and (b) a 180-day post-index or treatment follow-up period (Figure 2). During the pre-index period, all beneficiaries were being treated with a fixed-dose handheld ICS+LABA therapy and no nebulized ARF. The index date was the date of first prescription for nebulized ARF between January 1, 2012 and December 31, 2013 for beneficiaries who were on fixed-dose handheld ICS+LABA who switched to ARF after resolution of a respiratory event, defined as a COPD-related hospitalization or an emergency department (ED) visit < 30 days before ARF initiation. Beneficiaries in the control group continued to receive a fixed-dose handheld ICS+LABA, as ascertained by prescription claims within 30 days after the index date. The index date for controls was randomly assigned to correspond to the date of nebulized ARF initiation in the treatment group. All outcomes were measured at 180-days post-index.
Independent and Outcome Measures
The following independent measures were examined: (a) demographic characteristics including age, sex, race, and regional residence; (b) dual-eligibility status (i.e., Medicare and Medicaid beneficiary); (c) comorbid conditions as measured by the Chronic Illness and Disability Payment System (CDPS) diagnostic classification19; (d) COPD treatments including short-acting muscarinic antagonists (SAMAs), LAMAs, short-acting beta2-agonists (SABAs), LABAs, ICSs, systemic corticosteroids (CSs), methylxanthines, phosphodiesterase inhibitors (PDE4), non-specific PDE inhibitors, mucolytics, and antibiotics; and (e) oxygen therapy.
Outcomes measures included: (a) the frequency of exacerbations, defined by an ED visit or a hospitalization for acute exacerbation of COPD (ICD-9-CM 491.21 or 491.22); (b) HRU including pharmacy, durable medical equipment (DME) use, hospitalizations (COPD-related and all-cause), outpatient care, ED visits, skilled nursing facility use, and home health care visits; and (c) costs.
Statistical Analysis
To ensure balance in matching procedure, pairs were checked against caliper of one fifth of propensity score standard deviation and restricted to common support. Standardized differences for covariates were also calculated before and after matching. Descriptive statistics including means, standard errors, and frequencies were used to compare sociodemographic characteristics, COPD treatment, exacerbation frequency, and HRU between nebulized ARF users and controls. Differences in HRU outcomes between beneficiaries treated with nebulized ARF and controls were estimated using difference-in-difference (DID) regression models. DID estimates for count variables (e.g., number of exacerbations) were calculated using a zero-inflated negative binomial regression model. DID estimates for costs were analyzed using generalized linear regression with a gamma distribution. P < .05 denoted statistical significance. All analyses were performed with SAS Enterprise Guide 7.1.
Results
Baseline Characteristics
Beneficiaries treated with nebulized ARF were well matched to controls on sociodemographic and treatment characteristics (Table 1). The majority of beneficiaries were 70 years of age or older, female, non-Hispanic white, and from the South or Midwest regions of the United States. A higher proportion of beneficiaries in the control group were dual-eligible as compared to beneficiaries treated with nebulized ARF (54.4% versus 49.9%, respectively).
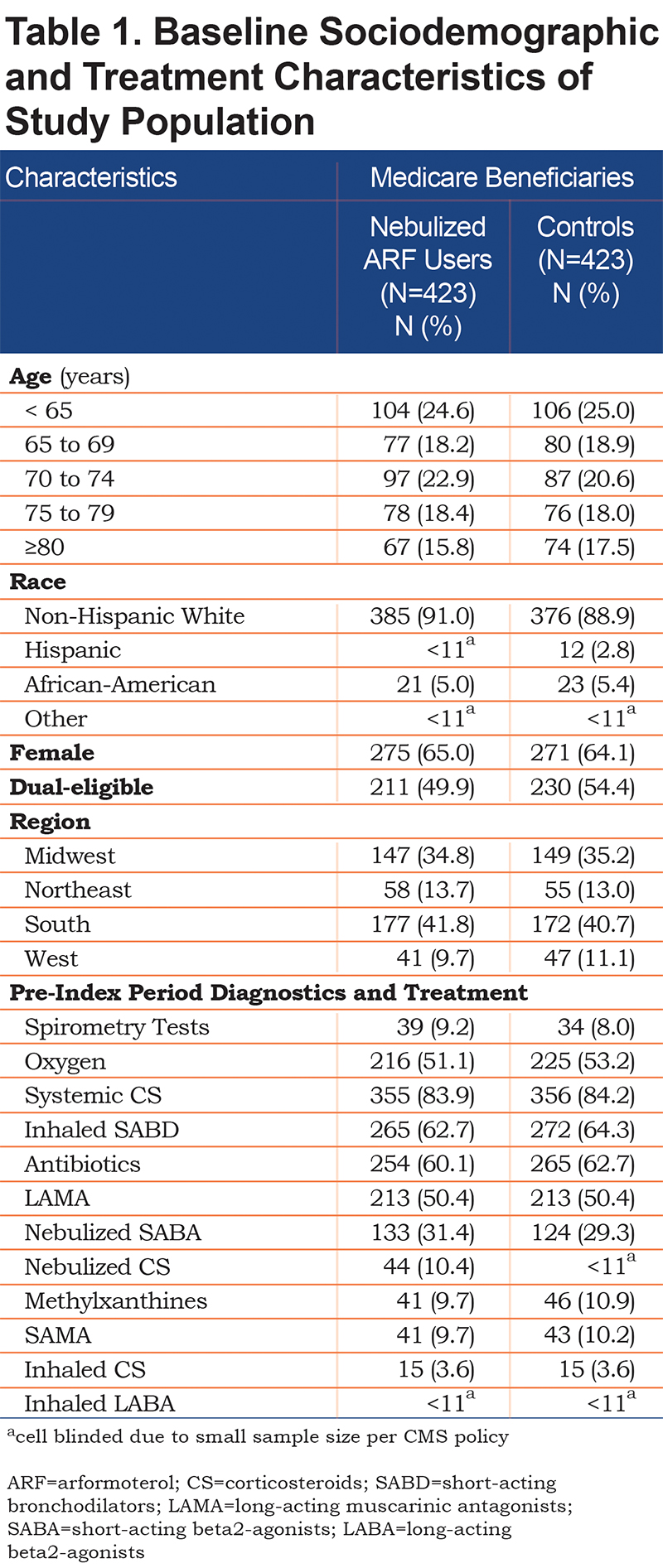
In addition to receiving handheld ICS+LABA therapy, more than half of the beneficiaries in both groups were receiving oxygen therapy, > 60% were on inhaled short-acting bronchodilators (SABDs) or antibiotics, and 50% were being treated with a LAMA (Table 1). About one-third of all beneficiaries were receiving a nebulized SABA.
Exacerbations, Health-Related Utilization and Costs
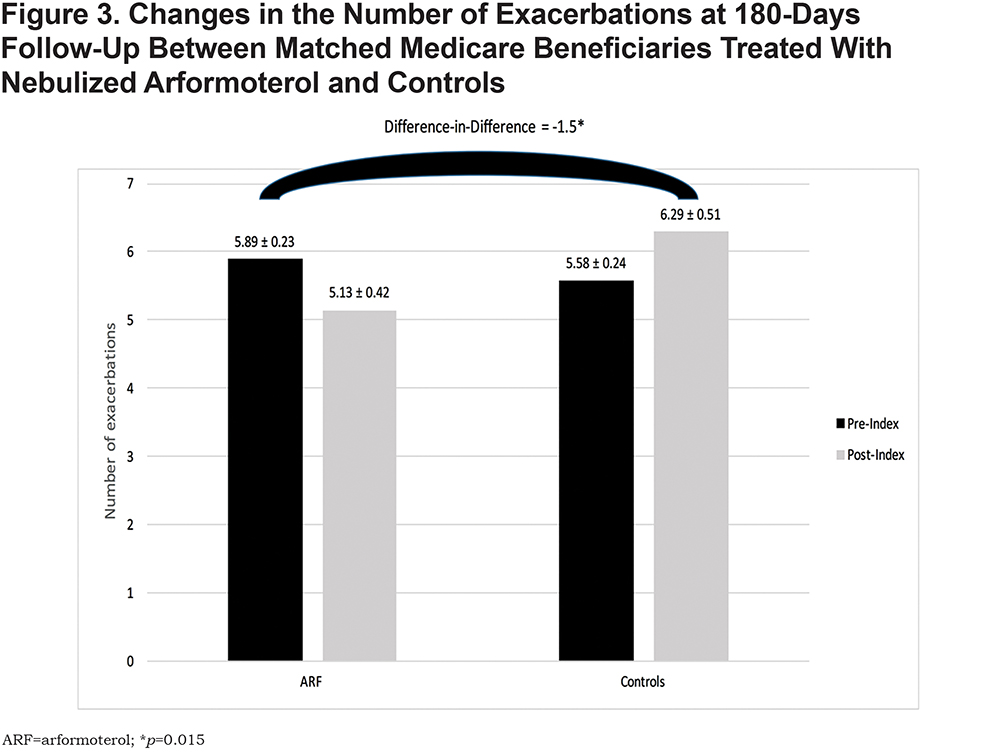
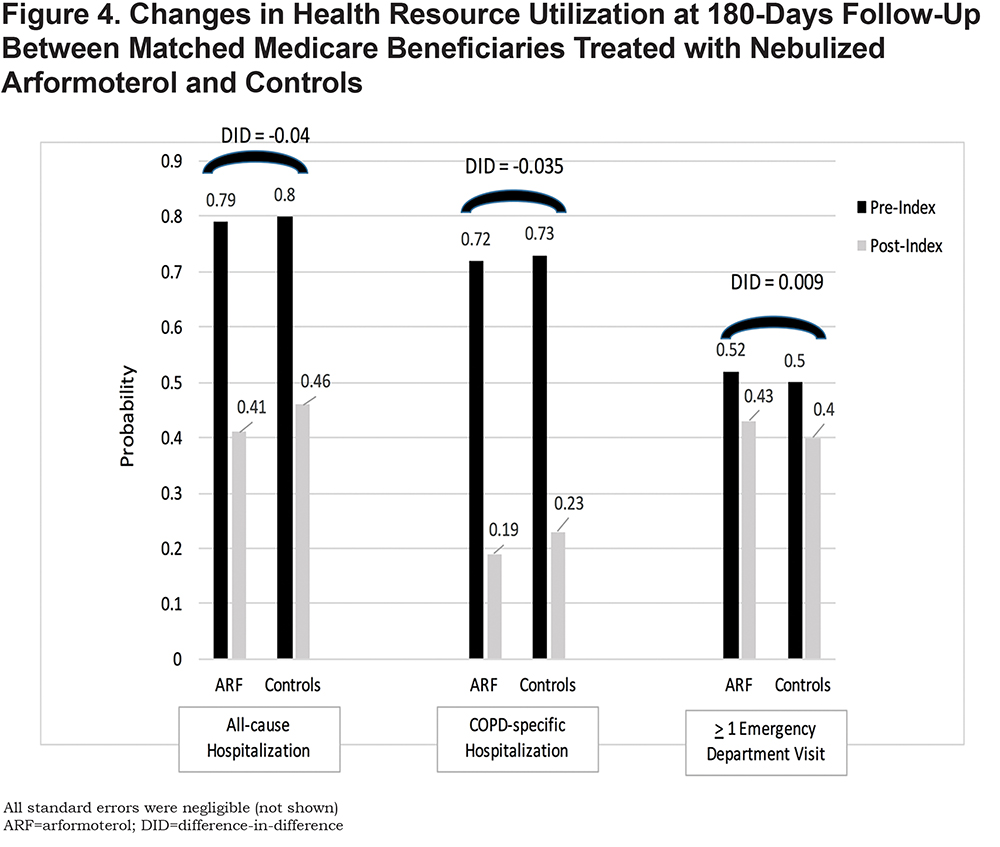
Beneficiaries treated with nebulized ARF had, on average, 1.5 fewer exacerbations at 180 days compared to controls (p=.015) (Figure 3). The probabilities of all-cause and COPD-related hospitalizations and ED visits were similar between the 2 groups (Figure 4).
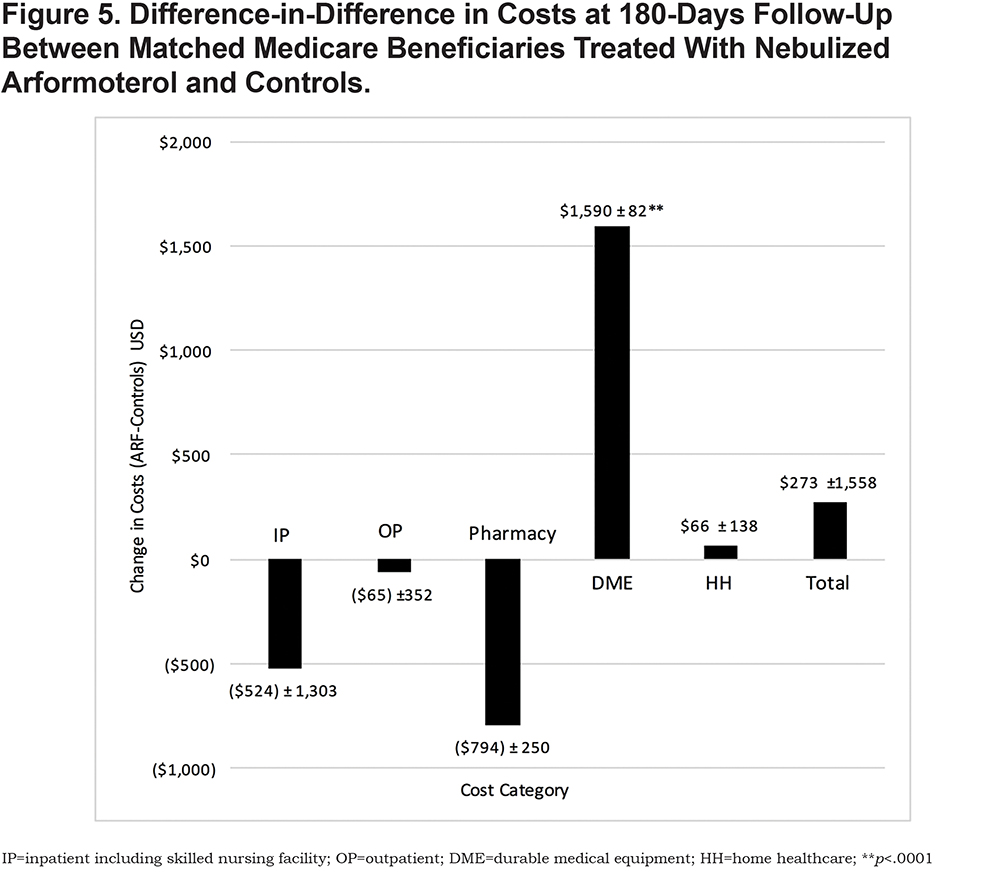
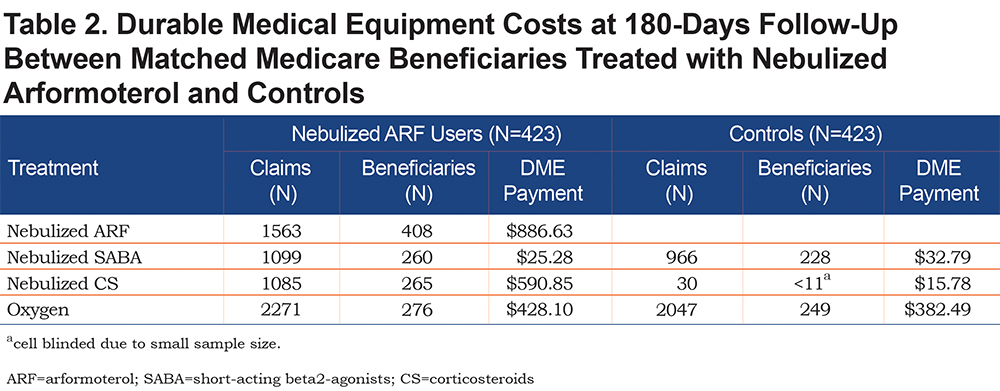
At 180-days follow-up, health care costs for inpatient care (-$524 ± 1303), outpatient office visits (-$65 ± 352), and pharmacy (-$794 ± 250) were lower for the nebulized ARF users but not significantly different from controls (Figure 5). By contrast, home health care costs were slightly higher for nebulized ARF users relative to controls ($66 ± 138), although the difference was not statistically different. On average, DME costs were significantly higher for beneficiaries on nebulized ARF ($1590 ± 82) relative to controls (p < .001). Further analysis of DME cost contributors revealed that nebulized ARF accounted for about 55% ($886.63) of the costs, with the remaining costs attributable to oxygen therapy ($428.10) and nebulized CS ($590.85) (Table 2). No significant differences in total costs were observed between the 2 groups.
Discussion
To our knowledge, our retrospective analysis is the first large study to compare exacerbations, HRU, and costs between Medicare beneficiaries with COPD who were treated with ICS+LABA and either switched to nebulized ARF or continued using handheld ICS+LABA following a respiratory event that included a COPD-related hospitalization or an ED visit ≤ 30 days before ARF initiation. We found that beneficiaries who switched to nebulized ARF had 1.5 fewer exacerbations at 180-days follow-up than those who continued treatment with handheld ICS+LABA at similar total health care costs.
In a previous retrospective cohort study conducted in a small sample of 23 Medicare beneficiaries with uncontrolled COPD symptoms despite regular use of handheld ICS+LABA, researchers examined the effects of switching the medication delivery system to a nebulized formulation of ARF+CS (budesonide).20 They found a significant decrease in combined ED visits and hospitalizations stemming from reduced exacerbations after beneficiaries switched to nebulized ARF+CS when compared to beneficiaries who continued handheld ICS+LABA (0.22 versus 0.99, respectively, p = 0.002). The study concluded that patients with severe COPD who are not achieving symptom control with a handheld ICS+LABA may benefit from switching to the same medication using a nebulized delivery system.
Our study expands the current knowledge base stemming from previous research by showing that, despite higher DME costs among nebulized ARF users compared to controls, the total health care costs were similar between the 2 groups due to cost offsets by ARF in lower pharmacy, inpatient, and outpatient care. Moreover, we determined that nebulized ARF accounted for only 55% of DME costs. Although previous studies among Medicare beneficiaries have shown that the use of maintenance medications (including handheld inhalers and nebulized therapies) lowers annual expenditures,21,22 we cannot directly compare our findings with these studies since we did not have a treatment naïve control group. Nonetheless, our results are consistent with past studies in suggesting that clinical decisions regarding the choice of maintenance therapies and delivery systems can impact COPD outcomes and costs.23,24
Preventing COPD exacerbations and repeat hospitalizations has become a key imperative in Medicare populations in light of the Medicare’s Hospital Readmission Reduction Program (HRRP) which imposes reimbursement penalties for unplanned readmissions.25,26 As such, researchers have explored the potential factors associated with recurrent exacerbations among Medicare beneficiaries. Poor adherence to inhaled treatment regimens has been proposed as one of the key drivers of unplanned readmissions.11,27 The reasons for suboptimal adherence among patients with COPD are multifactorial.28,29 One contributing reason is that some patients with COPD have difficulty using inhaler devices properly.11,30-35 In real-world settings, proper use of handheld inhalers requires patients to have hand-breath coordination, intact cognitive abilities to operate devices, the ability to hold their breath for up to 10 seconds, and the ability to generate adequate inspiratory force.13 Since these types of requirements can lead patients to stop inhaled therapy due to the perceived complexity of using handheld devices,36 the importance of matching the right inhalation device to the right patient cannot be over emphasized. Ultimately, prescribing a device that patients are unable to use properly can result in inadequate medication dose delivery which subsequently leads to more exacerbations, costly ED visits and hospitalizations.6,27-32,37
Our study had certain limitations inherent to retrospective observational studies that rely on administrative claims data including coding accuracy, missing or omitted data, and other indicators of reliability and validity associated with data quality. We did not have information on unobserved factors that may have potentially influenced medication management decisions such as the patient’s cognitive impairment, hand-breath coordination abilities, dexterity, inhalation device preferences, or medication adherence.15,27,28,31,38,39 There was also no available data on factors related to the risk of exacerbations such as physical activity40 and lung function as a direct measure of COPD disease severity.6 Finally, our analysis was based on fee-for-service Medicare beneficiaries and may not be generalizable to managed Medicare or other COPD populations. Despite these limitations, our study included a large number of Medicare beneficiaries, careful propensity-score matching between nebulized ARF users and controls, and a comprehensive evaluation of health and economic outcomes.
Conclusions
Nebulized ARF may be an appropriate treatment option among patients with COPD following a respiratory event such as a COPD-related hospitalization or an ED visit. In our retrospective study, Medicare beneficiaries who switched to nebulized ARF for maintenance therapy after experiencing multiple respiratory events had a significant reduction in the number of exacerbations at 180-days follow-up at similar overall costs when compared to beneficiaries who were treated on handheld ICS+LABA therapy. Selecting an inhalation device that best matches the patient’s clinical needs and his or her ability to use it successfully can increase the probability of positive outcomes.
Acknowledgments
We thank Vaidyanathan Ganapathy, PhD, formerly of Sunovion Pharmaceuticals Inc., for his support with study conceptualization and implementation. Authors’ contributions were as follows: MN, SC, and CD conducted literature searches and/or reviewed relevant articles identified through the searches; MN, BRC, SC, ZX, and TPG were responsible for study conceptualization, research design, and analytic plan development; ZX and TPG led data curation and analysis; and all authors were involved in results interpretation and presentation as well as preparation, review and final approval of the manuscript. An earlier version of this study was presented, in part, at the American Thoracic Society International Conference, May 18-23, 2018, in San Diego, CA.
Declaration of Interest
MN and SC are employed by Advance Health Solutions, LLC which received funding from Sunovion Pharmaceuticals Inc., to oversee this study. BRC received consultation remuneration as a member of the Medical Advisory Board at Advance Health Solutions, LLC. He has also been an expert pulmonologist consultant for GlaxoSmithKline, Boehringer Ingelheim, Astra Zeneca, Novartis, and Pulmonix. ZX and TPG are employed by the University of California San Diego which received a grant from Advance Health Solutions, LLC, to implement this retrospective study. CD is employed by Sunovion Pharmaceuticals Inc.