Running Head: Revefenacin for COPD Patients With Suboptimal PIFR
Funding Support: This study was funded by Theravance Biopharma, Ireland Limited Inc. (Dublin, Ireland). Mylan Inc., (Canonsburg, Pennsylvania) and Theravance Biopharma US, Inc., (South San Francisco, California) funded medical writing support.
Date of Acceptance: September 13, 2019
Abbreviations: chronic obstructive pulmonary disease, COPD; suboptimal peak inspiratory flow rate, sPIFR; dry powder inhaler, DPI; long-acting muscarinic antagonist, LAMA; forced expiratory volume in 1 second, FEV1; intent-to-treat, ITT; least squares, LS; confidence interval, CI; Global initiative for chronic Obstructive Lung Disease, GOLD; long-acting beta2-agonists, LABAs; forced vital capacity, FVC; inspiratory capacity, IC; adverse event, AE; standard deviation, SD; analysis of covariance, ANCOVA; optimal PIFR, oPIFR; tiotropium, TIO; revefenacin, REV; modified Medical Research Council, mMRC
Citation: Mahler DA, Ohar JA, Barnes CN, Moran EJ, Pendyala S, Crater GD. Nebulizedversus dry powder long-acting muscarinic antagonist bronchodilators in patients with COPD and suboptimal peak inspiratory flow rate.
Chronic Obstr Pulm Dis. 2019; 6(4): 321-331. doi:
http://doi.org/10.15326/jcopdf.6.4.2019.0137
Online Supplemental Material: Read Online Supplemental Material (393KB)
Introduction
Note: Data from this study were previously presented at the CHEST Annual Meeting, October 6—10, 2018, San Antonio, Texas
The Global initiative for chronic Obstructive Lung Disease (GOLD) strategy recommends inhaled bronchodilators including long-acting muscarinic antagonist (LAMAs) or long-acting beta2-agonists (LABAs) as maintenance treatment for patients with chronic obstructive pulmonary disease (COPD).1 Dry powder inhalers (DPIs) are one of the most frequently prescribed inhalation devices.2 However, for effective DPI use, patients must generate enough inspiratory force to overcome the internal resistance of the device to de-aggregate the powdered drug from its carrier into fine particles small enough for lung deposition.3 Results from in vitro and in vivo studies have demonstrated that a peak inspiratory flow rate (PIFR) ≥ 60 L/min against the internal resistance of an inhaler (e.g., Diskus®, GlaxoSmithKline) is optimal for effective DPI use.4-6 Because of lung hyperinflation, hypoxemia, and/or muscle wasting, many patients with COPD are unable to generate sufficient inspiratory force to use a DPI effectively.7 Suboptimal PIFR ([sPIFR]; < 60 L/min) has been observed in 19% to 78% of outpatients with COPD,6-9 suggesting that many patients with COPD may not be able to derive full benefit from DPIs.
We hypothesized that patients with sPIFR may achieve greater improvements in lung function with nebulized bronchodilator therapy than a similar dry powder bronchodilator. We compared the bronchodilation effect of the once-daily LAMA revefenacin inhalation solution via standard jet nebulizer with that of dry powder tiotropium delivered using HandiHaler® in patients with COPD and PIFR < 60 L/min against the simulated resistance of the Diskus.
Methods
Study Design and Conduct
This was a Phase 3b randomized, double-blind, double-dummy, active-comparator, parallel-group, 28-day multicenter study (NCT03095456. See online supplement for study design). It was conducted per the principles of the International Council on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guideline for Good Clinical Practice,10 and the code of ethics of the World Medical Association’s Declaration of Helsinki.11 All patients provided written informed consent. The protocol was approved by an institutional review board (Quorum Review IRB, 1501 Fourth Avenue, Suite 800, Seattle, Washington, 98101 [review file no. 32313]).
Patients and Treatments
We enrolled patients aged ≥ 40 years with moderate to very severe COPD and PIFR < 60 L/min against the resistance of Diskus. Other key eligibility criteria included a smoking history of ≥ 10 pack years, postipratropium forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio < 0.7, postipratropium predicted FEV1 < 80%, and screening FEV1 > 400 mL.
Key exclusion criteria included any chronic pulmonary condition other than COPD, including asthma, any medical condition that could preclude inhaled anticholinergics use, COPD hospitalization within 8 weeks of screening, and systemic corticosteroid or antibiotic use for respiratory tract infections within 8 weeks of screening. Patients with concurrent disease or condition, such as hepatic impairment, that in the opinion of the investigator, would interfere with study participation or confound the evaluation of safety and tolerability of the study drug were excluded. Patients were permitted to continue concurrent LABA or inhaled corticosteroid/LABA therapy.
Patients were randomized (1:1) in a double-blind manner to receive revefenacin 175 µg inhalation solution once daily via Pari LC® Sprint jet nebulizer (Pari Respiratory Equipment, Inc.) or active-comparator tiotropium 18 µg dry powder once daily via HandiHaler device (see Online Supplement for randomization details). Blinding of the study drugs was maintained by the double-dummy design. Revefenacin and its matched placebo were supplied as solutions in sealed plastic vials. Tiotropium was blinded, as described previously,12 using a color-matched placebo capsule and a foil overlay masking the placebo package and the commercial blister packing of tiotropium.
Endpoints
The primary endpoint was change from baseline in trough FEV1 at Day 29. Due to the expected enrollment of patients with mild airflow obstruction, a prespecified subgroup analysis based on airflow obstruction severity—including a subgroup with post-bronchodilator FEV1 < 50% predicted—was planned to compare efficacy in patients with severe to very severe disease. Key secondary efficacy endpoints were the effect of revefenacin versus tiotropium on trough FVC and inspiratory capacity (IC) at Day 29 and peak FEV1 and FVC at Day 29 (0-4 hours).
A post hoc analysis was performed to examine the effect of sPIFR levels on raw change from baseline in trough FEV1 by dividing sPIFR into quintiles (< 33 L/min, ≥ 33 to < 45 L/min, ≥ 45 to < 52 L/min, ≥ 52 to < 56 L/min, and ≥ 56 to < 60 L/min), with approximately the same number of participants within each quintile. Safety was assessed through adverse events (AEs) evaluation.
Assessments
PIFR was assessed at screening, randomization, and at the end of the study using In-Check™ DIAL (Alliance Tech Medical, Inc., Granbury, Texas) with resistance set to Diskus as well as HandiHaler. The relationship between Diskus and HandiHaler resistance was estimated using a multiple regression prediction model.13 Patients were instructed to exhale completely, place the mouthpiece of the device into their mouths, and inhale forcefully and as deeply as possible to quantify the PIFR; final values were recorded as the maximum of 3 sequential measurements.
Efficacy was assessed via pulmonary function tests, including trough and peak FEV1, FVC, and trough IC measurements using spirometry at baseline and/or peak on Days 1 and 29. Rescue albuterol medication use was also assessed.
Statistical Analyses
Sample Size
We planned to enroll approximately 200 patients (n = 100 patients/group) to ensure 150 evaluable patients, assuming a withdrawal rate ≤ 25% and assigned in a 1:1 ratio to revefenacin or tiotropium. The study had ≥ 90% power to detect a difference between revefenacin and tiotropium treatments ≥ 80 mL (assuming standard deviation [SD] = 250 mL, a model R2 of 0.65, and a 2-sided 5% significance level) in change from baseline to Day 29 trough FEV1. The statistical testing of hypotheses was conducted in a sequential manner starting with trough FEV1. If the primary endpoint was not statistically significant, all endpoints were declared nonsignificant. The results are reported as point estimates with 95% confidence interval (CI).
Efficacy
The primary endpoint of trough FEV1 was measured as a change from baseline after the 28th dose on Day 29 in the intent-to-treat (ITT) population and patients with evaluable FEV1 measurements. An analysis of covariance (ANCOVA) model was used to evaluate the difference between revefenacin and tiotropium treatments. Treatment group, maximum inspiratory pressure at baseline, ipratropium reversibility, smoking status, concomitant LABA use, sex, age < 65 years, supplemental oxygen use, baseline PIFR, and center and baseline FEV1 with interaction terms were incorporated as covariates using the ITT population.
Secondary endpoints of trough FVC and IC and peak FEV1 and FVC were assessed via an approach similar to that used for the primary endpoint.
Results
Study Population
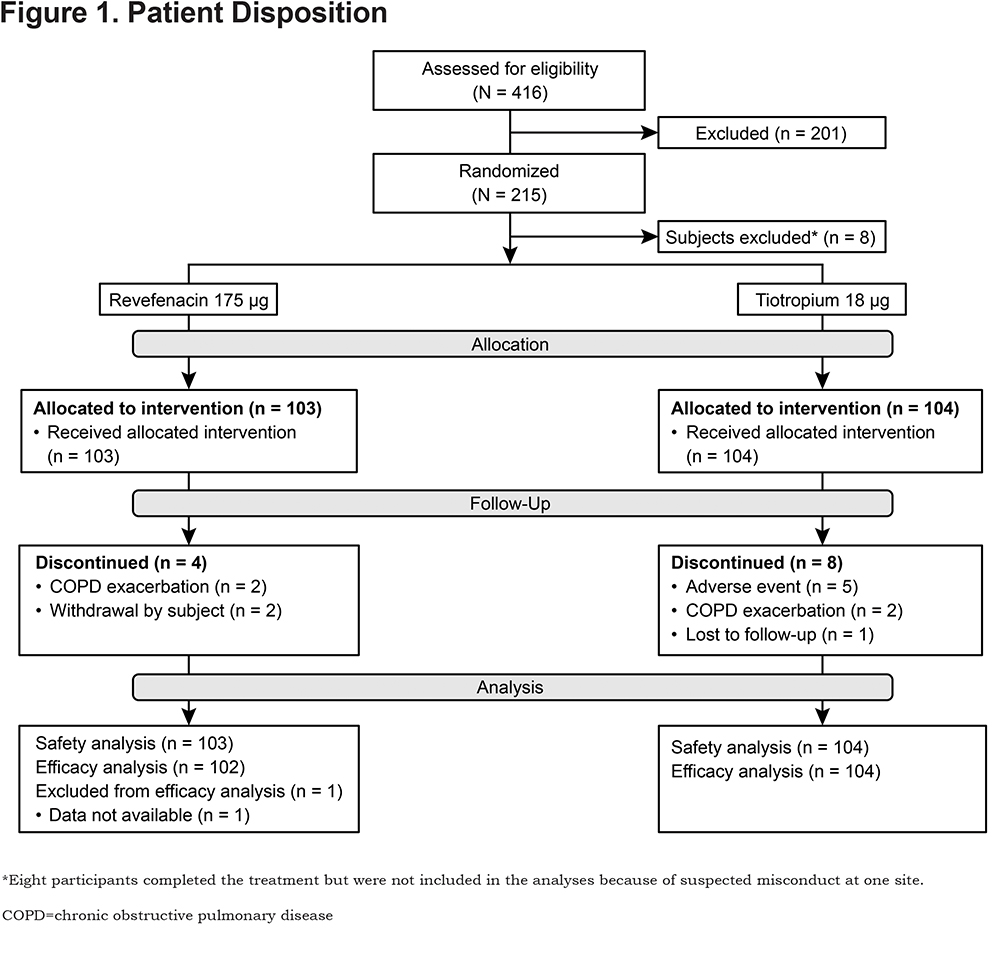
This trial was conducted at 38 study sites in the United States between March and November 2017. Of 215 patients who were randomized and received treatment, 8 were excluded from analyses due to suspected misconduct at 1 study site; therefore, 207 patients were included in the safety analysis set (Figure 1). Overall, 206 patients were included in the efficacy analyses as 1 patient did not have efficacy data available.
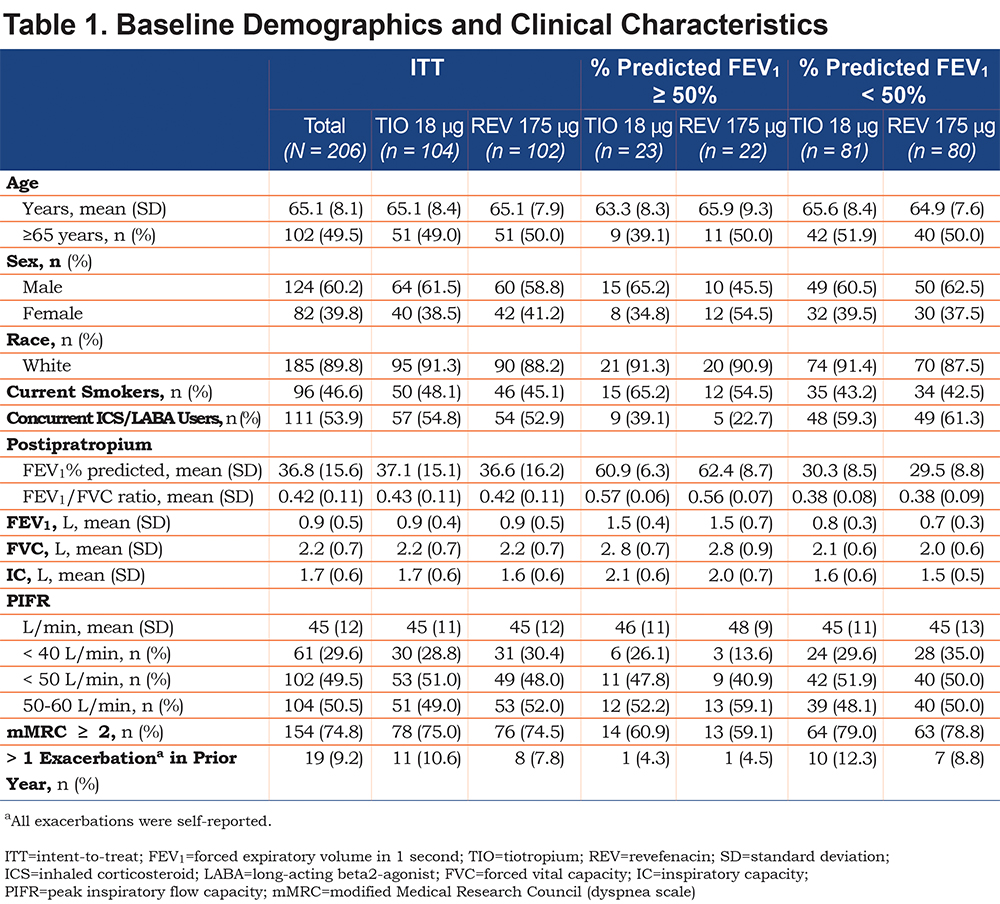
Patient demographics and baseline characteristics are summarized in Table 1. Overall, the study population had severe disease, as reflected by mean (SD) percent predicted FEV1 (37% [15.6%]) and baseline FEV1 (0.92 [0.455] L). There were noted differences in baseline lung function (FEV1, FVC, IC) across the airflow limitation categories; however, baseline PIFR values were similar between the 2 subgroups.
Efficacy Outcomes
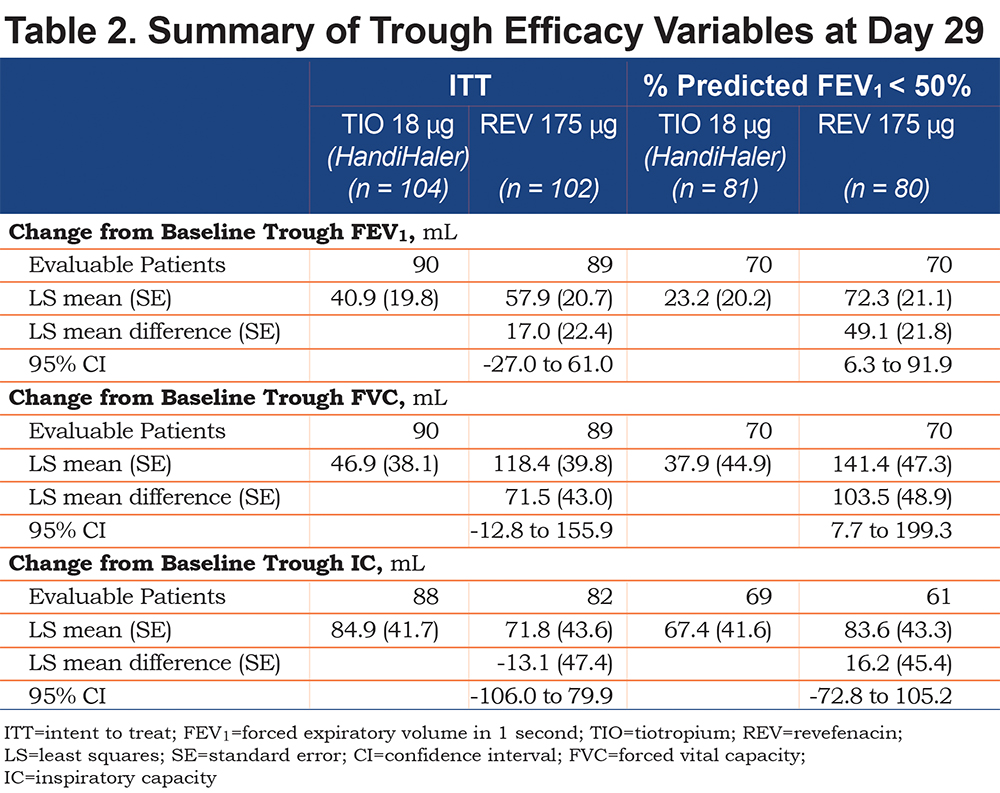
In the ITT population, revefenacin treatment produced numerically greater improvements from baseline in trough FEV1 versus tiotropium; however, the least squares (LS) mean difference was not statistically significant (Table 2). In patients with FEV1 < 50% predicted (78% of ITT population), revefenacin produced a greater change in trough FEV1 than tiotropium with LS mean difference of 49.1 (SD, 21.8; 95% CI, 6.3–91.9) mL (Table 2). The likelihood of > 80 mL change from baseline in trough FEV1 at Day 29 favored revefenacin with an odds ratio of 1.95 (95% CI, 1.03–3.68). The effect was driven by patients with FEV1 < 50% predicted, with an odds ratio of 3.57 (95% CI, 1.66–7.69). More patients achieved >100 mL change from baseline in trough FEV1 with revefenacin than tiotropium in ITT (37 [41.6%] versus 31 [34.4%]) and FEV1 < 50% predicted (29 [41.4%] versus 18 [25.7%]) populations.
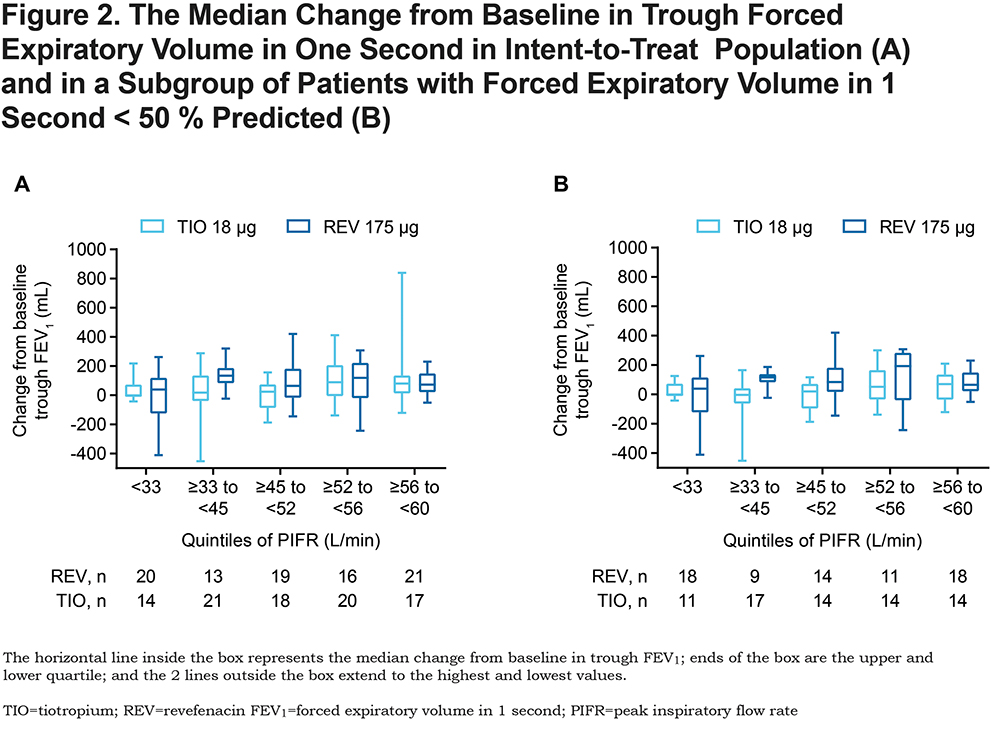
Additionally, changes in trough FEV1 were examined by dividing sPIFR into quintiles. In the ITT population, revefenacin treatment produced numerically greater changes in the median trough FEV1 than tiotropium in the ≥ 33 to < 45 L/min quintile of PIFR; no substantial differences were observed in other quintiles (Figure 2A). In the FEV1 < 50% predicted population, numerically greater improvements from the baseline in the median trough FEV1 were observed with revefenacin in the ≥ 33 to < 45 L/min , ≥ 45 to < 52 L/min, and ≥ 52 to < 56 L/min quintiles of PIFR; no substantial differences were observed in the < 33 L/min and ≥ 56 to < 60 L/min quintiles (Figure 2B). Substantial variability of FEV1 was observed in all quintiles, especially in the < 33 L/min quintile, which demonstrated greater instability than other subgroups.
Revefenacin produced numerically greater improvements in Day 29 trough FVC than tiotropium in the FEV1 < 50% predicted population (LS mean difference [SD], 103.5 [48.9] mL; 95% CI, 7.7–199.3 mL) and in the ITT population (LS mean difference [SD], 71.5 [43.0] mL; 95% CI, -12.8 to 155.9 mL; Table 2). More patients achieved > 200 mL increase in FVC from baseline, which is considered a clinically relevant change, with revefenacin than tiotropium in ITT (34 [38.2%] versus 29 [32.2%]) and FEV1 < 50% predicted (29 [41.4%] vsersus 20 [28.6%]) populations. No significant between-group differences were observed in trough IC at Day 29 (Table 2) or peak FEV1 or FVC on Days 1 and 29 (data not shown). Rescue albuterol use was not different between revefenacin and tiotropium groups (LS mean [standard error], 3.4 [0.42] versus 2.9 [0.41] puffs/day; LS mean difference, 0.6 [0.49] puffs/day; 95% CI for LS mean difference, -0.4 to 1.5 puffs/day).
Safety Outcomes
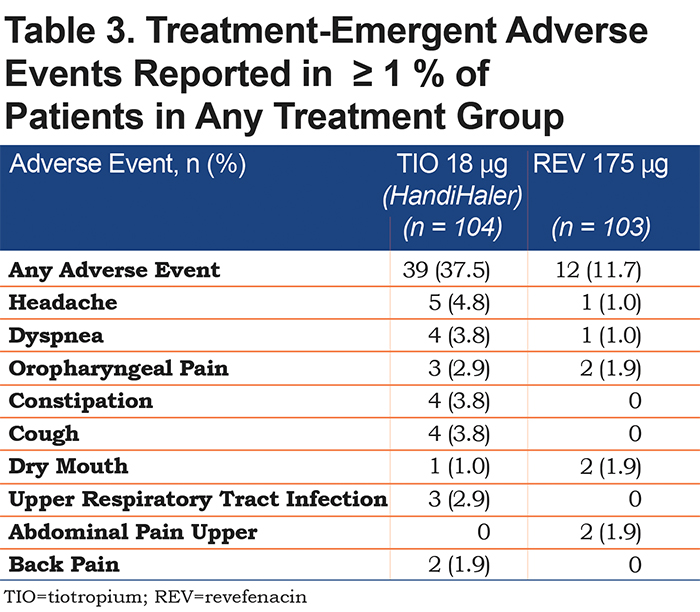
Very few AEs were reported for either group, but fewer occurred with revefenacin than with tiotropium (Table 3). Dyspnea and cough were the only treatment-related AEs reported in > 2% of patients in either group. Patients taking tiotropium reported more treatment-related AEs than patients taking revefenacin. The overall rate of antimuscarinic AEs (dry mouth and constipation) was low, but it was even lower in patients treated with revefenacin than with tiotropium. One serious AE (COPD exacerbation) was reported in a patient in the tiotropium group. No serious AEs were reported in patients from the revefenacin group. AEs leading to permanent discontinuation of study drug were reported in 5 (4.8%) patients, and all were in the tiotropium group.
Discussion
Efficacy of bronchodilators delivered through DPI in patients with COPD and sPIFR is unknown. In one study, a single dose of nebulized arformoterol provided significantly greater increases in FVC and IC (but not FEV1) at 2 hours than salmeterol Diskus in patients with COPD and sPIFR (<60 L/min) to Diskus.14 In this multicenter, multi-dose randomized trial, we compared the bronchodilator effects of 2 once-daily LAMAs delivered through nebulization versus DPI in patients with COPD and sPIFR.
In the ITT population, the changes from baseline in trough FEV1 and trough FVC were not significantly different between delivery systems, whereas, in patients with severe to very severe airflow obstruction, revefenacin for nebulization produced numerically greater changes than dry powder tiotropium. Results of a post hoc analysis based on PIFR quintiles suggest a greater benefit from nebulization than DPI in patients with PIFR ≥ 33 to < 45 L/min in the ITT population and PIFR < 56 L/min in the FEV1 < 50% predicted subgroup.
Because this is the first multi-dose trial examining patients with COPD and sPIFR, the sample size was calculated based on a projected difference of 80 mL in change from baseline in trough FEV1 at Day 29 between revefenacin and tiotropium. Revefenacin demonstrated efficacy in two 12-week, Phase 3 registration trials (NCT02459080 and NCT02512510), in which revefenacin 175 µg improved trough FEV1 at Day 85 by a mean value of 148 mL versus placebo.15 Comparator tiotropium, which has been available in the United States since 2003, demonstrated substantial improvements versus placebo in trough FEV1, ranging from 114 mL at Day 92 during a 13-week trial to 137 mL following 24 weeks of treatment.16,17 Patients in this 4-week trial, however, had more severe airflow obstruction (mean FEV1, 37% versus 55% predicted) and reported more dyspnea (modified Medical Research Council dyspnea scale score > 2, 75% versus 51%) than observed in the registration trials. Two LAMAs were compared in this trial, whereas revefenacin was compared with a placebo in the registration trials.
HandiHaler has the highest internal resistance of the DPIs.7 In an in vitro study, an inspiratory flow rate of 40 L/min or higher was shown to deliver an optimum dose of the powdered drug.18 Because our trial was a pilot study enrolling patients with COPD and sPIFR, we used the optimal PIFR of low-medium resistance DPI Diskus (60 L/min)19,20 as the cut-off for sPIFR. Additionally, Diskus resistance is the most frequently used resistance for reporting the prevalence of sPIFR.4,6,8,14,21,22 The 60-L/min PIFR against the Diskus resistance corresponded with 40-L/min PIFR against the HandiHaler in this study.13 Al-Showair and colleagues reported that a mean PIFR of 58 L/min for the Diskus resistance corresponded to 29 L/min for the HandiHaler resistance.4
Suboptimal PIFR has been demonstrated in 19% to 78% of outpatients and 32% to 52% of inpatients before discharge from the hospital after treatment for an exacerbation.6,8,21,22 In a recent study of 66 patients with COPD, 40% had sPIFR to prescribed DPIs.9 It has been hypothesized that clinical benefit may not be optimum with PIFR < 60 L/min against a specific DPI resistance. In the post hoc analysis based on PIFR quintiles in this study, numerically greater change from baseline in the median trough FEV1 was observed with nebulized therapy compared with a DPI in patients with PIFR ≥ 33 to < 45 L/min in the ITT population and < 56 L/min in patients with FEV1 < 50% predicted. For patients in the PIFR quintile < 33 L/min, inconsistent improvement in trough FEV1 was observed. This is likely due to lack of stability in this group, possibly because of difficulty in measuring inspiratory (lower limit of detection for InCheck DIAL is 20 L/min) and expiratory flow rates in patients with very low PIFR and airflow limitation. Prospective studies are required to further examine the most clinically relevant oPIFR value for use with DPIs. The secondary findings of improved lung function in the subgroup of patients with severe to very severe COPD and sPIFR (≥ 33 L/min to < 56 L/min) are also of interest. These data can be used to calculate an appropriate power effect and sample size for subsequent trials.
There are several study limitations. Because there was no difference in the primary outcome of the study in the ITT population, the findings in patients with FEV1 < 50% predicted should be interpreted with caution and require investigation in a future study. In addition, patient-reported clinical outcomes were not assessed in this trial and should be evaluated in future studies. Since this was a 4-week trial designed to evaluate the efficacy of revefenacin for nebulization versus dry powder tiotropium, the long-term safety of revefenacin in patients with COPD and sPIFR was not assessed. However, there were no new safety concerns in this patient population.
The 2019 GOLD strategy emphasizes a personalized approach to the treatment of COPD.1 Routine measurement of PIFR against the DPI being considered for treatment incorporates the principle of precision medicine.7 Patients with sPIFR are predominantly women and have a shorter height, lower percent predicted FVC and IC values, and reduced inspiratory muscle strength.6,8,21-23 Lung hyperinflation, which is common in outpatients with COPD and develops with an exacerbation, adversely affects respiratory muscle strength and the ability of patients to generate oPIFR.24,25 Prospective studies that compare clinical and physiological outcomes with a DPI and other delivery systems (pressurized metered-dose inhalers, slow mist inhalers, and nebulizers) are needed in patients with COPD who have sPIFR.
Conclusion
Although revefenacin administered once daily via a standard jet nebulizer produced numerically greater improvements from baseline in trough FEV1 and FVC than dry powder tiotropium, the differences were not significant. Revefenacin produced substantial improvements in lung function among patients with FEV1 < 50% predicted compared with tiotropium.
Acknowledgements
This study was funded by Theravance Biopharma, Ireland Limited Inc., (Dublin, Ireland). The authors acknowledge Ritu Pathak, PhD, and Autumn Kelly, MA, for medical writing and Frederique H. Evans, MBS, for editorial assistance in the preparation of the manuscript (Ashfield Healthcare Communications, Middletown, Connecticut).
Author Contributions: CNB, EJM, SP, and GDC take responsibility for the conception and design. DAM, JAO, CNB, EJM, SP, and GDC take responsibility for data analysis and interpretation. DAM, JAO, CNB, EJM, SP, and GDC take responsibility for drafting the manuscript for important intellectual content. All authors approve the submitted version of the manuscript and agree to be accountable for the accuracy and integrity of the work.
Declaration of Interest
DAM has served on advisory boards for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline plc, Grifols SA, Sunovion Pharmaceuticals Inc., Theravance Biopharma, Inc., and Trevi and is on the speaker’s bureau for AstraZeneca, Boehringer Ingelheim, and Sunovion Pharmaceuticals Inc. JAO has served on advisory boards for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline plc, Mylan Inc, Reckitt Benckiser Group plc, Sunovion Pharmaceuticals Inc., and Theravance Biopharma, Inc. EJM and GDC are employees of Theravance Biopharma US, Inc. CNB and SP were employees of Theravance Biopharma US, Inc. at the time this study was conducted.