Running Head: Differential Pulmonary Subtype Progression in COPD
Funding Support: The COPDGene® study is funded by the National Heart, Lung, and Blood Institute grants U01 HL089897 and U01 HL089856. The COPDGene® study is also supported by the COPD Foundation through contributions made to an Industry Advisory Committee comprised of AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion.
Date of Acceptance: October 29, 2019
Abbreviations: Global initiative for chronic Obstructive Lung Disease, GOLD; COPD Genetic Epidemiology, COPDGene®; high-risk airway-predominant disease only, APD-only; moderate-risk airway-predominant disease only, MR-APD-only; high-risk emphysema-predominant disease only, EPD-only; combined high-risk airway and emphysema-predominant disease, combined APD-EPD; combined moderate-risk airway and emphysema-predominant disease, combined MR-APD-EPD; preserved ratio-impaired spirometry, PRISm; odds ratio, OR; confidence interval, CI; chronic obstructive pulmonary disease, COPD; computed tomography, CT; 6-minute walk distance, 6MWD; longitudinal follow-up program, LFU; wall area percentage, WA%; Hounsfield units, HU; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; preserved ratio-impaired spirometry, PRISm; body mass index, BMI; standard deviation, SD; percentage low attentuation of area, LAA%; analysis of variance, ANOVA
Citation: Young KA, Strand MJ, Ragland MF, et al for the COPDGene® Investigators. Pulmonary subtypes exhibit differential Global Initiative for Chronic Obstructive Lung Disease spirometry stage progression: the COPDGene® study. Chronic Obstr Pulm Dis. 2019; 6(5): 414-429. doi: http://doi.org/10.15326/jcopdf.6.5.2019.0155
Online Supplemental Material: Read Online Supplemental Material (345KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous disorder.1 The Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines2 are currently used to grade the severity of spirometric disease. Previous studies have indicated that lung function declines and progression occurs across GOLD stages,3-6 although decline varies and many individuals remain stable. While smokers without spirometrically defined COPD commonly show indications of lung impairment and disease,7,8 the risk and pattern of progression to spirometrically defined COPD under GOLD stages is unclear. Previous studies have indicated that only 20% progress9 to defined COPD, but that may be an underestimation of the true number.10,11 Progression from preserved spirometry may depend on subtypes12 or clinical phenotypes such as increased air trapping.13,14
Recently, we identified continuous pulmonary disease axes based on the correlational structure of Phase 1 chest computed tomography (CT) and pulmonary function tests that were associated with mortality risk.15 The pattern of risk indicated a complex relationship, and we further classified individuals into high-risk pulmonary subtype groups based on mortality risk within the 2 underlying pathophysiologic disease axes (airway-predominant disease [APD] and emphysema-predominant disease [EPD]).16 These groups were associated with mortality independent of GOLD spirometry stage, and they appear to represent different disease pathways. We found that for the emphysema-predominant axis, marked increased risk for mortality occurred in the highest 2 deciles of the axis. In contrast, the airway-predominant axis showed a more robust differentiation of mortality risk with the top 60% showing an enhanced hazard ratio and the top 2 deciles the greatest increase in mortality hazard ratio (Figure 1).
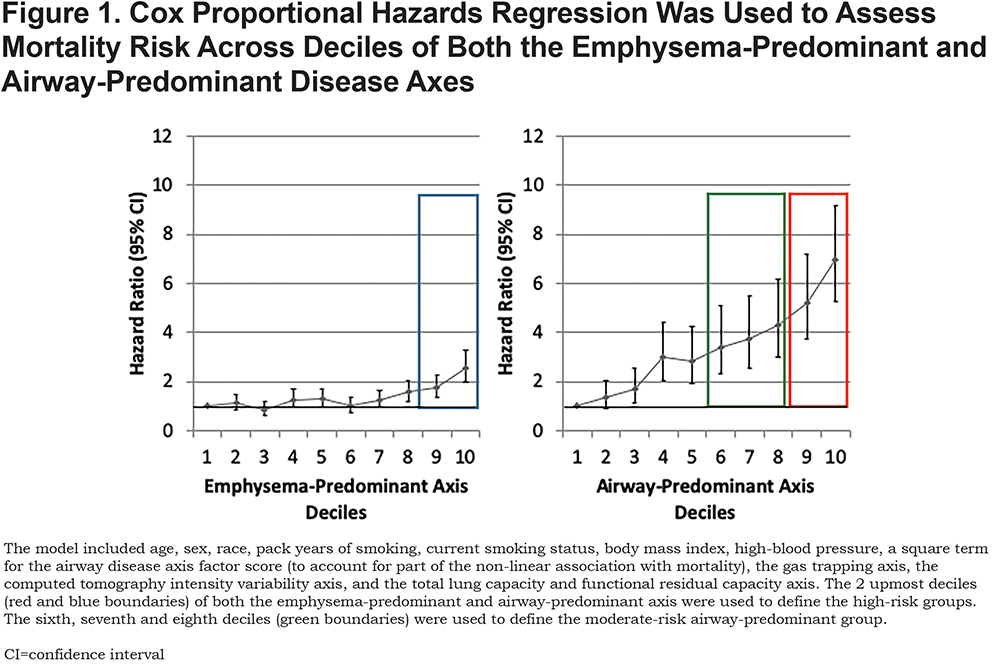
Because some high-risk individuals express characteristics of both the airway-predominant and emphysema-predominant subtypes, a further subdivision was done to create 6 groups that share common characteristics in terms of mortality risk and their relationship to the airway-predominant and emphysema-predominant subtypes. Our objective in the current analysis is to determine whether these high-risk groups are uniquely associated with GOLD spirometry stage progression.
Methods
COPD Genetic Epidemiology Study
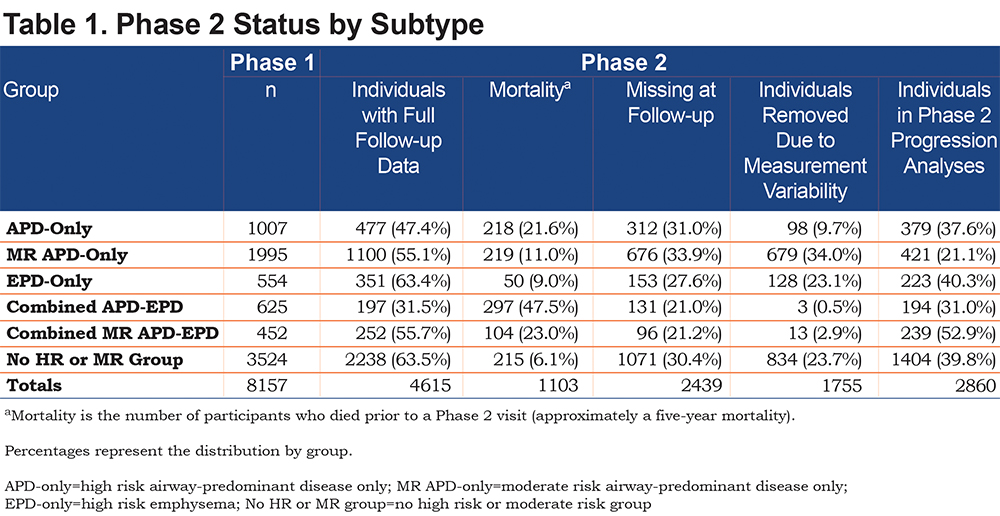
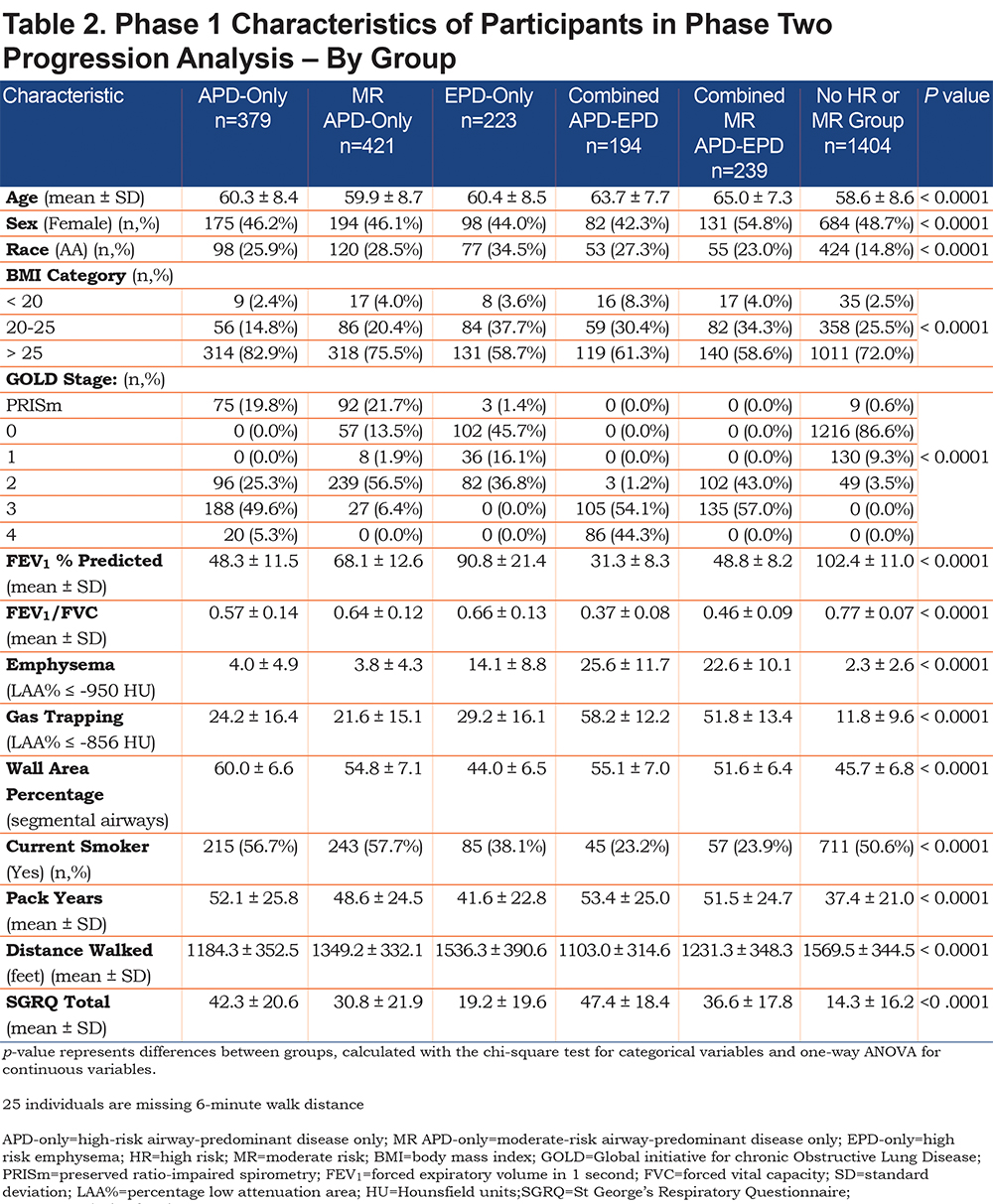
The Genetic Epidemiology of COPD (COPDGene®) study (www.copdgene.org) is a longitudinal multicenter observational study designed to identify genetic and epidemiologic factors associated with the development of COPD and to characterize disease progression using pulmonary function tests and volumetric CT scans.7 The COPDGene® study recruited 10,198 non-Hispanic whites and African Americans aged 45-80 years at 21 clinical centers across the United States beginning in 2008. Of the 10,198 participants enrolled at Phase 1 (baseline), 8157 had complete data required to assess disease axes.15 Of the 8157 Phase 1 participants, 4615 (3301 non-Hispanic whites and 1314 African Americans; 49% female) completed a 5-year Phase 2 follow-up with complete pulmonary subtype data and a defined GOLD spirometry stage at both Phase 1 and Phase 2 (Table 1). In order to assess disease-related progression to a different GOLD stage, we removed individuals who were sufficiently close to a GOLD boundary in Phase 1 that change across the GOLD boundary could likely be attributed to intra-individual measurement variability alone. Thus, 1755 individuals were removed from the Phase 2 progression analysis to reduce the impact of this random variability (see detailed methods below), leaving 2860 individuals for analysis. Descriptive characteristics of the study population stratified by these high-risk groups are reported in Table 2.
The COPDGene® cohort is made up of individuals with a heavy smoking history. All were current and former smokers with least 10 pack years of smoking history; however, the mean smoking history for all groups ranged from 37-53 pack years.17 The COPDGene® study was approved by the institutional review board at each center, and all participants provided written informed consent. In addition, all protocols were approved by the institutional review boards at National Jewish Health and the University of Colorado-Denver. Individuals underwent detailed baseline characterization, including demographics; anthropometrics; medical and smoking history; pre- and post-bronchodilator spirometry as well as 6-minute walk distance (6 MWD) testing according to American Thoracic Society standards18,19; and inspiratory and expiratory CT scans using a standardized protocol.7 Enrollment in Phase 1 of the COPDGene® study took place between 2008 and 2011, and participants were prospectively followed every 6 months by telephone and web-based inquiry as part of a longitudinal follow-up program (LFU) to determine mortality, comorbid disease events and disease status.20 Participants enrolled in Phase 1 were invited to participate in a 5-year follow-up study visit (Phase 2, 2012–2016).
Thoracic CT scans were acquired at full inspiration at both Phase 1 and Phase 2 using the same standardized protocol.7 Quantification of airway wall thickness, emphysema and gas trapping was performed by Thirona (Thirona, The Netherlands). Lung and airways structures were automatically extracted from inspiratory CT scans and visually approved by trained analysts. Airway wall thickness was assessed using wall area percentage (WA%) for segmental airways.21,22 In this analysis we report WA% which we found to be the most robust airway measurement for our primary assessment of airway disease. Emphysema was defined as the percentage of low-attenuation area below −950 Hounsfield units (HU) (% emphysema).23
Current smoking status and pack years of smoking were determined by questionnaire. COPD was grouped as spirometric grades 1–4 based on the GOLD guidelines.2 Participants without spirometric evidence of airflow obstruction (forced expiratory volume in 1 second [FEV1] to force vital capacity [FVC] ratio ≥ 0.70 and FEV1 ≥ 80% predicted) were classified as GOLD 0. Participants with FEV1/FVC ≥ 0.70 and FEV1 < 80% predicted were classified as preserved ratio-impaired spirometry (PRISm).8
Mortality Ascertainment
Vital status (mortality assessed through January 31, 2018) was determined through the COPDGene® participant tracking LFU program and augmented with searching the Social Security Death Index on the subset of participants with available social security numbers. Results were aggregated centrally. Participant follow-up time was the time between their Phase 1 study visit and identified death or most recent active LFU participation. Mortality for these analyses was limited to those who died between Phase 1 and Phase 2 (5 years) to account for their removal from the progression analyses.
Identification of High-Risk Groups Based on the Airway-Predominant and Emphysema-Predominant Disease Axes
Factor analysis of 26 spirometry and CT variables were used to classify 8157 COPDGene® participants onto disease axes. The loading of the variables on the axes described different disease processes. Two of these disease processes, the emphysema-predominant and airway-predominant axes, had increased mortality (Figure 1); in addition, a synergistic interaction between these 2 disease axes indicated that mortality was significantly increased at higher values of both axes in combination.15
To account for the non-linear association with mortality observed in our previous work and to determine if the association remained significant after adjustment for additional covariates, a Cox proportional hazards regression was used to assess mortality risk across deciles of both the emphysema-predominant and airway-predominant axes. The model included age, sex, race, pack years of smoking, current smoking status, body mass index (BMI), high-blood pressure, a square term for the airway disease axis factor score (to account for part of the non-linear association with mortality), the gas trapping axis, the CT intensity variability axis, and the total lung capacity and functional residual capacity axis.15 While increased risk of mortality was observed across most deciles of the airway-predominant axis, the 9th and 10th deciles had the highest risk of mortality. The next 3 deciles (6th, 7th and 8th) also showed an increased mortality hazard ratio (Figure 1). Across the emphysema-predominant axis, increased mortality was observed only in the 9th and 10th deciles of the emphysema-predominant factor. Based on these results, individuals were classified into 6 high-risk groups (Figure 2; Supplementary Figures S1-S6):
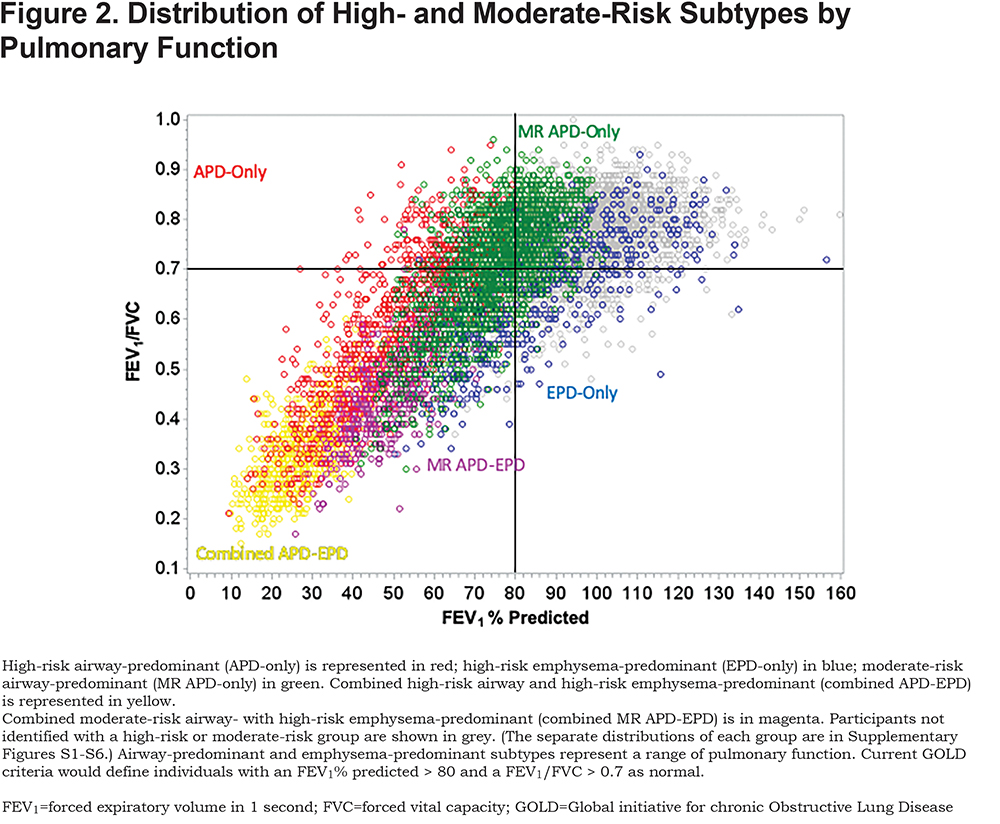
- APD-Only: High-risk airway-predominant disease —participants in the 9th or 10th deciles of the airway-predominant axis who are not also in the 9th or 10th deciles of the emphysema-predominant axis.
- MR APD-only: Moderate-risk airway-predominant disease only—participants in the 6th, 7th or 8th deciles of the airway-predominant axis and who are not also in the 9th or 10th deciles of the emphysema-predominant axis.
- EPD-only: High-risk emphysema-predominant disease only—participants in the 9th or 10th deciles of the emphysema-predominant axis who are not also in the 9th or 10th deciles of the airway-predominant axis.
- Combined APD-EPD: Combined high-risk airway and emphysema-predominant disease—participants in the 9th or 10th deciles of the airway-predominant axis who are also in 9th or 10th deciles of the emphysema-predominant axis.
- Combined MR APD-EPD: Combined moderate-risk airway and emphysema-predominant disease—participants in the 6th, 7th or 8th deciles of the airway-predominant axis and who are also in the 9th or 10th deciles of the emphysema-predominant axis.
- No HR or MR: No high-risk or moderate-risk pulmonary group—participants not in the 6th to 10th deciles of the airway-predominant axis and also not in the 9th or 10th deciles of the emphysema-predominant axis.
Statistical Analyses
Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, North Carolina). P-values less than 0.05 were considered statistically significant. Data are presented as means (standard deviation [SD]), n (percentages [%]), or odds ratios (OR) and 95% confidence intervals (CI) where appropriate. Phase 1 demographic and clinical characteristics between groups were compared with the chi-square test for categorical variables and one-way analysis of variance (ANOVA) for continuous variables.
Change in smoking status between Phase 1 and Phase 2 was classified into 4 categories: current-current, former-former, current-former, and former-current. Outcomes were dichotomized as yes/no for GOLD spirometry stage progression from Phase 1 to Phase 2 for:
1) GOLD 0 to PRISm (FEV1% predicted < 80%, FEV1/FVC > 0.70);
2) GOLD 0 to GOLD 1;
3) GOLD 0 to GOLD 2–4;
4) PRISm to GOLD 2–4;
5) GOLD 1 to GOLD 2–4.
Due to the small number of individuals in the comparison group (No HR or MR) in PRISm (n=9), multivariable regression models were not run for PRISm to GOLD 2–4 for this group. Therefore, we used 4 logistic regression models of the progression outcomes on the high-risk and moderate-risk groups to calculate OR and 95% Cis compared to the No HR MR group after adjustment for age, sex, race, and change in smoking status. Due to a priori hypotheses, we did not correct for multiple testing.
Intra-Individual Measurement Variability of Pulmonary Function
Twenty-nine participants in COPDGene® had duplicate visits within 6 months during which full clinical assessment was completed including both replicate CT scans and replicate pulmonary function studies. These participants were used to assess intra-individual measurement variability in repeat spirometry. The coefficient of variation of these pairs (mean/1 SD) for FEV1% predicted = 15.7% and for FEV1/FVC = 10.5%. To reduce the impact of measurement variability on our assessment of progression across GOLD boundaries, we removed individuals whose Phase 1 and Phase 2 FEV1% predicted was within 15.7% of 80% (67.44–92.56%) or whose Phase 1 and Phase 2 FEV1/FVC ratio was within 10.5% of 0.70 (0.6265–0.7735). This resulted in excluding 1755 individuals from the Phase 2 progression analysis. Thus, 2860 individuals with complete data and for whom a change across a GOLD boundary would likely represent disease progression and not just acute changes in pulmonary function were used for the Phase 2 progression analysis. The exclusion of all participants close to the GOLD boundaries from progression analysis will likely result in a conservative estimate of progression that would be expected to underestimate total progression. In addition, disease progression that does not result in crossing a GOLD boundary is not considered in this analysis. Changes in GOLD stage irrespective of intra-individual measurement variability are presented in Figure S7 in the online supplement.
Results
There were marked differences in all-cause mortality among the different high-risk groups defined by unbiased factor analysis. Five-year all-cause mortality for the high-risk and moderate-risk airway-predominant subtypes (APD-only and MR-APD-only) were 21.6% and 11.0% while 5-year mortality for the high-risk emphysema subtype (EPD-only) was 9% (Table 1). Participants in the combined APD-EPD group had a 5-year mortality of 47.5% and those in the combined MR-APD-EPD group had a 23.0% mortality (Table 1). Participants in the combined high-risk groups were primarily in GOLD 2–4 stages at the time of their Phase 1 visits (Figures 2, and S4 and S6 in the online supplement). The airway-predominant groups have higher mean values of measures of airway disease (WA%), while the emphysema-predominant groups have higher mean values of measures of emphysema (Table 2).
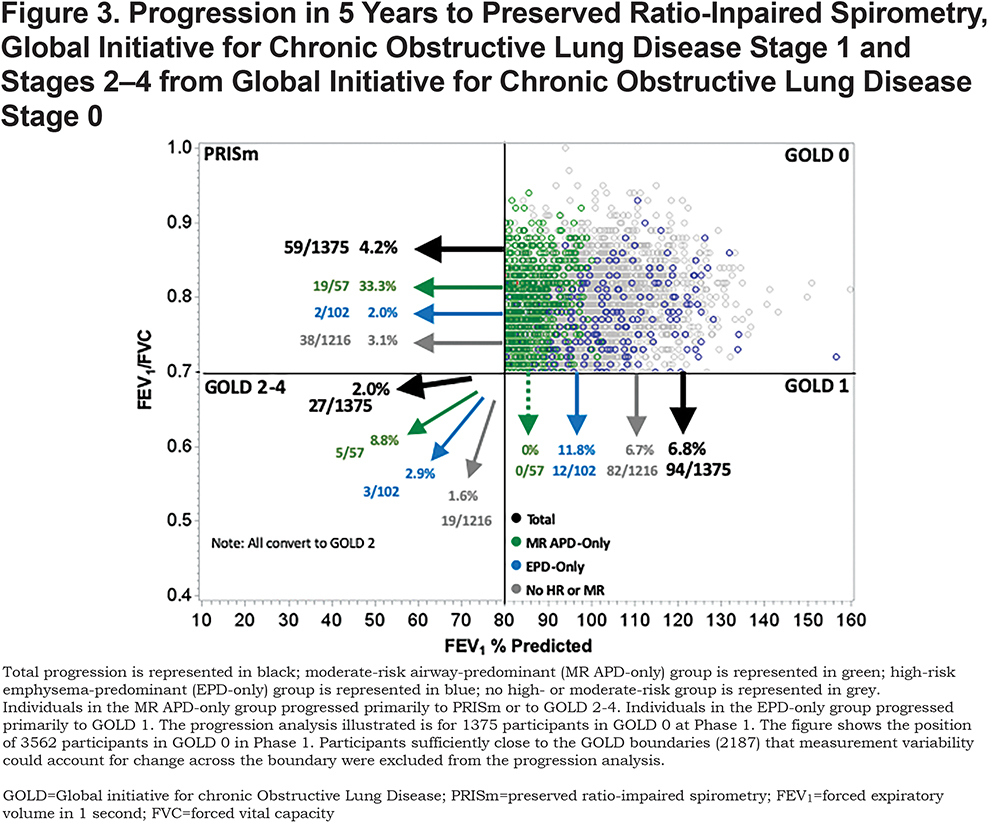
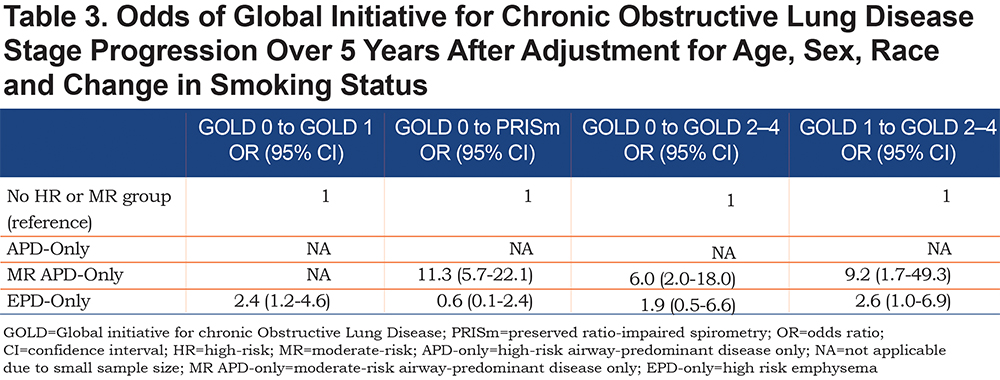
Of the 1375 participants available for progression analysis in GOLD 0 at Phase 1, 4.2% converted to PRISm, 6.8% converted to GOLD 1, and 2.0% converted to GOLD 2—4 at Phase 2 (5-year follow-up) (Figure 3). Individuals in the MR-APD-EPD group converted to PRISm (33.3%), but not to GOLD 1 (0.0%). Of individuals in the EPD-only group, 11.8% converted to GOLD 1, but few converted to PRISm (2.0%). Conversion to GOLD 2—4 occurred in both the MR-APD-only group (8.8%) and the APD-only group (2.9%). The MR-APD-only group was associated with conversion from GOLD 0 to PRISm (OR 11.3, 95% CI 5.7—22.1) and to GOLD 2—4 (OR 6.0, 95% CI 2.0—18.0) after adjustment for age, race, sex and change of smoking status (Table 3). The EPD-only group was associated with conversion from GOLD 0 to GOLD 1 (OR 6.0, 95% 2.0—18.0) after adjustment (Table 3).
Thirty-six percent of the 179 participants available for progression analysis in PRISm at Phase 1 converted to GOLD 2–4 at Phase 2 (Figure 4). No conversion from PRISm to GOLD 0 or GOLD 1 occurred at Phase 2. A large proportion of individuals in the APD-only group (30.7%) and the MR-APD-only group (37.8%) in PRISm at Phase 1 converted to GOLD 2–4 at Phase 2 (Figure 4). While a large proportion of the EPD-only group and the No HR MR group converted to GOLD 2–4, this is due to the small numbers of individuals in these groups (n=3 and n=9, respectively) in PRISm. Due to the small number of individuals in the comparison group (No HR MR) in PRISm, multivariable regression models were not run.

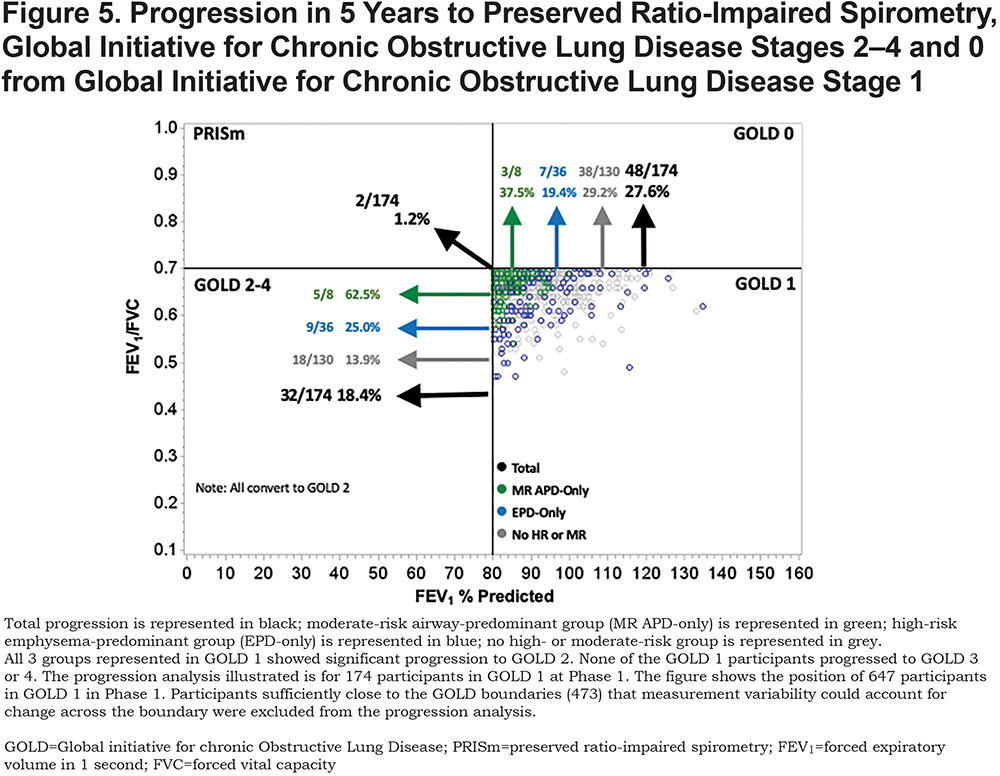
In the 174 participants available for progression analysis in GOLD 1 at Phase 1, 18.4% converted to GOLD 2–4, 1.2% converted to PRISm, and 27.6% converted back to GOLD 0 at Phase 2 (Figure 5). Individuals in the EPD-only group converted to GOLD 2–4 (25.0%) and GOLD 0 (19.4%). While a large proportion of individuals in the MR-APD-only group converted to GOLD 2–4 (5/8) and GOLD 0 (3/8), this represents small numbers of individuals with this group in GOLD 1 (8 total). The EPD-only group was associated with conversion from GOLD 1 to GOLD 2–4 (OR 2.6, 95% CI 1.0–6.9), after adjustment (Table 3). All participants progressing from GOLD 1 to GOLD 2–4 were categorized as GOLD 2 at Phase 2, and none progressed to GOLD 3 or 4 in this 5-year interval.
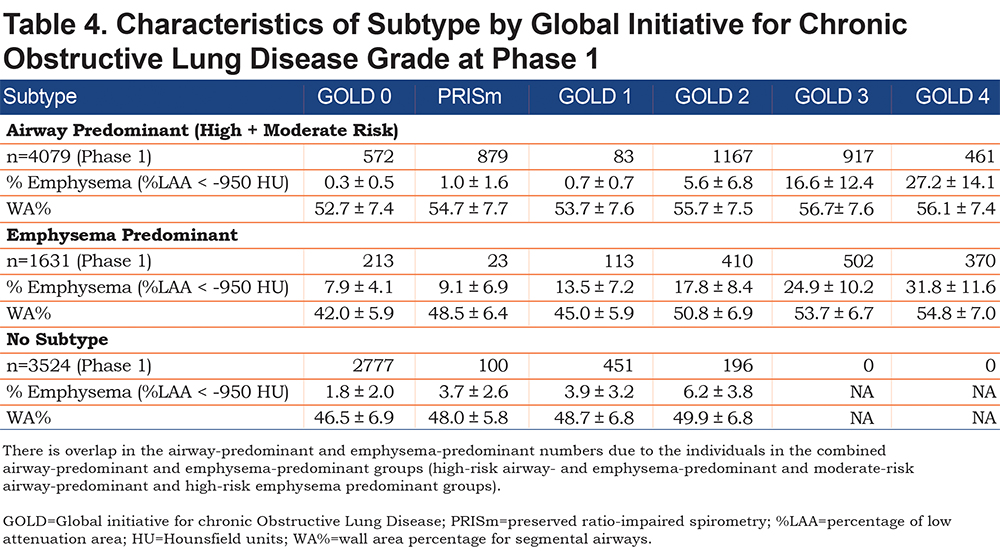
Participants in GOLD 0 and PRISm who have the airway-predominant subtype (combined APD-only and MR-APD-only) at Phase 1 have minimal emphysema (Table 4) but have an elevated WA% suggesting airway inflammation. A large number of the MR-APD-only group are located in GOLD 0 at Phase 1. Progression of these participants into PRISm at Phase 2 is not associated with development of emphysema on CT scan. When participants with airway-predominant disease initially progress from PRISm to GOLD 2–4, there is only a modest increase in emphysema. These participants appear to develop emphysema only late in the course of their disease as they progress to GOLD 3 and 4 (Table 4). These individuals, who do have a larger percentage of emphysema when in GOLD Stages 3 and 4, are also associated with a marked increase in 5-year mortality (Table 1).
In contrast, the participants in the EPD-only group develop CT-quantifiable emphysema early, while in the GOLD 0 stage, and move through GOLD 1 to GOLD 2 with a progressive increase in emphysema (Table 4). High-risk emphysema-predominant participants who also qualify for moderate- or high-risk airway-predominant disease demonstrate the highest degree of emphysema (Table 4) and the highest mortality (Table 1).
Analysis of 5-year progression of participants in GOLD 2 at Phase 1 was restricted to 252 participants who were considered appropriate for progression analysis after removing participants for whom change in GOLD stage could be attributed to intra-individual measurement variability. Of these 252 participants, 197 were in the MR-APD-only or APD-only groups and 71 (36%) of these progressed to GOLD 3–4 over 5 years. Forty-eight of the 252 participants were in the EPD-only group and 16 (33%) of these progressed to GOLD 3–4. After accounting for intra-individual measurement error, other changes involving participants in GOLD 2 at Phase 1 included: 6 (2.4%) went to GOLD 0, 6 (2.4%) went to GOLD 1, and 7 (2.8%) went to PRISm at Phase 2.
In a post-hoc analysis, recognizing that we took a conservative approach to deal with intra-individual variability, we examined total movement across all boundaries (Figure S7 in the online supplement). This analysis had no correction for measurement variability for FEV1 % predicted and FEV1/FVC and thus included individuals who changed GOLD stage at Phase 2 due to measurement variability rather than being due to a significant change in disease state. As shown in Figure S7 (online supplement), this analysis identified substantial movement in both directions across GOLD boundaries. However, overall net change in GOLD stages mimicked the progression to worse pulmonary function observed after accounting for intra-individual variability – as shown in Figures 3, 4 and 5.
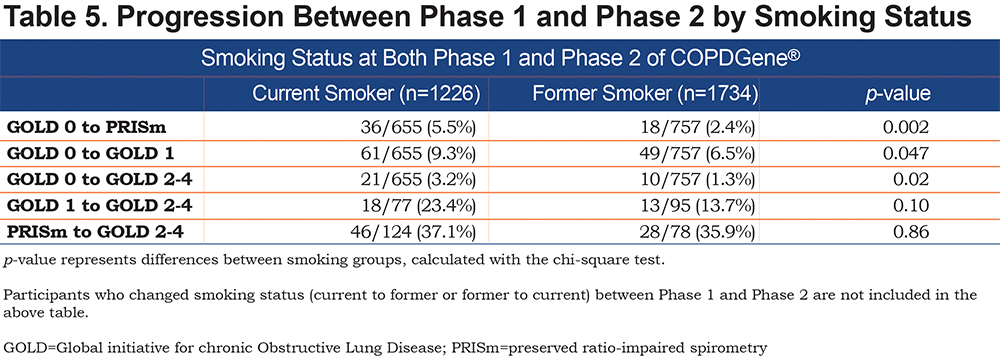
We assessed the impact of smoking cessation on progression of GOLD stage between Phase 1 and Phase 2 of COPDGene® (Table 5). After accounting for intra-individual measurement variability, 14.8% of individuals who were current smokers at both Phase 1 and Phase 2 progressed, whereas 6.8% of individuals who were former smokers at both Phase 1 and Phase 2 progressed in GOLD spirometry stage between Phase 1 and Phase 2 (p<0.0001). As shown in Table 5, the primary gain for smoking cessation in terms of progression across GOLD stage was for individuals in GOLD 0 or GOLD 1. Of current smokers in GOLD 0, 18.0% progressed to PRISm, GOLD 1 or GOLD 2–4. Of former smokers in GOLD 0, only 10.2% progressed to PRISm, GOLD 1 or GOLD 2–4. This contrasts with the findings for participants demonstrating PRISm physiology at baseline. Of current smokers in PRISm, 37% progressed to GOLD 2–4, and 35.9% of former smokers in PRISm progressed to GOLD 2–4.
Discussion
Previous work has defined 2 underlying pathophysiologic processes related to pulmonary function: airway-predominant disease and emphysema-predominant disease which are associated with differential risk of mortality.15 We now find that individuals with excess risk of mortality based on these disease axes demonstrate unique patterns of disease progression through GOLD stages towards more severe COPD. We have chosen to use a conservative approach to assess the magnitude of progression by excluding participants so sufficiently close to the GOLD boundaries that intra-individual measurement variability could account for change. We also did not assess progression within a GOLD stage that does not cross a boundary. Our results, by design, likely underestimate the true magnitude of progression. We recognize that participants close to a boundary are at greatest risk for progressing across that boundary. The current analysis is directed at evaluating the relative progression of the 6 subtypes identified by unbiased factor analysis and mortality risk.
Participants with airway-predominant disease or emphysema-predominant disease associated with excess mortality are identified within GOLD 0 and demonstrate higher levels of conversion to more advanced GOLD stages than individuals without 1 of these 2 underlying disease processes. The primary pattern for progression of the airway-predominant disease is from GOLD 0 to PRISm and from PRISm to GOLD 2–4 while the pattern for progression of emphysema-predominant disease is primarily from GOLD 0 to GOLD 1 and from GOLD 1 to GOLD 2–4. Fifty percent of the entire cohort (8157 individuals with a substantial smoking history) are associated with moderate or high-risk airway-predominant disease (combining APD-only, MR-APD-only and the combined APD-EPD groups [Figures 1 and 2]). Participants with airway-predominant disease appear to be on a pathway of disease progression that has not previously been well appreciated and that is associated with a markedly elevated 5-year all-cause mortality. Twenty percent of the entire cohort was identified as having high-risk emphysema-predominant disease (Figures 1 and 2) with a different pattern of disease progression and who also show a significant elevation in 5-year all-cause mortality. Identification of these 2 distinct patterns of COPD progression offer opportunities to identify participants for unique interventions to disrupt specific underlying disease processes related to pulmonary function decline.
Individuals in the MR-APD-only group represent 4.1% of GOLD 0. One-third (33.3%) of these progressed to PRISm within 5 years, while none progressed to GOLD 1, and a small proportion progressed to GOLD 2–4 (5/57). The vast majority of individuals in PRISm have airway-predominant disease (167/179) with only 1.7% having emphysema-predominant disease and 2.8% being in the no high-risk group category. Of the COPDGene® participants who were in PRISm at baseline, greater than 1/3 progressed to GOLD 2–4 over 5 years while none moved into GOLD 0 or GOLD 1. Thus, PRISm represents an advanced, progressive disease state associated with airway-predominant disease that shows no improvement in pulmonary function in 5 years. (Note: PRISm participants in the COPDGene® cohort are heavy current and former smokers, and participants with a clinical diagnosis of interstitial lung disease were excluded from this cohort.)
Participants with airway-predominant disease who progressed from GOLD 0 to PRISm and from PRISm to GOLD 2–4 over 5 years had minimal to no detectable emphysema and no significant change in emphysema from Phase 1 to Phase 2 (Table 5). Participants with airway-predominant disease who were in GOLD 2–4 at Phase 1 had substantial amounts of emphysema with marked differences between GOLD stages 2, 3 and 4 (Table 4). This suggests that development and progression of emphysema in the airway-predominant groups is late in the disease process. In comparison, the high-risk emphysema-predominant group had significant emphysema early in their disease process (i.e., in GOLD 0) at Phase 1. The high-risk emphysema-predominant group was associated with progressively greater amounts of emphysema associated with being in GOLD stages 1, 2, 3 and 4 in the Phase 1 cross-sectional analysis (Table 4).
Individuals at high risk of mortality associated with emphysema-predominant disease represent 7.4% of GOLD 0 participants. Participants with emphysema-predominant disease appear to be relatively stable within GOLD 0 with only 16.7% changing GOLD stage over 5 years (11.8% converted to GOLD 1, 2.0% converted to PRISm, and 2.9% converted to GOLD 2–4). GOLD 1 appears to be a relatively unstable stage with a total of 46.0% changing GOLD stage over 5 years (18.4% progressed to GOLD 2–4 and 27.6% moved back to GOLD 0). The majority of GOLD 1 participants changing GOLD stage were categorized as not being in a high-risk group. Participants with emphysema-predominant disease showed significant progression from GOLD 1 to GOLD 2–4 (25.0%, OR=2.6); however, 19.4% of GOLD 1 participants with emphysema-predominant disease moved back to GOLD 0.
Smoking cessation was found to have a substantial impact on decreasing the probability of an individual progressing in GOLD stage when smoking cessation occurred prior to progression to PRISm or to GOLD 2–4 (Table 5). All groups of participants in the COPDGene® cohort had a history of heavy smoking. Former smokers in GOLD 0 or GOLD 1 at baseline had a marked decrease in risk of progression over the next 5 years. In contrast, former smokers who were in PRISm at baseline carried the same risk of progression to GOLD 2–4 as did current smokers. This suggests that heavy smoking, when associated with airway-predominant disease and associated with progression into PRISm physiology, carries a poor prognosis even after smoking cessation.
The purpose of the current study was to investigate the progression of underlying pathophysiologic disease processes through clinically-defined categories of COPD (GOLD spirometry stages) over 5 years. Key strengths of the study are a large, well characterized cohort of current and former smokers with both CT scans and pulmonary physiology measures. In addition, intra-individual measurement variability of pulmonary function was derived from within the COPDGene® study. This allowed for removing noise associated with the well-recognized variability of the pulmonary function test.24,25 Our post-hoc analysis that eliminated consideration of intra-individual measurement variability showed a substantial amount of apparent movement of individuals across GOLD boundaries. However, net change in GOLD classification in this post-hoc analysis (Figure S7, online supplement) was found to closely mimic the primary findings of progression of pulmonary function to higher (worse) GOLD stages (Figures 3-5). Although by design, conservative estimates of progression were obtained, and it should be recognized that this approach will mainly identify rapid progressors. Further analyses examining movement within GOLD spirometry grade and including data from the ongoing COPDGene® Phase 3 will enable a better understanding of progression within the airway-predominant and emphysema-predominant disease subtypes.
Much previous work in COPD has shown that individuals with a low FEV1/FVC are at risk of rapid progression and loss of FEV1, and that these patients also have a poor overall prognosis.26 The question of disease progression in less advanced phases of lung disease remains open. The Lung Health Study looked at differences between those who quit smoking compared to those who continued to smoke in a cohort of smokers with largely mild to moderate disease, finding that continued smokers declined steadily in mean FEV1 at a rate of approximately 60 ml/year while quitters declined at approximately half that rate.27 There was no consideration in either of these studies of high-risk disease subtypes nor patterns of progression from smokers without defined spirometric disease.
This study identified 2 discrete patterns of disease progression from GOLD 0 to GOLD 2–4 among smokers. One pathway is characterized by those with airway-predominant disease and demonstrates an initial decline in both FEV1% predicted and FVC which would move them over 5 years toward or into PRISm physiology. A high percentage of COPDGene® participants who were in PRISm at baseline were found to move into GOLD 2–4 after 5 years of observation. This was associated with a further decline in FEV1% predicted and a decline in FEV1/FVC. A second pathway is defined by an initial 5-year decline in the FEV1/FVC ratio from GOLD 0 to GOLD 1 and is the major pathway seen among those with emphysema-predominant disease. While GOLD 1 appears to be a relatively unstable stage, the emphysema-predominant subtype shows an overall subsequent progression to GOLD 2–4. The characterization of these discrete patterns of disease progression associated with underlying pathophysiologic disease processes offers a unique opportunity for personalized targeted intervention to prevent disease progression and pulmonary function decline among smokers.
Acknowledgements
Author Contributions
All authors contributed to study design, critical review of the manuscript for important intellectual content, and interpretation of data. Statistical analysis was done by KAY, GLK, JEH, MJS, and EAR.
Declaration of Interest
EKS has received grant and travel support from GlaxoSmithKline. All other authors declare they have no conflicting interests.