Running Head: Perspective: A New Diagnostic Paradigm
Date of acceptance: November 5, 2019
Abbreviations: chronic obstructive pulmonary disease, COPD; computed tomography, CT; COPD Genetic Epidemiology, COPDGene®; Global initiative for chronic Obstructive Lung Disease, GOLD; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; preserved ratio-impaired spirometry, PRISm; Subpopulation and Intermediate Outcome Measures in COPD, SPIROMICS; St George’s Respiratory Questionnaire, SGRQ; American Thoracic Society, ATS
Citation: Make BJ. COPD: a new diagnostic paradigm. Chronic Obstr Pulm Dis. 2019; 6(5): 438-443. doi: http://doi.org/10.15326/jcopdf.6.5.2019.0172
Introduction
The disease we know as chronic obstructive pulmonary disease (COPD) continues a circuitous journey in its definition, characterization and clinical course. The article by Lowe et al in this issue of Chronic Obstructive Pulmonary Diseases: Journal of the COPD Foundation expands our understanding of the diagnosis, progression and mortality of this heterogeneous group of disorders.1 The definition of COPD is expanded and a new diagnostic term is introduced – COPDGene® 2019.
This perspective provides insights on the importance of this manuscript to clinicians, clinical researchers, and patients. Four questions are raised by this new definition of COPD:
· Does COPDGene® 2019 differ from the current definition of COPD?
· Is COPDGene® 2019 clinically important?
· Are there opportunities for future research to improve the implementation and management of COPDGene® 2019?
· How can clinicians employ COPDGene® 2019 in the management of their patients?
COPDGene® 2019 Represents a Logical and Patient-Centric Expansion of the Previous Definitions of COPD
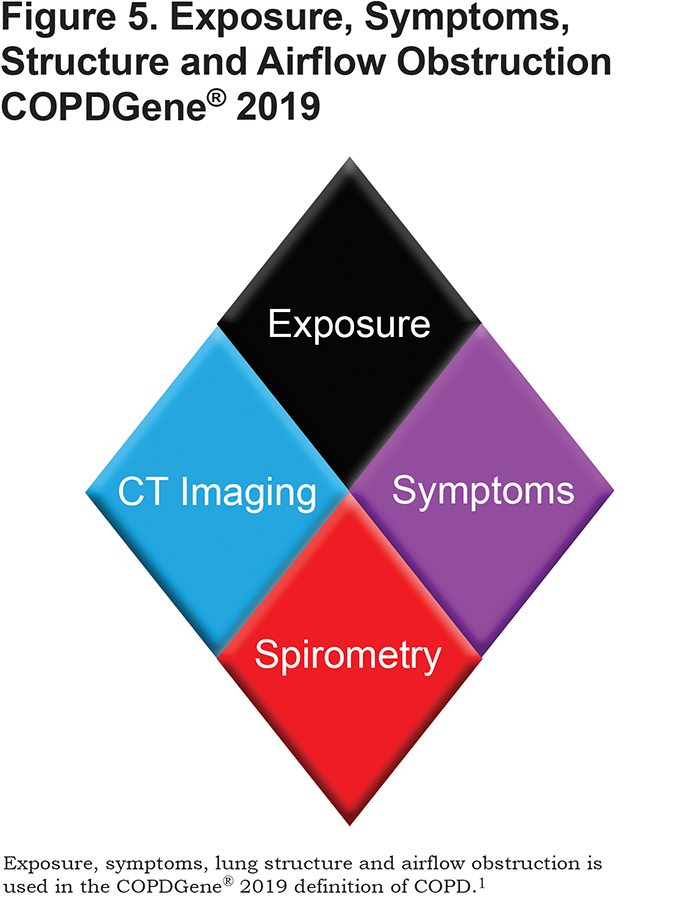
Four cardinal clinical features are included in the diagnostic criteria of COPDGene® 2019:
(1) exposure to toxic inhalants (cigarette smoking),
(2) respiratory symptoms (dyspnea and chronic bronchitis),
(3) structural lung disease (chest computed tomography [CT] assessment of lung pathology), and
(4) physiologic measure of airflow obstruction (spirometry).
Clinicians are familiar with these aspects of COPD and routinely evaluate them in clinical practice. Clinicians always assess smoking history in patients with respiratory symptoms. Similarly, shortness of breath, cough and sputum are queried in all patients with suspected respiratory disease in order to refine the differential diagnosis and assess disease severity. Spirometry is universally recommended in the assessment of patients with respiratory symptoms. Chest CT scans are frequently obtained in patients with a history of cigarette smoking as part of lung cancer screening, as recommended by the U.S. Preventative Services Task Force and endorsed by multiple professional societies.2 Chest CT scans are also ordered clinically as part of the diagnostic evaluation to determine the presence and nature of the underlying lung disease.
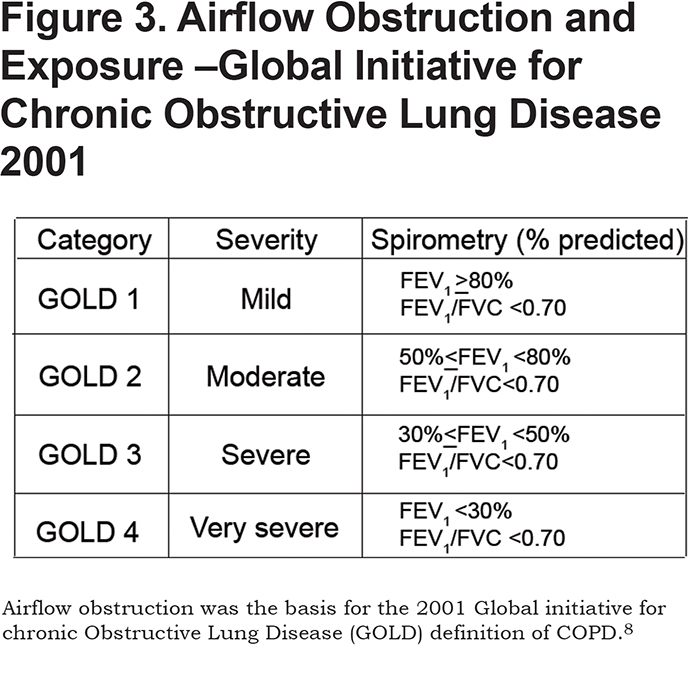
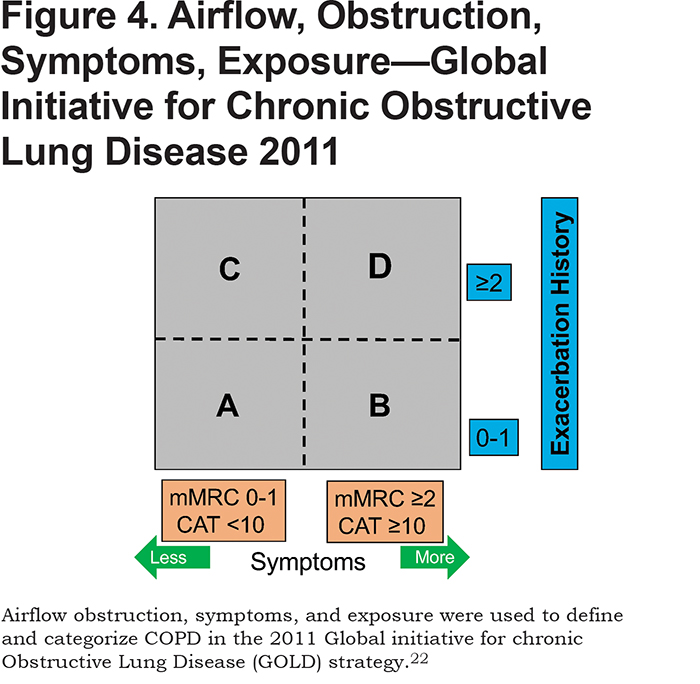
The 4 cardinal COPDGene® 2019 features are not new and are components of previous efforts to diagnose smoking-related lung disease. However, not all of these 4 features were included in previous definitions of COPD, as depicted in the figures at the end of this article (See Figures 1, 2, 3, 4, 5). Incorporating recent findings from the COPD Genetic Epidemiology study (COPDGene®) and other cohorts of individuals with a history of cigarette smoking, COPDGene® 2019 is a logical extension of previous definitions of COPD. The earliest descriptions of the pulmonary effects we now relate to cigarette smoking were those of airway disease based on symptoms of cough and sputum (chronic bronchitis) and structural airspace disease based on lung pathology (emphysema).3,4 The ability to physiologically measure lung function led to the incorporation of airflow obstruction as the major diagnostic feature of the term COPD and was used in the definitions of COPD proposed in the 1990s.5-7 The first Global initiative for chronic Obstructive Lung Disease (GOLD) strategy used only airflow obstruction and exposure to define COPD.8 Most recently, the GOLD reports since 2011 continued to use physiology as the major diagnostic criterion, also referring to respiratory symptoms, exposure, and structure without clear delineation of how to incorporate them in the diagnosis. Symptoms, but not structure nor physiology, were used to define categories of disease that were then linked to pharmacotherapy.9-11 COPDGene® 2019 incorporates all 4 features, exposure, symptoms, structure and physiology, and represents a logical step in redefining COPD.1
COPDGene® 2019 incorporates recent findings in 2 groups of patients with a history of cigarette smoking without classic COPD, i.e., without “classic” airflow obstruction as defined by a low forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio > 0.70. These 2 groups of patients are those (1) without “classic” airflow obstruction (a previously unnamed condition), and (2) without “classic” airflow obstruction and a low FEV1 (i.e., preserved ratio-impaired spirometry [PRISm]). These 2 groups of patients are included in COPDGene® 2019. Smokers without classic airflow limitation have been reported from the National Heart, Lung, and Blood Institute (NHLBI) supported COPDGene® and the Subpopulations and Intermediate Outcome Measures in COPD (SPIROMICS) studies to have significant impairments with respiratory symptoms, dyspnea, acute respiratory illnesses and related hospitalizations, structural changes and functional limitations.12,13 Poor outcomes have been shown in PRISm individuals in the COPDGene® study14 and recently validated in a general population cohort in Rotterdam.15
COPDGene® 2019 is unique in its incorporation of all 4 clinically significant features in a novel way to diagnose smoking-related lung disease. COPDGene® 2019 expands the definition of patients with a history of cigarette smoking to include those without classic spirometric airflow obstruction and those with chest CT scan structural abnormalities.
COPDGene® 2019 Is Clinically Important
The clinical significance of respiratory symptoms and emphysema on CT scan without classic airflow limitation are clarified by COPDGene® 2019.
Primary care physicians are perplexed by the changing COPD diagnostic landscape. They and their patients are frustrated that respiratory symptoms seem to have been ignored or relegated to minor importance in recent definitions of COPD. Clinicians frequently encounter patients with a history of cigarette smoking who present with cough, sputum, and dyspnea, but when spirometry is performed, airflow obstruction (as classically defined) is not present and thus the classic diagnostic criterion of COPD is not fulfilled. These patients may now be diagnosed with possible, probable or definite COPD under COPDGene® 2019, based upon an integrated assessment of respiratory symptoms and other features.
Similarly, patients with a history of cigarette smoking who have a chest CT scan clinically interpreted as showing emphysema or airway disease are frequently encountered and the clinical significance of their emphysema has been unclear. Up to 30% of lung cancer screening participants without a diagnosis of COPD have emphysema.16,17 These individuals without classic airflow obstruction do not fulfill the current GOLD definition of COPD but do fulfill the COPDGene® 2019 definition.
Most importantly, individuals with possible and probable COPD as defined by COPDGene® 2019 are at high risk for very poor outcomes. These patients have a high risk for substantial declines in lung function (FEV1 decline > 50 ml / year) and may progress to classic COPD. The finding that these patients have a high risk for all-cause mortality should sound the alarm to find therapies that can prevent disastrous outcomes.
COPDGene® 2019 Presents Clinical Research Opportunities
There is little doubt that the COPDGene® 2019 results as described by Lowe et al are clinically important.1 The most important research opportunity is to replicate the findings from the 8784 COPDGene® participants in other studies in the United States and around the world. Evidence from multiple investigations would assist in the dissemination of the importance of the expanded diagnosis of COPD in COPDGene® 2019 and spur clinical trials targeting patients in the possible and probable COPD categories. Further attempts to revise the diagnosis of the complex syndrome called COPD should take into account the heterogeneity of symptoms, structure, exposure, physiology, progression and mortality.
There are opportunities to simplify the implementation of the COPDGene® 2019 definition of COPD in clinical practice. COPDGene® 2019 used the classic definition of chronic bronchitis with cough and sputum production for 3 months or more in 2 years or more using the 6 questions in the American Thoracic Society (ATS) respiratory questionnaire.18 Kim et al found that the 2 cough and sputum questions from the St George’s Respiratory Questionnaire (SGRQ) identified patients at high risk for exacerbations.19,20 Would the 2 simple SGRQ questions replace the 6 more complex ATS questions in the COPDGene® 2019 definition?
COPDGene® 2019 presents an opportunity to bring chest CT scans into routine clinical practice. Quantitative measures of emphysema and airway disease were used in COPDGene® 2019 but not readily available from clinical chest CT imaging. Manufacturers of CT scanners can play a role in defining COPDGene® 2019 by routinely calculating and reporting computer quantification of emphysema and airway disease. However, standardization of the methods for measuring emphysema and airway disease, the impact of different CT protocols on these metrics and the use of different cut points for the amount of emphysema are needed. To simplify implementation of COPDGene® 2019 into clinical practice, would visual assessment of the presence of emphysema replace computer quantification of emphysema used in COPDGene® 2019?
The clinical features, along with the spirometric progression and mortality risk shown in Table 4 of the COPDGene 2019 Lowe et al manuscript,1 mandate that new therapies and currently available management modalities are tested in this group of vulnerable patients in rigorously designed clinical trials. The role of standard COPD therapies (e.g., vaccinations, pulmonary rehabilitation, pharmacotherapy) has not been studied in patients with possible or probable COPD as defined in COPDGene® 2019. Prescribing vaccinations and regular exercise is beneficial to all individuals and their potential role in improving outcomes for COPDGene® 2019 patients should be considered. Would reduction of acute respiratory events and hospitalizations prevent progression and mortality? Would pulmonary rehabilitation with continued regular exercise mitigate acute respiratory events and reduce mortality? Are there treatments that will slow the decline in lung function in COPDGene® 2019 patients? For example, in one recent trial in patients with mild-to-moderate COPD, tiotropium reduced the decline in lung function.21 Would pharmacotherapy have the same results in patients diagnosed with possible or probable COPD based on COPDGene® 2019? Smoking cessation reduces decline in lung function and improves survival in patients with mild COPD; would smoking cessation have the same results in COPDGene® 2019 patients? What novel therapies to reduce airway inflammation and reduce emphysema progression can be developed and tested in this population to prevent lung function decline and reduce mortality?
COPDGene® 2019 Can Be Implemented Into Clinical Practice
Primary care and pulmonary specialist clinicians can consider implementing COPDGene® 2019 in patients who are current or former cigarette smokers. Most importantly, clinicians should recognize the importance of assessing symptoms in current and former smokers and ask appropriate questions to elicit symptoms. Chest CT scans should be considered to search for structural abnormalities to identify COPDGene® 2019 patients. A clinical algorithm is suggested to implement COPDGene® 2019 into clinical practice:
- Ask all patients about current or former cigarette smoking. This is currently recommended and is a clinical practice quality metric. In current and former cigarette smokers, continue to steps 2 through 6.
- Ask about shortness of breath, cough and phlegm. The presence of either chronic bronchitis or dyspnea identifies patients with respiratory symptoms.
- The presence of significant dyspnea can be assessed in response to the question: On level ground, do you walk slower than people of the same age because of breathlessness, or do you have to stop for breath when walking at your own pace?
- Chronic bronchitis is defined as cough and phlegm for at least 3 consecutive months for 2 or more years.
- Perform spirometry pre-bronchodilator. In the presence of respiratory symptoms, spirometry is indicated to evaluate symptoms. If evidence of airflow obstruction is present, perform post-bronchodilator spirometry.
- Consider a chest CT scan. Lung cancer screening with CT scan is recommended in patients age 55-80 with ≥ 30 pack-year cigarette smoking history and smoking cessation < 15 years.2 In patients with respiratory symptoms, a chest CT scan may be indicated to assess the presence and character of intrinsic lung disease.
- Quantitative measures of emphysema are used in COPDGene® 2019. In the absence of emphysema quantification, interpretation by a chest radiologist may find evidence of structural lung disease.
- Determine the number of positive clinical features identified in steps 1 through 4.
- Two clinical features indicates Possible COPD as defined by COPDGene® 2019.
- Three clinical features indicates Probable COPD as defined by COPDGene® 2019.
- Four clinical features indicates Definite COPD as defined by COPDGene® 2019.
- For patients with Possible, Probable and Definite COPD per COPDGene® 2019 consider further management.
- Educate patients about the risk of disease progression and mortality.
- Institute aggressive and repeated attempts at smoking cessation.
- Consider additional COPD nonpharmacologic management – vaccinations, education about symptoms, education about exacerbation recognition, reduction and management, and avoidance of risk factors.
- Consider symptom reduction with pharmacotherapy.
- Consider other non-pharmacologic therapies, such as regular exercise.
- Monitor patients longitudinally for progression and symptom control.
The lack of clinical trials in COPDGene® 2019 should not deter clinicians from considering therapy and assessing response to the treatment for individual patients (“n of 1” trial). What treatments can reduce current symptoms? As shown in the COPDGene® and SPRIOMICS studies, these patients are frequently treated for dyspnea with inhaled bronchodilators, for which there is no evidence base – only because these patients have heretofore not been studied. The fact that these patients are receiving pharmacotherapy suggests that prescribing physicians and their patients have found these therapies to be beneficial. Treatment responses should be assessed by respiratory symptoms, lung structure, physiology, and progression as appropriate in individual patients based on their COPDGene® 2019 features and category and the treatment prescribed.
Smoking cessation is of paramount importance and has been shown to reduce decline in lung function and reduce mortality. Clinicians should target these patients with high risk of poor outcomes for aggressive smoking cessation efforts. Educating patients newly diagnosed as having possible or probable COPD, based on COPDGene® 2019 criteria, about the increased risk of morality and decline in lung function may serve as a motivating factor to quit cigarette smoking.
Although there are challenges in the implementation of COPDGene® 2019 in a wide variety of clinical practices, the algorithm above provides clinicians with a roadmap to more thorough assessment, monitoring and potential treatment of these patients today while awaiting the results of further research investigations.
COPDGene® 2019 represents a paradigm shift in the diagnosis of COPD by incorporating multiple components —patient-centered symptoms of chronic bronchitis and dyspnea, structural abnormalities on chest CT scan, airflow obstruction and cigarette smoking exposure. COPDGene® 2019 patients are at high risk of poor outcomes, and clinical trials should be designed to determine the best therapies to prevent progression and improve survival.