Running Head: Short Physical Performance Battery Test in COPD
Funding Support: This study was funded by a grant from Innovate UK. The awarding body did not have a role in study design, study conduct or interpretation of results. GlaxoSmithKline, a consortium partner, made in-kind contributions. The United Kingdom Clinical Research Network contributed towards the study at all participating sites. Participants from the Wales Heart Research Institute, Cardiff, were simultaneously recruited to the ARCADE study (ClinicalTrials.gov Identifier NCT01656421), which was funded by GlaxoSmithKline. Ian Wilkinson received funding support from the National Institute for Health Research (NIHR) Cambridge Comprehensive Biomedical Research Centre. Part of the work was undertaken at the NIHR Respiratory Biomedical Research Unit at the Royal Brompton and Harefield National Health Service Foundation Trust and Imperial College London, which partially funded Divya Mohan and Michael Polkey’s salaries.
Date of acceptance: October 10, 2019
Abbreviations: Short Physical Performance Battery, SPPB; 4-meter gait speed, 4mGS; 5-repetition sit-to-stand, 5STS; Evaluation of the Role of Inflammation in Chronic Airways Disease, ERICA; chronic obstructive pulmonary disease, COPD; principal component analysis, PCA; Committee for Medicinal Products for Human Use, CHMP; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; sniff nasal inspiratory pressure, SnIP; quadriceps maximal volitional contraction, QMVC; 6-minute walk test, 6MWT; St George Respiratory Questionnaire, SGRQ; COPD Assessment Test, CAT; modified Medical Research Council, mMRC; standard deviation, SD; interquartile range, IQR; odds ratio, OR; confidence interval, CI; body mass index, BMI; Body-mass index, airflow Obstruction, Dyspnea, and Exercise, BODE; activities of daily living, ADL; minimal clinical important difference, MCID; constant work rate, CWR
Citation: Mohan D, Benson VS, Allinder M, et al. Short physical performance battery: what does each sub-test measure in patients with chronic obstructive pulmonary disease? Chronic Obstr Pulm Dis. 2020; 7(1): 13-25. doi: http://doi.org/10.15326/jcopdf.7.1.2019.0144
Online Supplemental Material: Read Online Supplemental Material (462KB)
Introduction
The Short Physical Performance Battery (SPPB) is a well-established measure of lower limb function in gerontology which can predict future risk of nursing home admission and mortality. The SPPB consists of 3 individual sub-tests – standing balance, 4-meter gait speed (4mGS) and 5-repetition sit-to-stand (5STS). All 3 are simple and quick to perform in the outpatient setting and require only limited equipment. Although the SPPB was intended for use in seniors, there is increasing interest in its use in younger, but physically frail, populations and in particular those with chronic diseases, including chronic obstructive pulmonary disease (COPD).1 Use of this test is likely to increase given the European Medicine Agency’s 2016 statement that the SPPB is the frailty assessment of choice for trials addressing sarcopenia.2 More recently, it has been adopted by the Committee for Medicinal Products for Human Use (CHMP) for identifying patients with frailty.3 It remains unclear, however, which measure of frailty should be used as an outcome measure in clinical trials for patients with COPD.
In COPD, the 4mGS is the most well characterized of the 3 SPPB sub-tests, and could arguably be used as a single surrogate measure for frailty since it relates well to the overall SPPB score,4 as well as exercise capacity, health status and risk of hospital readmission in COPD.5,6 However, despite many useful test properties, including the identification of COPD patients at increased risk of hospital readmission following exacerbation6 and 1-year mortality,7 it is unknown whether 4mGS is a measure of functional limitation in COPD patients or whether one of the other tests may be more suitable, either for identification of functional limitation or as an outcome measure in interventional studies. Like the 4mGS, in COPD, the 5STS is closely associated with exercise capacity,8 as well as with mortality,9 and, in addition, other variants of this test, for example the 1 minute sit-to-stand test, also have prognostic power.10 To our knowledge only 1 study has reported on the standing balance in COPD.11
Few studies in COPD have compared individual SPPB sub-tests within the same population, but based on data from a single center, Bernebeau-Mora et al proposed the 5STS to be the most discriminative measure to identify functional limitation in COPD patients.12 In a subsequent analysis, Bernebeau-Mora et alalso reported that quadriceps strength was consistently associated with all 3 individual SPPB sub-tests in COPD,11 and an earlier study reported its association with SPPB total score.13 That study also provided a biological validity for SPPB in COPD by demonstrating a relationship between total SPPB score and other known features of skeletal muscle involvement in COPD, specifically Type II fiber switch, and muscle atrophy assessed by ultrasound.
Our objectives were firstly to identify clinical and demographic factors associated with the SPPB test scores overall and with each of the 3 SPPB sub-tests individually, and secondly to formally evaluate which of the 3 sub-tests is most discriminative in COPD patients. Our study will seek to validate findings from much smaller studies, and to add to the small body of existing literature.
Methods
Data from the Evaluation of the Role of Inflammation in non-pulmonary disease manifestations in Chronic Airways disease study (ERICA) was used for this analysis. The study protocol has been published previously14 (briefly summarized below) as has cross-sectional data from this study.15 The ERICA study is a prospective cohort study that recruited COPD participants from hospital outpatients and community settings at 5 United Kingdom sites. Baseline, cross-sectional data was used for this analysis. The study was approved by the Cambridge South Research Ethics Committee (reference 11/EE/0357) and local research and development departments at participating sites and was registered with the UK Clinical Research Network Study Portfolio. All participants provided written, informed consent.
Participants
Participants were aged between 40-90 years, male or female, with a clinical diagnosis of COPD (defined as post-bronchodilator forced expiratory volume in 1 second [FEV1] < 80% predicted, FEV1/forced vital capacity [FVC] ratio of < 0.7, and >10 pack-year smoking history). Relevant exclusion criteria was: known severe alpha-1-antitrypsin deficiency or significant co-existing pulmonary disease other than COPD; and individuals had to be clinically stable for > 4 weeks before assessments. Comorbid disease was not an exclusion criterion.
Assessment Measures
Baseline assessments were performed over 2 visits, within a maximum of a 3-month interval; physical performance tests were typically carried out on the first visit. The 3 SPPB sub-tests were scored from 0 to 4, with a higher score denoting better performance; total SPPB test scored from 0 to 12.1 Other assessments included demographics, self-reported exacerbation history, anthropometry, body composition by bioimpedance, post-bronchodilator spirometry, sniff nasal inspiratory pressure (SnIP), quadriceps maximal voluntary contraction (QMVC),16 blood pressure, 6-minute walk test (6MWT), health-related quality of life questionnaires including the St George’s Respiratory Questionnaire (SGRQ), the COPD Assessment Test (CAT) and modified Medical Research Council (mMRC), self-reported comorbidities, and inflammatory markers, including fibrinogen. Details of these assessments were reported in the study protocol.14
Statistical Analysis
Descriptive statistics were calculated using frequencies and proportions for categorical variables and means and standard deviations (SDs) for continuous variables; medians and interquartile ranges (IQRs) were calculated for all continuous variables that were not approximately normally distributed.
Proportional odds models were used to calculate odds ratios (OR) and 95% confidence intervals (CI) for determinants of SPPB total score and each of its 3 sub-tests.
For the outcome measures, SPPB score was treated as an ordinal variable and cut into categories based on differing degrees of functional limitation (0-6, 7-9, 10-12 [as described in Patel et al 2014]).13 Scores for the 5STS, 4mGS, and standing balance were cut into 3 ordinal categories based on their distribution in the population (5STS score was cut at 0-1, 2-3 and 4; 4mGS score and standing balance score were cut at 0-2, 3 and 4). Univariable models were fitted, modeling the effect of each explanatory variable as measured by the probability of a one-category reduction in the outcome variable, i.e., the probability of an increase in functional limitation. Explanatory variables with a p-value ≤ 0.15 and not highly correlated with another variable of interest were used to build a multivariable model.
All multivariable models were initially minimally adjusted for age, sex, and exacerbation history (0-1, 2+ exacerbations in the 12 months prior to study entry) as fixed effects and site as a random effect. Starting with the minimally adjusted model, a semi-automated stepwise approach was used to identify variables that significantly contributed to the model fit where p-value ≤ 0.05. Only patients with complete data for all assessed variables were used in the model building process (missing data was infrequent: most variables had a few missing values, but none had more than 10% missing). The final multivariable model was applied to all patients who had complete data for the variables included in the final model. The proportional odds model assumptions were checked for every final multivariable model fitted (further details provided in the supplemental material). Three sensitivity analyses were conducted for SPPB total score and each of the 3 sub-tests, namely: (1) excluding patients with a high sensitivity C-reactive protein concentration > 20mg/l in an attempt to remove patients with subclinical exacerbations; (2) excluding patients who completed pulmonary rehabilitation within the last year; and (3) excluding patients who completed pulmonary rehabilitation within the last 2 years.
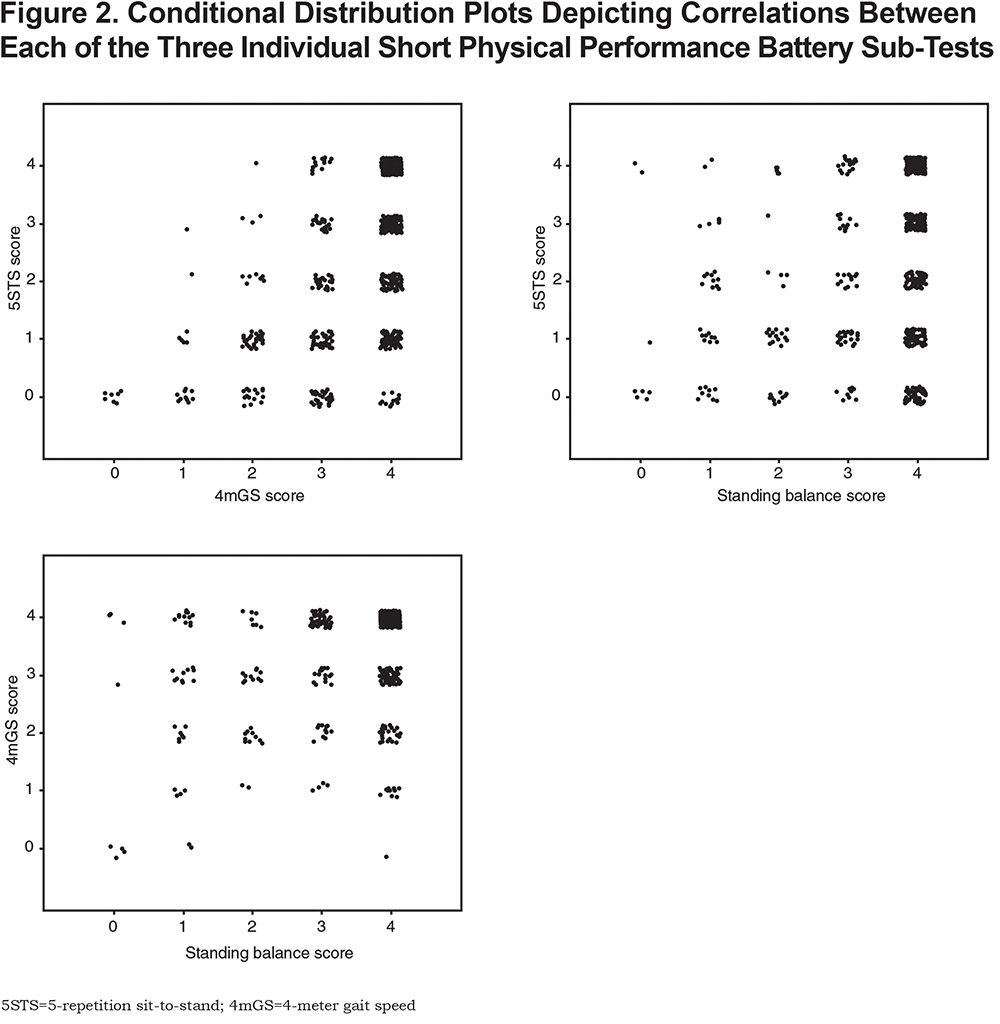
A principal component analysis (PCA) was used to evaluate how the 3 sub-tests of the SPPB relate to each other in COPD and how much of the variance of the SPPB can be explained by each of the 3sub-tests. Conditional distribution plots were used to illustrate the correlations between each of the 3SPPB sub-tests.
All analyses were performed using SAS version 9.3.
Results
The ERICA study enrolled 729 patients, of which 717 had full SPPB data and thus were available for analysis. Mean age was 67 years and 60% were male. A total of 76% of patients had evidence of some functional limitation, manifested by SPPB total score < 12 (Figure 1). With regard to the individual sub-test scores, a score less than the maximum (4 points) was observed in 71%, 29%, and 22% of participants for the 5STS, 4mGS, and standing balance tests of the SPPB, respectively.

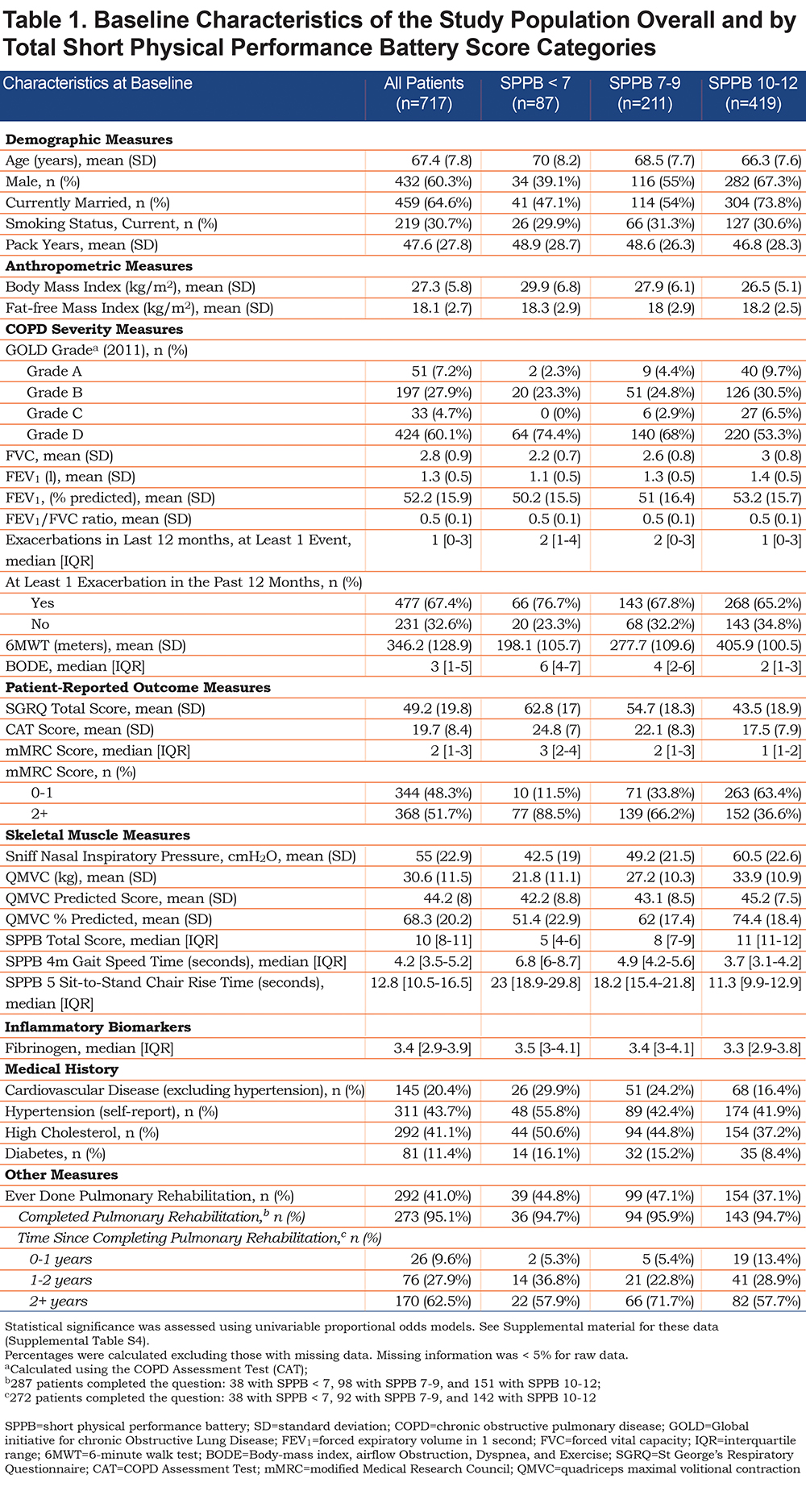
Table 1 shows the patient characteristics overall and by SPPB score category. A total of 419 patients (58%) showed little or no functional limitation (SPPB score 10-12), 211 (30.8%) showed some limitation (SPPB score 7-9) and 87 (12%) showed major limitation (SPPB score 0-6). Patients with a lower SPPB score were more likely to be older, female, unmarried, with a higher body mass index (BMI), lower FEV1, shorter 6MWT, higher (i.e., worse) SGRQ and CAT scores, lower SnIP and quadriceps strength (measured as QMVC), more comorbidities, and were less likely to have completed a pulmonary rehabilitation program within the last year. Baseline characteristics for 5STS, 4mGS and standing balance subtest scores are provided in the online supplement material (Online supplement Tables S1-S3).
Predictors of SPPB Total Score
Univariable analyses performed during the model build (where p-value ≤ 0.15) are documented in Supplemental Table S4.
Table 2 shows the variables significantly associated with SPPB total score after adjustment for age, sex, exacerbation history, and site. For a 30m increase in distance walked for 6 minutes (the 6MWT), the odds of being in a lower SPPB category (i.e., being more functionally limited) decreased by 26%. A 1kg increase in QMVC also decreased the odds of being in a lower category, by 5%, and being married decreased it by 47%. For a 1-year increase in age, the odds of being more functionally limited increased by 4%. Self-reported hypertension and increasing mMRC dyspnea scale score also increased the odds of increased functional limitation. These results were not substantially changed in the 3 sensitivity analyses (results not shown).

Predictors of SPPB Sub-Tests
Univariable analyses performed during the model build (where p-value ≤ 0.15) are documented in Supplemental Tables S5-S7.
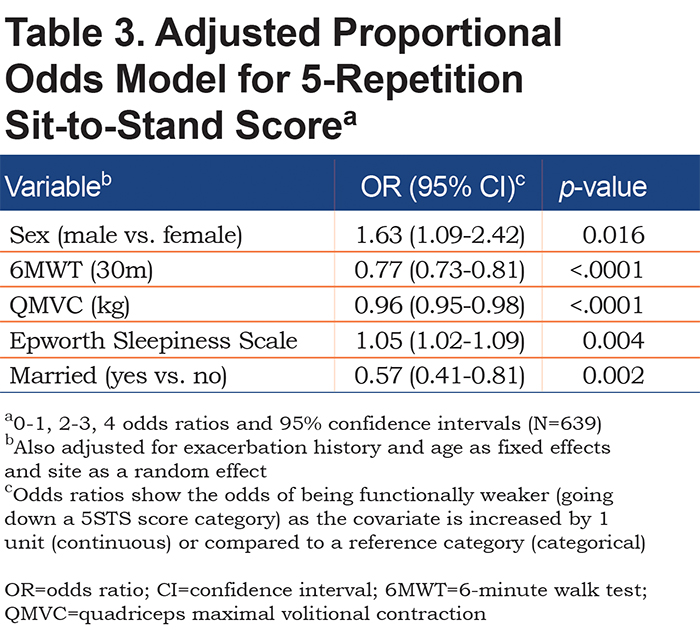
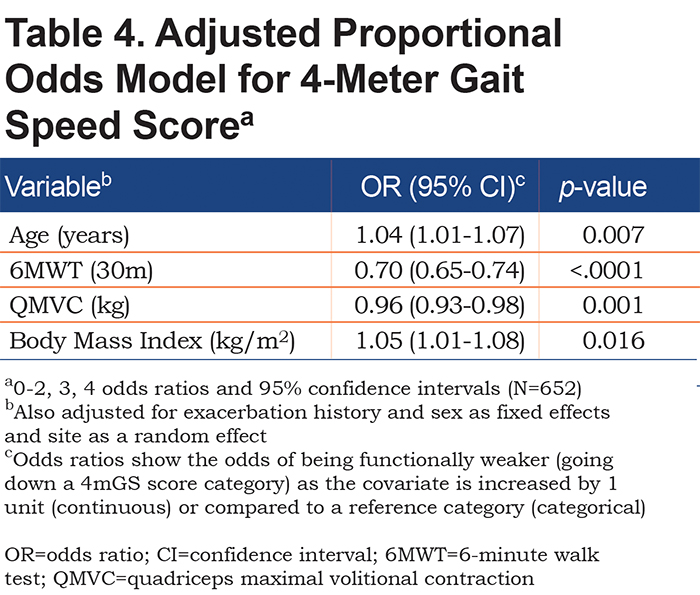
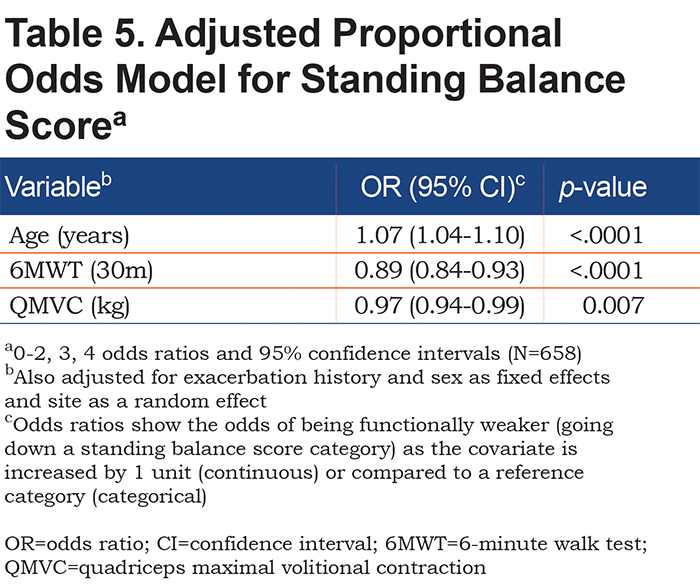
A longer 6MWT and greater QMVC decreased the odds of dropping a score category for all 3 sub-tests (for example, patients with a longer 6MWT were more likely to complete the 5STS quicker than those with a lower 6MWT). Each of the 3 SPPB sub-tests had additional factors significantly associated with it: for 5STS, being male and having symptoms of daytime somnolence increased the odds of being in a lower category; being married decreased the odds (Table 3); for 4mGS, older age and higher BMI increased the odds of being in a lower category (Table 4); and for standing balance, older age increased the odds of dropping a category (Table 5). These results were not substantially changed in the 3 sensitivity analyses for each sub-test (results not shown).
Principal Component Analysis
A PCA evaluated whether the SPPB sub-tests should have equal weight in COPD. In the PCA, the first principal component accounted for 61% of the variation in SPPB sub-test scores. Each of these SPPB tests had similar positive weightings for this component (0.6 for 5STS, 0.63 for 4mGS, and 0.5 for standing balance), indicating that they reflect a common underlying variable. The joint distributions of the tests are shown in Figure 2. These distributions show that a score of 4 in one test is not closely correlated with a score of 4 in another. i.e., in COPD patients, each of the SPPB sub-tests are bringing different information to the SPPB total score.
Availability of Data and Material
The data that support the findings of this study may be made available on request from the corresponding author.
Discussion
In the ERICA cohort, we found that up to 76% of COPD patients had evidence of some functional limitation (SPPB total score < 12) reflected by 71%, 31%, and 22% of participants scoring less than the maximum score for 5STS, 4mGS, and standing balance, respectively. In addition, SPPB total score and all 3 individual sub-tests were significantly associated with 6MWT and QMVC; which is unsurprising given the SPPB was designed as a test of lower limb function. Additionally, all 3 sub-tests have individual utility in clinical trials, with our PCA suggesting that all 3 sub-tests bring different but equally important information to the SPPB, which is a novel finding for COPD. Consequently, we recommend that total SPPB score should be utilized rather than the individual sub-tests. However, the distribution of the 3 sub-test scores in this cohort shows that the 5STS may have the greatest variation, and therefore, where only one test is possible, the 5STS may prove the most discriminatory for identifying muscle weakness in clinical studies in COPD.
Significance of Our Findings
There were some differences that emerged among the explanatory variables associated with individual SPPB sub-tests; worse 5STS (i.e., lower score) performance was associated with symptoms related to daytime sleepiness as well as with being male and not being married. Whilst it is not surprising that alertness was associated with physical performance, the association with marital status has not been reported before for the 5STS in COPD, although being married is known to be associated with increased physical activity in the general elderly population,17 which in turn can influence (or be influenced by) lower limb strength and performance. A higher BMI was associated with worse 4mGS performance, which likely reflects lower fitness and exercise performance. For the standing balance test, older age was associated with worse balance, which likely reflects multiple factors including neurodegenerative processes related to aging, sensory neuropathy18 and possibly polypharmacy with associated side effects for older individuals. In agreement with our study, Bernabeu-Mora et al11 reported QMVC as a significant determinant of 5STS, 4mGS, and standing balance tests; 6MWT was also a significant determinant of 5STS and 4mGS but not standing balance score, which is possibly because the score was dichotimized (< 4 versus 4) therefore losing power to detect possible differences.11
Of the 3 individual SPPB sub-tests, our results suggest that the 5STS test has multiple modifiable factors associated with it. For example, a change in alertness and/or lower limb strength or performance from an exercise or anabolic intervention could impact the 5STS potentially more than the other 2 SPPB sub-tests. Furthermore, given that the spread of the 5STS scores is greater than that of either 4mGS or the standing balance tests (for which most COPD patients appear to score a maximum of 4 points, suggesting a ceiling effect), the 5STS is the test with most room for improvement following an intervention. Therefore, in agreement with Bernabeu-Mora et al, we propose that if an individual sub-test was required, the 5STS might be most suitable as an outcome measure for interventional studies because it is less likely to have a ceiling effect.12 However, as our PCA showed that each sub-test was important and contributed some unique phenotypic information to the SPPB, we argue that an individual sub-test should not normally be used as a full equivalent for the whole SPPB test in COPD. Specifically, much will depend on the nature of the clinical trial and whether the SPPB is used as an outcome measure or a stratification measure to identify frailty/muscle weakness for inclusion in a study. It is also possible that different tests have different decline trajectories in COPD, although longitudinal data are required to assess this.
Multiple medical conditions and indeed age itself may cause impairment of physical performance and thus a low SPPB score. In particular, ceiling effects for balance tests and gait speed tests are noted in community-dwelling older individuals,19 whereas the 5STS and 4mGS appear to suffer from a floor effect in unselected nursing home populations.20 Interestingly, in another study the 3 tests appear to be associated with different comorbidities, gait speed being particularly reduced in patients with COPD and renal failure compared to those with heart failure or high cardiovascular risk (identified on the basis of coronary artery, peripheral vascular disease, stroke or diabetes with adverse biomarkers).21 Conversely, 5STS time was most reduced in renal patients compared to those with the other conditions, and balance tended to be nearest to 4, the maximum.21 The SPPB total score also relates to a greater degree of comorbidity, or the severity of the index disease, whether that is renal disease,22 pneumonia, congestive heart failure, COPD or stroke.23 Against that background, it is perhaps surprising that after model adjustment, comorbidities were not significantly associated with SPPB total score or any of the 3 sub-tests scores in multivariable models. We would highlight, however, that these factors were significant in univariable observations (consistent with other studies, although notably our inclusion criteria permitted significant comorbidities unlike many other studies). Comorbidities may be more prevalent among those with a lower SPPB score perhaps because the factors that cause comorbidities such as low levels of physical activity and poor nutrition may also cause a lower SPPB score. In this respect, the SPPB may be a more attractive tool to adopt in the COPD field than the BODE score, which takes into account scores for Body-mass index, airflow Obstruction, Dyspnea, and Exercise. BODE scores are specific to COPD and are not widely adopted due to the necessity to carry out a 6MWT, which is not amenable to primary care, and many secondary care settings.
Investigators should be aware that where the whole SPPB score is used as an outcome measure in clinical trials, the change in overall score is possibly driven by changes in the 5STS test, especially for trials of anabolic medications such as testosterone or emerging new therapies.24,25 Our findings differ from those in the general, elderly population where the 4mGS has been shown to be the most discriminative test for predicting disability and mortality, being almost as good as a standalone to the SPPB total score.4 The inverse association of age and SPPB score in our cohort is also reported in the older, hospitalized population.23 SPPB is unsurprisingly linked to measures of cognitive function as well as lower limb function and ability to independently perform activities of daily living (ADLs) in the elderly, as well as in other disease populations, such as those with chronic kidney disease.22,23 However, we did not record cognitive or ADL measures in the ERICA study, which is a limitation of our dataset. Whilst FEV1 was not included in the multivariable analysis due to model instability with other variables in the final multivariable model, it was significantly associated, albeit a small association, in the univariable analyses for total score and all 3 sub-tests. This finding may indicate that in COPD the airflow limitation additionally impacts lower limb physical performance, but perhaps to a lesser extent than peripheral muscle dysfunction, consistent with our prior observations where lower limb function was evaluated as maximal voluntary contraction force or bulk.26
The SPPB is currently favored as a stratification measure by the European Medicines Agency.2 The meaningful clinically important difference (MCID) of 1 point for the overall tool1 makes it unlikely to be a useful tool as an outcome measure unless trial clinicians intentionally stratify by low SPPB score at entry; individual tests with raw time values, rather than categorical scores such as SPPB, may therefore prove more useful as an outcome measure, particularly in early phase studies, but further work from interventional studies is required to evaluate this in COPD.
We show that the SPPB test can be reliably collected across multiple centers, and by this means we also recruited a more diverse population than the single-center Bernabeu-Mora study.11 In this regard, the SPPB is much easier to standardize and administer in a multi-center study than other performance tests such as the 6MWT and constant work rate (CWR), which require more space, time and, in the case of CWR, specialized equipment. Whilst the SPPB test and sub-tests may have a learning effect, this is not uncommon in functional measures that are simple to perform, however, the SPPB is quick to complete and can be performed in the office making it a favorable measure of functional limitation in COPD. Moreover, our study is based on one of the largest COPD cohorts with SPPB measures to date.
Limitations
Our study does have limitations, most notably that we examine cross-sectional data, and cannot therefore comment on repeatability or stability of the SPPB test in COPD. Additionally, 41% of participants had ever completed or partially completed pulmonary rehabilitation; much higher than the ~6% seen in COPD populations.27,28
Previous work has suggested that depression (as measured by the Hospital Anxiety and Depression Scale) in COPD is an important determinant of 4mGS12; ERICA, which was conceived before that report, did not include questionnaires on these topics, therefore, we cannot adjust for the possible effects of these measures.
Finally, ERICA is an observational study and therefore the value of the SPPB as an outcome measure cannot be reliably assessed via our data; data from interventional trials will be needed to evaluate whether the SPPB is a useful outcome measure.
Conclusion
In conclusion, the majority of COPD patients in this cohort have some evidence of functional limitation. All 3 individual sub-tests of the SPPB make largely independent contributions to the SPPB total score, and should be used together, unless specific scientific reasons make it inappropriate to do so. The 5STS may prove to be the most discriminative test in trials examining lower limb performance. However, data from interventional studies is required to further evaluate this.
Acknowledgments
We would like to thank members of the ERICA consortium for their contributions to the project.
Author Contributions: DM, VSB, MA and MIP designed the study. The source data set came from a study conceived and directed by RT-S, IBW and MIP and with CEB, WM and JRC as co-investigators who organized and carried out the clinical study. DM, CEB, WM, JRC, and MIP obtained the data. VSB and MA conducted the analysis and produced the results, figures and tables. NG provided statistical support. DM and VSB wrote the initial draft of the manuscript. DM, VSB, MA, NG and MIP contributed to the writing of the manuscript. All authors critiqued and commented on the manuscript. All authors have approved the final version of the manuscript prior to this submission and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of Interest
VSB, DM, NG, MA and RT-S are employees of (or were during the time of this study) and hold stock in GlaxoSmithKline. MIP has received payment to his institution or himself for advice on skeletal muscle weakness in COPD from GlaxoSmithKline, Novartis, AstraZeneca, Pfizer, Lilly and Astellas. CEB reports grants from GlaxoSmithKline and Pfizer; consultancy fees from Boehringer Ingelheim and honorarium from Chiesi in the past 3 years. JRC reports grants from Technology Strategy Board/Medical Research Council, during the conduct of the study; personal fees from GlaxoSmithKline, outside the submitted work. WM received research support from GlaxoSmithKline and Pfizer, and was on advisory committees of Almirall, GlaxoSmithKline, Novartis and Pfizer; he was a speaker for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Janssen and Novartis. IBW reports grants from Technology Strategy Board and GlaxoSmithKline during the conduct of the study, and grants from GlaxoSmithKline outside the submitted work.