Running Head: Characterization of COPD in U.S. Primary Care
Funding Support: This study was funded by GlaxoSmithKline plc (GSK study HO-14-14653/204942). GlaxoSmithKline plc was involved in the design of this study, the collection, analysis, and interpretation of data, the writing of this manuscript and the decision to submit for publication.
Date of acceptance: October 10, 2019
Abbreviations: COPD Assessment Test, CAT; chronic obstructive pulmonary disease, COPD; electronic diary, eDiary; EXAcerbations of COPD Tool, EXACT; Global initiative for chronic Obstructive Lung Disease, GOLD; modified Medical Research Council, mMRC; Short Form 12-item Health Survey version 2, SF-12v2; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; Lung Function Questionnaire, LFQ; Evaluating Respiratory Symptoms in COPD, E-RS: COPD; Clinical Global Impressions, CGI; standard deviation, SD; Physical Component Summary, PCS; Mental Component Summary, MCS; analysis of variance, ANOVA; St George’s Respiratory Questionnaire for COPD, SGRQ-C
Citation: Stanford RH, Tabberer M, Kosinski MA, et al. Assessment of the COPD Assessment Test within U.S. primary care. Chronic Obstr Pulm Dis. 2020; 7(1): 26-37. doi: http://doi.org/10.15326/jcopdf.7.1.2019.0135
Introduction
Effective management of chronic obstructive pulmonary disease (COPD) requires good communication between the physician and patient. Good communication is essential to ensure that patients receive more accurate diagnoses, adequate disease education, improved treatment choices, and hence, optimal outcomes. 1 There is a need to improve communication between patients and physicians with regard to COPD management and assessment tools have been developed to ameliorate some of the dissatisfaction that exists. 2-4 Patient dissatisfaction may arise due to an unmet need for sufficient and accurate information on COPD. 1,5 In contrast, for physicians, frustrations may arise due to the perceived failure of patients to adequately convey the severity of their symptoms. 2,4
The COPD Assessment Test (CAT) was developed in recognition of the need for a validated, short, simple instrument with the ability to permit the quantification and monitoring over time of COPD disease burden in routine clinical practice. 3,6,7 The underlying aim of the CAT was to aid health status assessment and communication between the patient and their physician. 6 The CAT consists of 8 items with item response values ranging from 0 to 5 (0 = no impairment). An overall score is calculated by adding the response values from each item with total scores ranging from 0 to 40, and higher scores indicating a greater impact of the disease on patient well-being and daily life. 6 It is a patient-completed instrument, which provides immediately available results without the need for any calculation, apart from summing the scores on individual items. 8 Within the CAT, score ranges of 0–9, 10–20, 21–30, and 31–40 have been described as representing mild, moderate, severe, and very severe clinical impacts of disease on patients’ well-being and daily life (herein referred to as Low Impact, Moderate Impact, High Impact, Very High Impact). 9,10 However, little is known about how these CAT score impact groups align with several other measures of COPD and about the performance of the CAT in the primary care setting. Although Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines 11 recommend using a CAT score threshold of ≥ 10 to define symptomatic patients and define risk, 12 there has been limited research aimed at relating to higher CAT scores with other measures of disease severity.
The aim of this study was, therefore, to assess how these categories of CAT scores align with other measures of COPD severity and disease control and to assess the feasibility of implementing CAT within a U.S. real-world primary care clinical practice setting.
Methods
Study Objectives
The primary objective of this study was to assess how the CAT aligns with other COPD measures of severity and disease control. Specifically, 4 levels of COPD impact, as derived from CAT score categories (0–9, 10–20, 21–30, and 31–40), were linked concurrently and prospectively to: COPD clinical status (assessed via GOLD 11 grading, spirometry, and clinician severity ratings); respiratory symptoms (breathlessness assessed via the modified Medical Research Council [mMRC] Dyspnea Scale); frequency, severity, and duration of pulmonary exacerbations (EXAcerbations of COPD Tool [EXACT]), 12 and physical and mental health status (Short Form 12-item Health Survey version 2 [SF-12v2]).
Patients
Patients receiving primary care in the United States were eligible to participate in the study. Patients were included if they met the following criteria: males and females aged ≥ 40 years, a clinical diagnosis of COPD with spirometry-confirmed airflow obstruction (pre- and post-bronchodilator forced expiratory volume in 1 second/ forced vital capacity [FEV1/FVC] < 0.70), and ability to complete patient-reported outcome instruments electronically.
Patients with a current clinical diagnosis of asthma or other significant lung disease were excluded. Patients were also ineligible if they were pregnant, had poorly controlled COPD or comorbid conditions (that would put the patient at risk through study participation per the judgement of the investigator), were unable to complete spirometry, had known or suspected alcohol or drug abuse, had spirometry contraindications present, or had participated in an interventional study or used an investigational drug within 30 days or 5 half-lives of final administration of the drug.
The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and the 2008 version of the Declaration of Helsinki. The study was approved on October 30, 2015 by the Sterling Institutional Review Board, Atlanta, Georgia (IRB ID# 5277-001). All patients provided written informed consent before participation.
Study Design
This was a multicenter, prospective, observational, longitudinal study of U.S. patients with COPD, conducted between January 2016 and January 2017. The target investigator population was community-based primary care physicians treating patients with COPD. Enrollment was capped at 201 patients across all study sites. Study investigators identified potentially eligible patients with COPD via a search of the site’s electronic health record system and those meeting the study-specific eligibility criteria were recruited. Patients were screened and enrolled during the baseline visit (Visit 1). Study eligibility was assessed via a patient-administered screening survey and spirometry testing. The screening survey included the CAT, mMRC Dyspnea Scale, and Lung Function Questionnaire (LFQ), a 5-item questionnaire that is used to determine whether a patient is appropriate for diagnostic evaluation for COPD using spirometry, with a total score ranging from 5 to 25. 13,14 Respondents with an LFQ total score ≤ 18 are considered at risk for COPD and are likely to meet the FEV1/FVC ratio of < 0.7; therefore, the LFQ was used to determine whether a patient is appropriate for diagnostic evaluation for COPD using spirometry to confirm airway restriction. 13,14 Patients with a total LFQ score ≥ 19, or who were unable to complete spirometry procedures, or who did not have spirometry-confirmed airway restriction after completion of the spirometry procedures, were excluded from study enrollment. Baseline procedures were subsequently completed for enrolled participants during Visit 1 (demographics, smoking status, SF-12v2). Patients were issued and trained on an electronic diary (eDiary) device to complete the baseline EXACT assessment each evening, with the first diary day collected at Visit 1. The EXACT is a 14-item daily diary assessing the symptoms and impact of COPD, allowing an evaluation of the frequency, severity, and duration of events in clinical studies of COPD. An EXACT event is defined as an increase in EXACT score ≥ 9 points for 3 days or ≥ 12 points for 2 days, above baseline. 15 The severity of an event is defined by the maximum EXACT score during the event. 15 Duration of an event was defined as the time from EXACT event initiation to recovery, where recovery is defined as a decrease in EXACT score ≥ 9 points from the maximum value, sustained for ≥ 7 days. 15 The eDiary and EXACT measurement approach has been used previously to analyze exacerbations. 16 The Evaluating Respiratory Symptoms in COPD (E-RS: COPD) is an 11 item sub-set of the EXACT focusing on the daily measurement of symptoms. The Clinical Global Impressions (CGI) scale assessment was completed by the site investigator.
Starting the day after Visit 1, patients initiated a 12-week follow-up period in which they completed the EXACT on a daily basis via the eDiary device, as well as a 6-week survey that included the CAT.
Twelve weeks (±5 days) following Visit 1, patients returned to the investigational site to complete a second and final study visit (Visit 2) to collect additional assessments and return the eDiary device. Daily compliance with the eDiary was monitored by the eDiary vendor and in-device notifications were sent if patients missed 1 day of completion. Non-compliant patients were withdrawn from the study.
Statistical Analyses
Descriptive analyses (mean, median, standard deviation [SD] and range) were conducted at baseline for COPD severity, mMRC dyspnea scale, SF-12v2 Health Survey, and the CGI scale. All statistical analyses were conducted using the SAS software package (version 9.4; SAS Institute Inc., Cary, North Carolina). Chi-square (Fisher's Exact) tests were used for binary measures. Analysis of variance (ANOVA) models were used for continuous measures.
Analyses were conducted to profile patients with COPD stratified by the 4 impact categories derived from the CAT scale. Patients were categorized into the following 4 groups based on their baseline CAT scale score: Low Impact (CAT < 10); Medium Impact (10 ≥ CAT ≤ 20); High Impact (20 > CAT ≤ 30); and Very High Impact (CAT > 30). Baseline CAT impact categories were compared to patients’ baseline clinical statuses, described in terms of % predicted FEV1 values, GOLD categories, LFQ, assessment of COPD severity, the mMRC scale, and SF-12v2 scores. Longitudinal analyses were performed on the EXACT measures from the 12-week follow-up, comparing to the baseline CAT impact categories.
Data Sharing Statement
To request access to patient-level data and documents for this study, please submit an enquiry via www.clinicalstudydatarequest.com.
Results
Patients
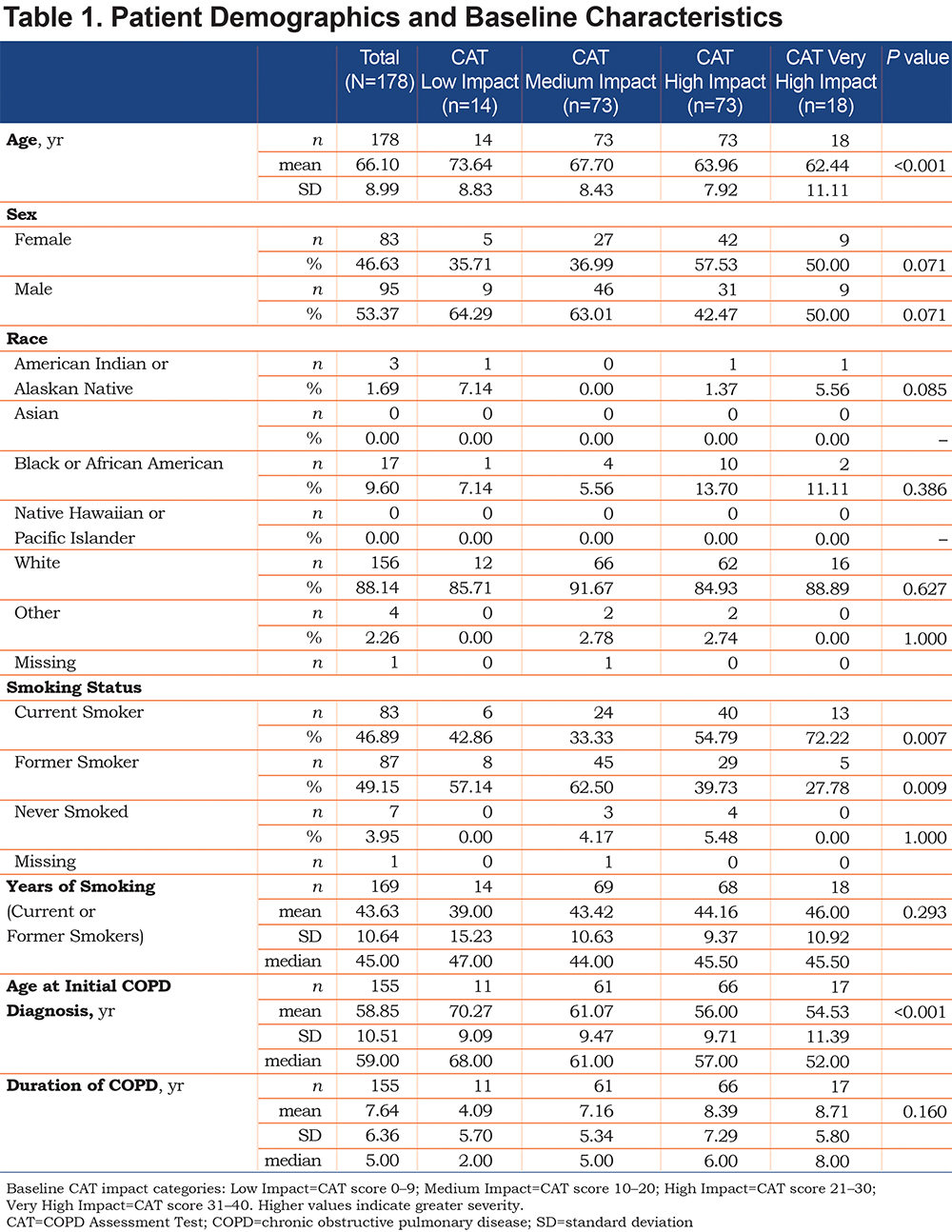
In total, 321 patients were screened and 201 enrolled into the study from 30 centers across the United States (geographical distribution: Midwest 20% (n=6), Northeast 20% (n=6), South 27% (n=8), West 33% (n=10); 178 patients met baseline requirements and had a completed baseline CAT. Patient demographics are shown in Table 1. Mean patient age was 66.1 years, with patients predominantly white (88%) and a slightly higher proportion of males than females (53%). On average, patients were diagnosed with COPD at 58.9 years of age and had a mean disease duration of 7.6 years. The mean baseline FEV1 % predicted value for patients in the study was 53.3. Average baseline CAT total score was 20.00; 14 patients (8%) were categorized as Low Impact, 73 (41%) as Medium Impact, 73 (41%) as High Impact, and 18 (10%) as Very High Impact. Mean baseline SF-12 Physical Component Summary (PCS) score was 38.77, indicating relatively poor health states among the patients studied; mean Mental Component Summary (MCS) score was 50.00, with scores ≥ 47 considered indicative of a normal mental health. The findings of PCS and MCS were both considerably lower than age- and sex-adjusted norms for PCS (45.6) and MCS (53.7) derived from the general U.S. population, indicating below-normal physical and mental health status. There were no differences in prescribed medications across all groups. A total of 169 patients had sufficient follow-up eDiary data available for analysis.
Association Between Disease Severity and CAT Scores
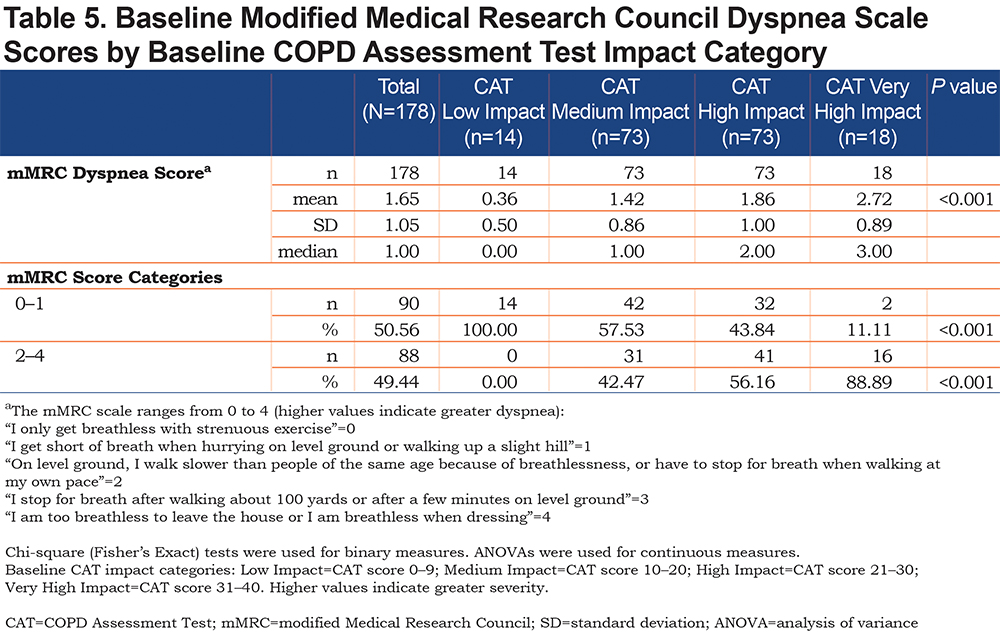
COPD severity; respiratory symptoms; frequency, severity, and duration of pulmonary exacerbations; and overall physical and mental health status were all shown to be linked concurrently and prospectively to CAT impact score categories. Typically, differences in each of these measures across the 4 CAT impact categories were in the expected direction. For example, patients in the High and Very High Impact categories were considerably worse than those in the Low Impact category on the majority of other measures of disease severity. Similarly, patients in the Medium Impact category demonstrated worse status than patients in the Low Impact category; however, this was less marked than in the High and Very High Impact categories. (Tables 2–5).
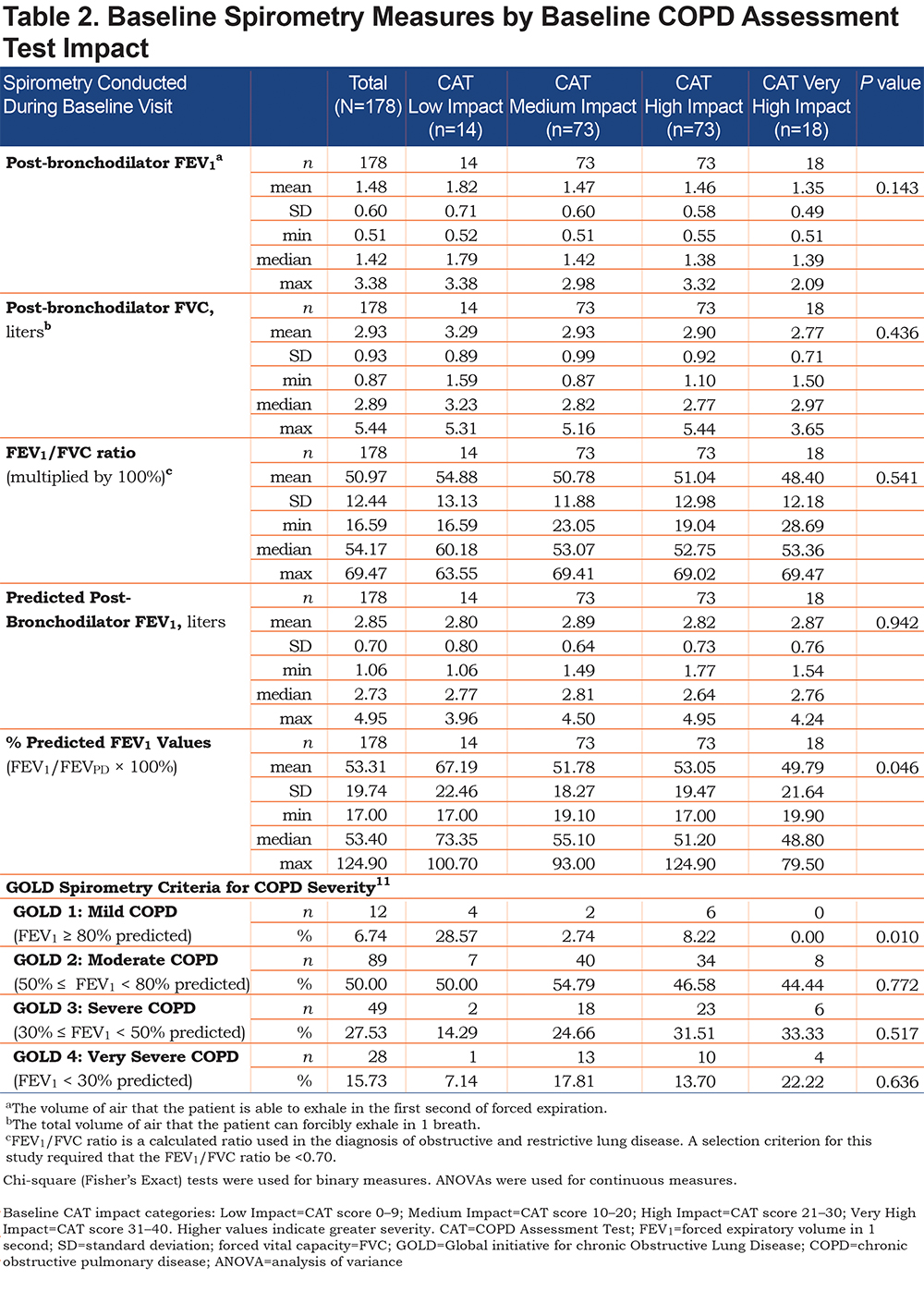
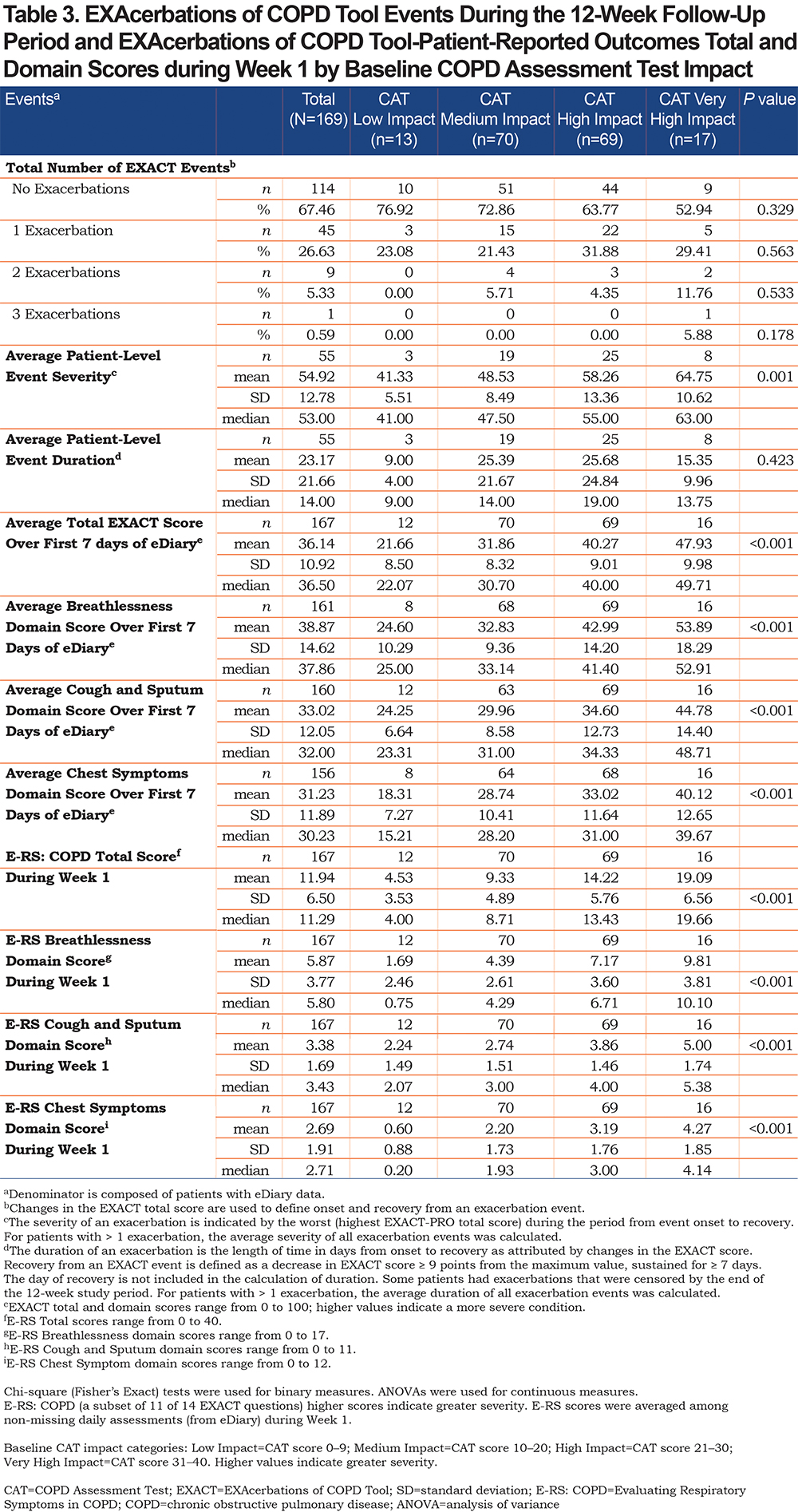
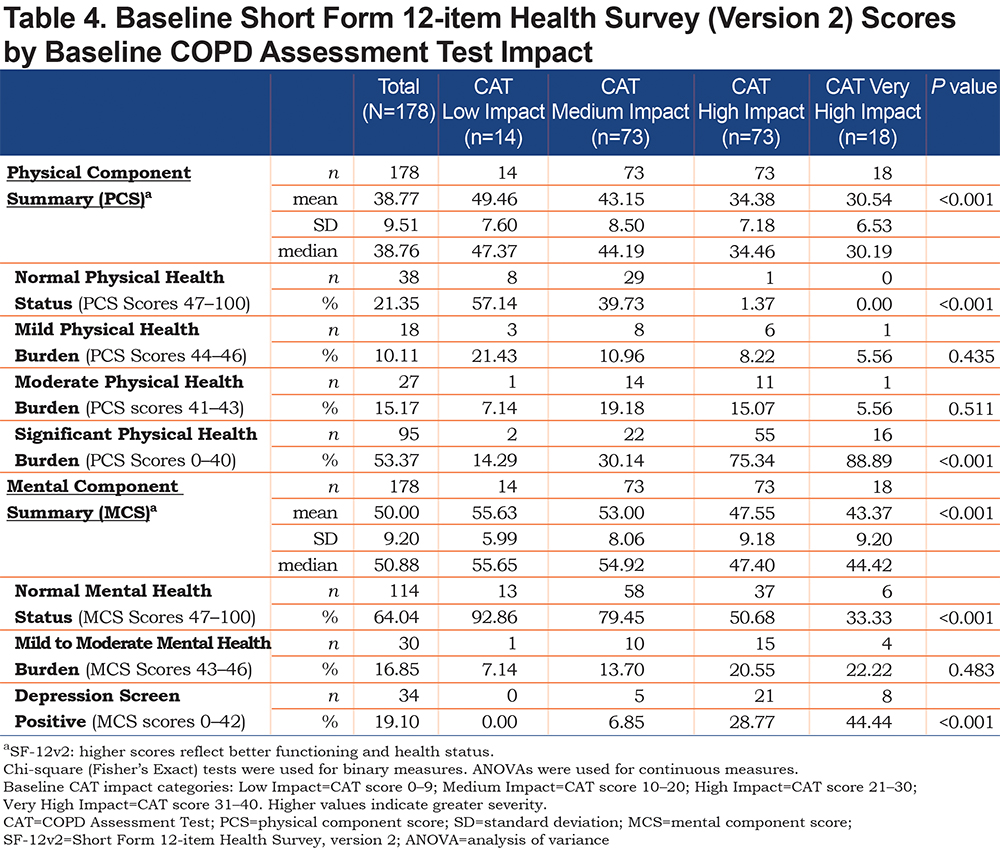
Stratification by COPD Assessment Test Impact Category
Higher mean mMRC Dyspnea Scale scores were observed in patients in the Very High Impact CAT score group when compared with those in the Low, Medium, and High Impact groups, with a greater proportion of patients in the Low Impact group having mMRC = 0 (Table 5). A greater proportion of patients in the Low Impact group were GOLD Grade 1 (Mild) at baseline (Table 2) and had higher mean LFQ scores than patients in the Medium, High, and Very High Impact groups (14.29 versus 11.78, 10.00, and 8.33, respectively;P< 0.001), which were indicative of their less severe disease state.
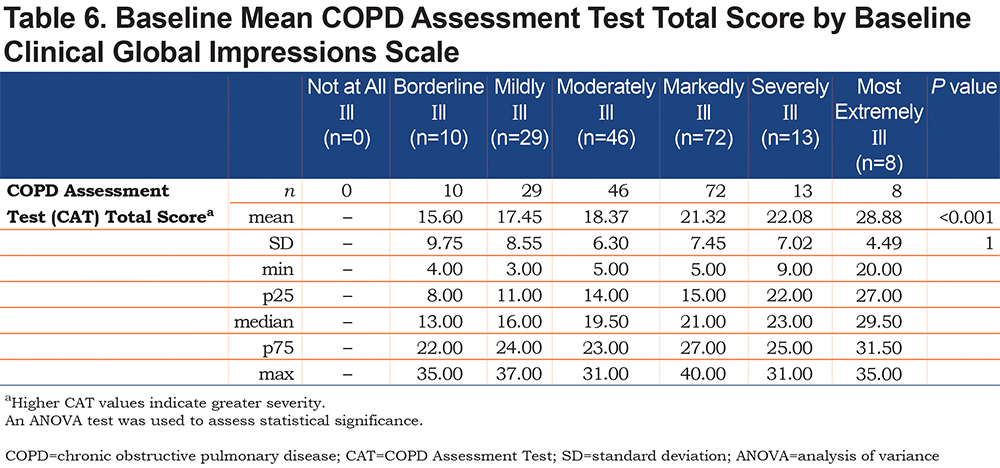
A comparison of patients in each of the CGI scale categories at baseline, by mean CAT total baseline scores, demonstrated that patients in the Most Extremely Ill category had higher mean CAT scores than patients in the Borderline Ill, Mildly Ill, Moderately Ill, Markedly Ill, and Severely Ill groups (Table 6). When compared with patients in the Low, Medium, and High Impact CAT score groups at baseline, those in the Very High Impact CAT score group demonstrated higher average EXACT total scores over the first 7 days of the study (47.93 versus 21.66, 31.86, and 40.27, respectively;P<0.001). In addition, EXACT Breathlessness domain scores (53.89 versus 24.60, 32.83, and 42.99, respectively;P<0.001), EXACT Cough and Sputum domain scores (44.78 versus 24.25, 29.96, and 34.60, respectively;P<0.001), and EXACT Chest Symptoms domain scores (40.12 versus 18.31, 28.74, and 33.02, respectively;P<0.001) over the first 7 days were all higher in the Very High Impact versus the Low, Medium, and High Impact CAT score groups (Table 3).
A comparison of the rate of EXACT events occurring between the 4 CAT impact groups during the follow-up period revealed no statistically significant differences. However, among those patients who experienced an EXACT event (n/N, 55/169; 33%), patients in the Very High Impact CAT score group were found to have greater mean EXACT event severity, defined as the worst (i.e., highest) EXACT total score, during the period from event onset to recovery, when compared with patients in the Low, Medium, and High Impact CAT score groups (64.75 versus 41.33, 48.53, and 58.26, respectively;P=0.001; Table 3). Baseline SF-12 PCS scores were lowest in those categorized as Very High Impact, with mean baseline MCS scores lowest in this group (Table 4).
Discussion
The findings of this study demonstrate that CAT impact score categories are linked concurrently and prospectively to COPD severity; respiratory symptoms; frequency, severity, and duration of pulmonary exacerbations; and overall physical and mental health status. In the primary care setting, the CAT could therefore be used as a convenient assessment over time, with the ability to reliably assess the severity of disease in patients with COPD, due to the good correlation observed between the CAT and other validated measures.
The CAT was developed to be a complementary practice tool for use with standard clinical measures for COPD, such as lung function or dyspnea, as these measures alone do not provide insight into the patient-perceived burden of COPD on their lives. For the CAT to be useful in clinical practice, clearly defined interpretation guidelines about the meaning of the CAT impact scores, as well as differences and/or changes in CAT scores, are essential. Moreover, a 2011 analysis conducted by Jones and colleagues 9 developed interpretation guidelines by mapping the 4 CAT impact score categories to item content of the St George’s Respiratory Questionnaire for COPD (SGRQ-C). Our findings indicate that COPD impacts individuals’ lives differently in each category of CAT severity, providing insights on their clinical and functional status. In the Low CAT Impact category, all patients had mMRC dyspnea scores of 0 (“I only get breathless with strenuous exercise”) or 1 (“I get short of breath when hurrying on level ground or walking up a slight hill”) and a mean EXACT Breathlessness domain score of 24.60. Conversely, in the Very High Impact CAT category, 89% of patients had mMRC dyspnea scores of 2 (“On level ground, I walk slower than people of the same age because of breathlessness, or have to stop for breath when walking at my own pace”), 3 (“I stop for breath after walking about 100 yards or after a few minutes on level ground”), or 4 (“I am too breathless to leave the house or I am breathless when dressing”) and higher mean EXACT Breathlessness domain score of 53.89. As expected, patients in the Medium and High Impact CAT score groups experienced a mixed intensity of dyspnea impacting on their lives.
In addition, Gruffydd-Jones and colleagues 4 have noted the utility of the CAT as a disease-specific instrument that is capable of assisting physician assessment of COPD. These findings align with the original purpose of the CAT, and it is not surprising that the authors note that the use of this instrument does not appear to improve the detection of non-COPD symptoms or comorbid conditions. The significant relationship that exists between the CAT, FEV1, and COPD disease severity has also been noted in another study, 17 which found the CAT to provide a reliable score of exacerbation severity. In this study of 165 patients with COPD, significant associations were observed between FEV1 % predicted values, frequency of patient hospitalization, and CAT scores.
Furthermore, higher CAT scores were recorded in those patients with a history of frequent exacerbations of COPD compared with those who were infrequent exacerbators (24.8 ± 6.7 versus 17.5 ± 6.5 [mean ± SD];P<0.0001), together with a significant increase in CAT score with increasing frequency of exacerbation (P<0.0001). 17 Similar elevations in CAT scores were also observed in those individuals with COPD who can be classified as frequent exacerbators compared with infrequent exacerbators (19.5 ± 6.6 versus 16.8 ± 8.0;P=0.025) in a 2012 study conducted by Mackay and colleagues, 18 which also noted a significant increase in CAT allied to the decrease in FEV1 at the onset of an exacerbation.
A review of the role of the CAT in the assessment of COPD, which also compared the CAT with other health-related quality-of-life questionnaires, concluded that data provided by the CAT can assist in a comprehensive assessment of COPD, which can subsequently guide clinicians’ decision making and patient management. 19 Moreover, the CAT was noted to be a simple and valid instrument with the potential to improve communication between physicians and their patients with COPD during routine clinical visits. This study adds to that body of evidence and is the first to assess the CAT in this way within a U.S. primary care population.
In addition, this study has also highlighted the high symptomatic burden of COPD in a U.S. primary care setting. For example, over half of the patient population were within the High or Very High CAT IMPACT score groups at baseline. The higher degree of physical and mental symptoms in these patients emphasize the need for improved patient monitoring and management in U.S. primary care.
Potential limitations of this study include the short follow-up time and relatively small number of patients in the CAT Low Impact and Very High Impact groups. Strengths of the study include its multicenter approach, use within a U.S. primary care setting and the longitudinal nature of the study, which allowed for evaluation over time of trends in the clinical and functional status of patients with COPD.
In conclusion, the findings of this study contribute to the growing body of information guiding the interpretation of CAT scores by demonstrating that factors such as COPD severity, respiratory symptoms, the frequency, severity, and duration of pulmonary exacerbations, and overall physical and mental health status are linked concurrently and prospectively to CAT impact score categories. Stratification of patients according to the 4 CAT impact scores, and the application of clinical and functional health status information to these categories, greatly enhances our interpretation of the CAT.
Acknowledgments
Editorial support (in the form of writing assistance, collating author comments, assembling tables and/or figures, grammatical editing, fact checking, and referencing) was provided by Sarah Birch, PhD, of Gardiner-Caldwell Communications (Macclesfield, United Kingdom) and was funded by GlaxoSmithKline plc. All trademarks are the property of their respective owners.
Author Contributions
RHS and MT contributed to the study conception/design and data analysis/interpretation; MK, PTJ, and NAT contributed to the study conception/design, data collection and data analysis/interpretation; JW and MC contributed to data collection and data analysis/interpretation. All authors wrote/reviewed the manuscript. All authors approved the final draft of the manuscript.
Declaration of Interest
The authors declare the following conflicts of interest during the last 3 years in relation to this article: RHS was formerly, and MT and NAT are employees of and own shares in GlaxoSmithKline plc. MK, PTJ, JW, and MC are employees of and own shares in Optum plc. Optum plc received funding from GlaxoSmithKline plc to conduct this study, but were not paid for manuscript development. This study was funded by GlaxoSmithKline plc (GSK study HO-14-14653/204942). GlaxoSmithKline plc was involved in the design of this study, the collection, analysis, and interpretation of data, the writing of this manuscript and the decision to submit for publication.