Running Head: COPD Treatment Ratio and Severe Exacerbation Risk
Funding Support: Sponsored and funded by GlaxoSmithKline plc (HO-16-17938).
Date of Acceptance: December 19, 2019
Abbreviations: chronic obstructive pulmonary disease, COPD; COPD treatment ratio, CTR; confidence interval, CI; Body mass index, airflow Obstruction, Dyspnea, and Exercise, BODE; emergency department, ED; Medicare Advantage with Part D coverage, MAPD; International Classification of Diseases, 9th Revision, Clinical Modifications, ICD-9-CM; odds ratio, OR; asthma medication ratio, AMR
Citation: Stanford RH, Korrer S, Brekke L, Reinsch T, Bengston LGS. Validation and assessment of the COPD treatment ratio as a predictor of severe exacerbations. Chronic Obstr Pulm Dis. 2020; 7(1): 38-48. doi: http://doi.org/10.15326/jcopdf.7.1.2019.0132
Online Supplemental Material: Read Online Supplemental Material ()
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive, treatable disease of the airways associated with substantial morbidity and mortality.1 In the United States, COPD was the third leading cause of death in 2015, with an estimated age-adjusted prevalence of 5.9%.2 The medical costs attributable to COPD were approximately $36 billion in 2010 and are projected to reach $49 billion by 2020.3
Exacerbations are experienced by patients with COPD at all stages of disease severity.4 Hospital visits associated with acute exacerbations are linked to increased morbidity, increased mortality, and reduced quality of life,5 and account for 85% of direct medical costs due to COPD.6,7 To improve patient outcomes and reduce health care utilization and costs, population-based risk assessment tools are needed to identify patients who may benefit from interventions that prevent COPD exacerbations.8 Therefore, developing valid predictors of COPD exacerbations through a more robust understanding of associated treatment factors, such as maintenance and rescue medication use, is an important goal.
Exacerbation risk models using clinical information have been developed previously, such as that by Niewoehner et al9 and the Body-mass index-airflow Obstruction-Dyspnea-Exercise (BODE) index,10 which predicted exacerbations based on advanced age, COPD severity, unscheduled clinic or emergency department (ED) visits, cardiovascular comorbidity, and prednisone use; and body mass index, airflow obstruction, functional dyspnea, and exercise capacity, respectively. More recently, 2 further studies utilized exacerbation history, lung function, smoking, and vascular disease history in their validation dataset,11 and patient responses to the COPD Assessment Test,12 to determine exacerbation risk. In the past, risk scores used to predict acute COPD exacerbations have relied on information from medical records that were not always readily available to external researchers.11,12
The COPD treatment ratio (CTR) is a new type of risk score and modifiable measure that uses accessible prescription data within health insurance claims, that has been developed as an easy method to assess exacerbation risk, without the need for patient history data.13 It is defined as the total units (30-day equivalent) of maintenance medication dispensed, divided by total units of maintenance plus rescue medications dispensed. The CTR has been validated in previous retrospective studies using health insurance data and has the potential to identify patients with an increased exacerbation risk who may benefit from interventions to improve treatment adherence, which in turn, may allow for the development of modifiable patient management.13,14
We compared the CTR13,14 as a predictor of severe exacerbations versus other COPD exacerbation predictors available in claims data: prior exacerbation, and use of rescue and maintenance medications.
Methods
Data Source
Data were extracted from the Optum Research Database, a large, geographically diverse U.S. administrative claims database that includes medical and pharmacy claims for individuals enrolled in Medicare Advantage with Part D (MAPD) health plans.
Study Design and Sample
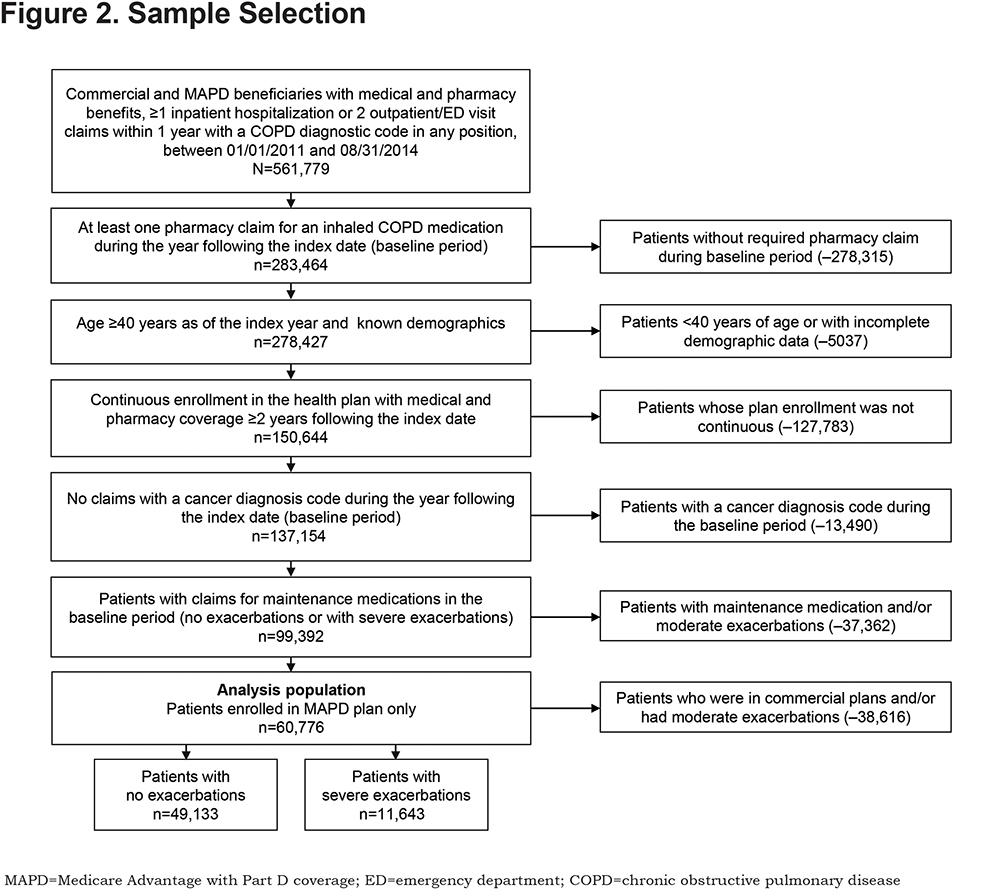
This retrospective, observational study used health care claims among MAPD beneficiaries diagnosed with COPD to validate CTR as a modifiable measure of COPD exacerbation risk. Medical and pharmacy data, and enrollment information from January 1, 2011 to August 31, 2016, were used (Figure 1). Eligible patients were ≥ 40 years of age as of the year of the index date, had ≥ 1 pharmacy claim for a COPD maintenance medication during the baseline period, continuous medical and pharmacy coverage for ≥ 2 years following the index date, and had at least 1 inpatient hospitalization or 2 outpatient and/or ED visits within 1 year (defined by an International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] COPD diagnosis code of 491.x, 492.x, or 496 in any position [the identification period ended on August 31, 2014; therefore only ICD-9 codes and not ICD-10th Revision (ICD-10) codes were included]).The index date was defined as the earlier of either: (a) the discharge date of the first COPD inpatient hospitalization or (b) the date of the second COPD-related ambulatory (outpatient or office visit) and/or ED visit within a 1-year period; the second visit date was used to ensure the eligibility criteria had been met at the index date and to prevent confounding of the exacerbation income. The index date was used to identify a baseline period (1-year post-index) and a subsequent at-risk period (1-year post-baseline; Figure 1).
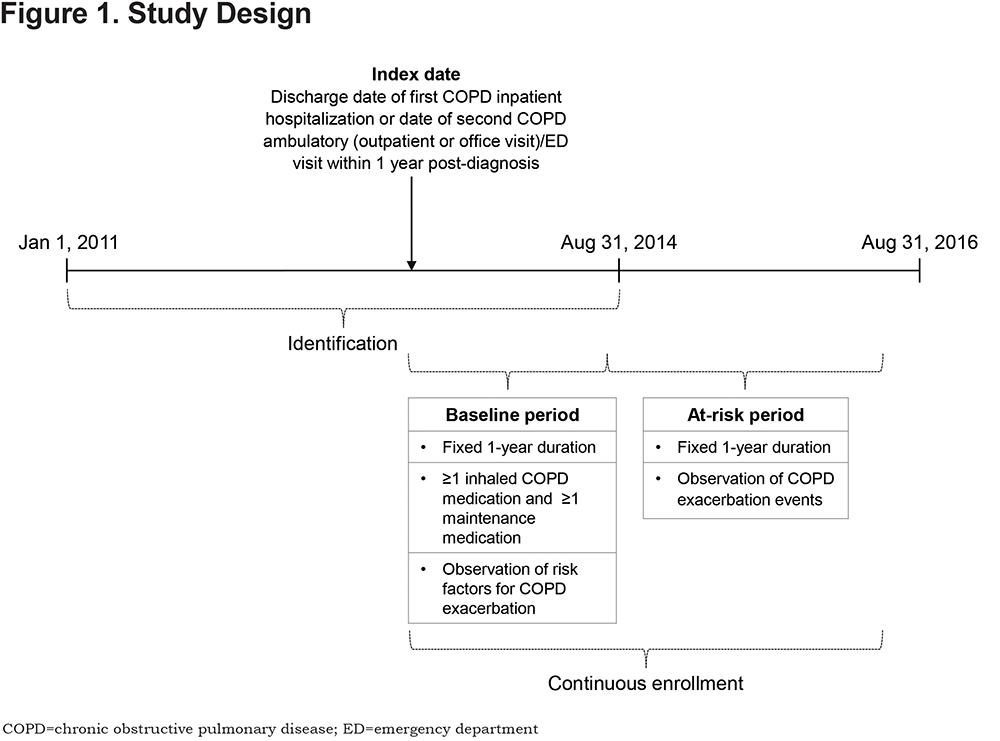
As use of maintenance medication during the baseline period was required, 24,967 patients with a CTR of 0 (no maintenance medication) were excluded. Patients diagnosed at baseline with any type of cancer were excluded, except for those with ≥ 1 non-diagnostic claims at baseline with an ICD-9-CM diagnosis code for a cancer with typically little effect on lung function (i.e., breast, prostate, and non-melanoma skin cancers).13
Severe exacerbations were the focus of analysis due to previous evidence for the ability of the CTR to better predict severe exacerbations and more consistently predict time to first severe exacerbation.14
Medication Classes Used to Calculate the COPD Treatment Ratio
Two classes of medication were used to calculate the CTR: maintenance medication and rescue medication. Maintenance medication included long-acting anti-muscarinic agents, long-acting-beta2-agonists, inhaled corticosteroids, methylxanthines, and phosphodiesterase-4 enzyme inhibitor rescue. Rescue medication included short-acting beta2-agonists and short-acting anti-muscarinic agents, either alone or combined.
Predictor Variables and Covariates
The 4 predictor variables of interest were: (1) baseline CTR (defined as the total units of maintenance medication dispensed, divided by total units of maintenance plus total units of rescue medications dispensed during the baseline period); (2) any (severe or moderate) baseline exacerbation; (3) count of baseline rescue units dispensed (1 unit of a rescue medication was defined as a canister of a rescue medication [containing 100 doses] or 100 doses of a nebulized rescue medication); and (4) count of baseline maintenance units dispensed (1 unit of maintenance medication was defined as a 30-day supply). Medication units were determined using pharmacy claims. Further information on the classification of patients by baseline exacerbation history is in the online data supplement.
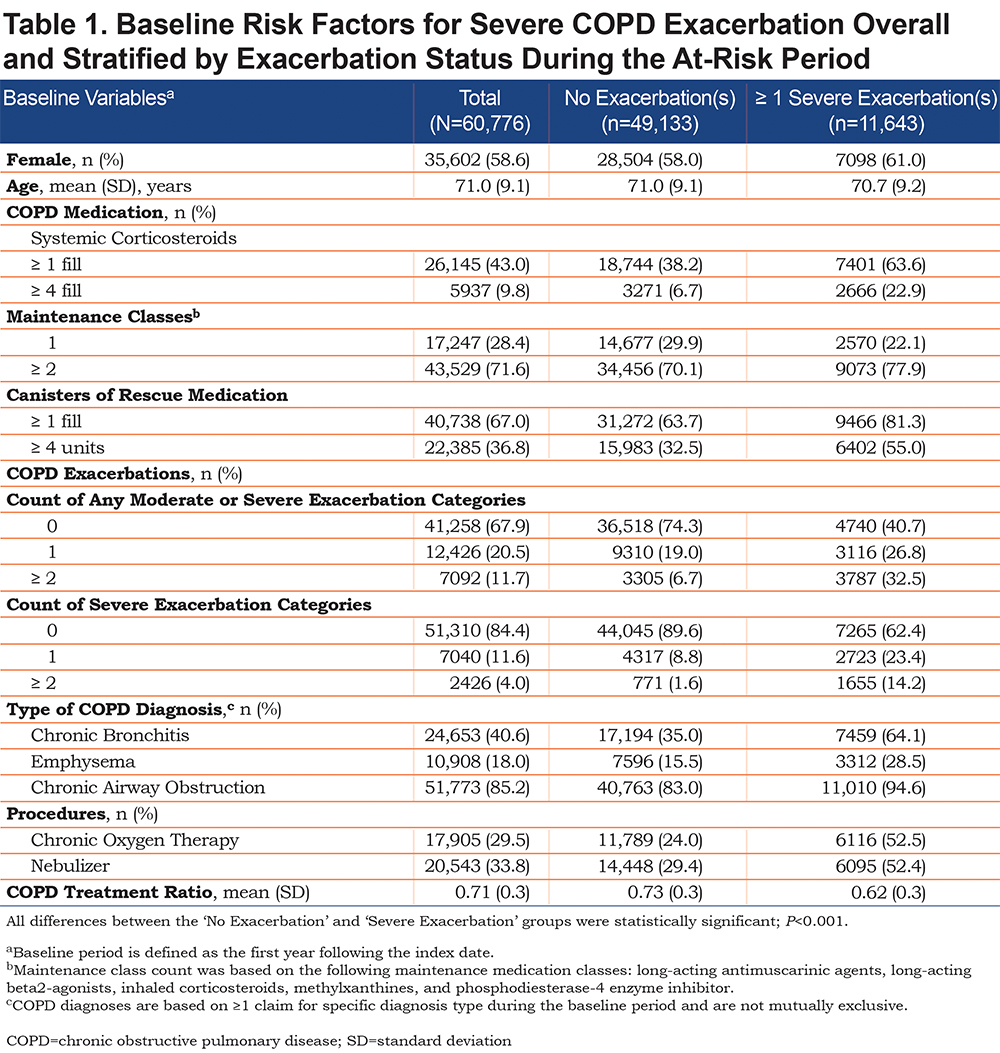
Clinical characteristics examined at baseline included: COPD medication use, COPD exacerbations, type of COPD diagnosis, and procedures (Table 1).
Outcome
The primary outcome was the occurrence of severe exacerbations during the 1-year at-risk period. The definition of a severe exacerbation during the at-risk period was identical to the definition used in the baseline period, with the addition of the corresponding ICD-10 codes: J41–J44, for an inpatient hospital stay with a diagnosis of COPD as a primary diagnosis; or J80, J9600–J9602, or J9620–J9622 as a secondary diagnosis with a primary diagnosis of respiratory failure. Exacerbations beginning during the baseline period and continuing into the at-risk period were attributed to the baseline period.
Statistical Analysis
Baseline demographics and clinical characteristics were described overall and stratified by severe exacerbation occurrence during the at-risk period. Unadjusted and adjusted logistic regression models were estimated for the association between the outcome of severe exacerbation versus no exacerbation and the 4 predictors of interest. Variables included in the adjusted model were age, geographic region in which the person was enrolled,15 chronic oxygen, and nebulizer use. The C-statistic was used to compare the predictive ability of each of the predictors. The odds ratio (OR) with 95% confidence interval (CI), P-value, and C-statistic were calculated for each model. The a priori alpha level for all inferential analyses was set at 0.05, and all statistical tests were 2-tailed. All statistical analyses were performed using SAS 9.4 software.
Results
Study Population
In total, 60,776 MAPD beneficiaries were identified for inclusion in this analysis; 49,133 had no exacerbation and 11,643 had ≥ 1 severe exacerbation during the at-risk period (Figure 2).
Descriptive Analysis
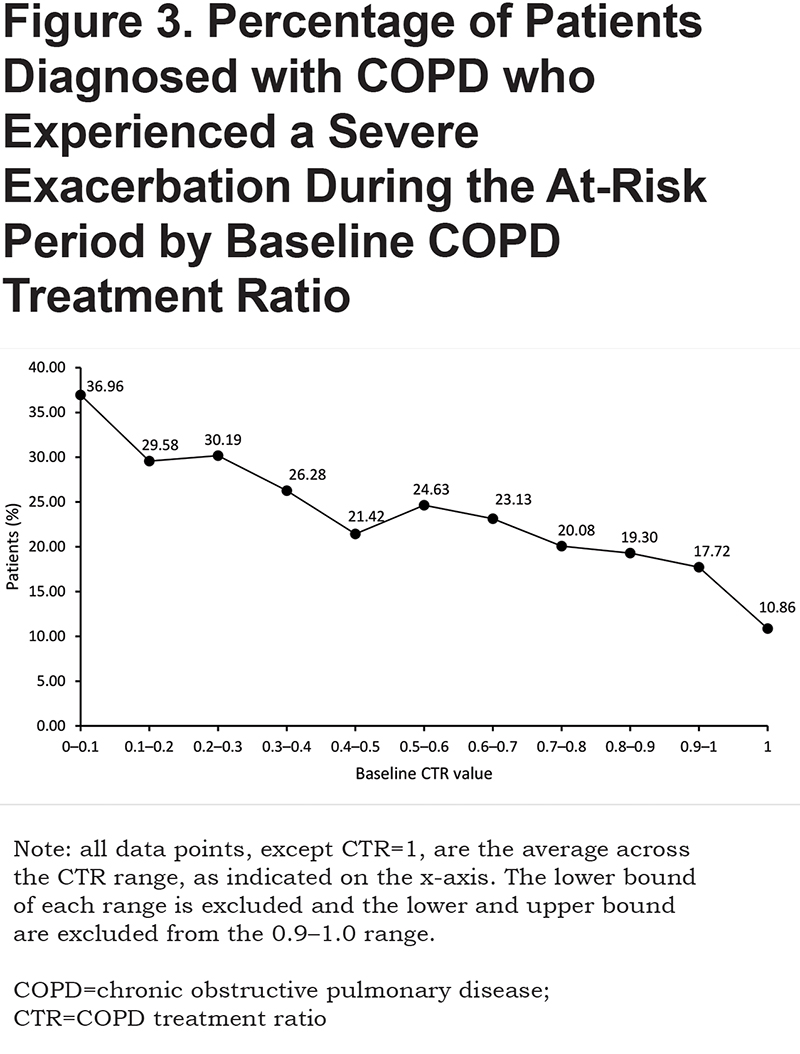
The study population had a mean age (± standard deviation) of 71.0 (± 9.1) years and was predominantly female (58.6%; Table 1). Compared with patients who experienced no exacerbation, those who experienced ≥ 1 severe exacerbation during the at-risk period used significantly more COPD medications (including frequent [≥ 4 fills] systemic corticosteroids use [22.9% versus 6.7%], use of ≥ 2 maintenance medication classes [77.9% versus 70.1%], and frequent [≥ 4 units] rescue medication use [55.0% versus 32.5%]), experienced significantly more baseline COPD exacerbations (≥ 2 severe exacerbations: 14.2% versus 1.6%, respectively; P<0.001), and had more procedures (chronic oxygen therapy and nebulizer) during the baseline period (Table 1). Individuals with a CTR of < 0.1 during baseline were most likely to experience an exacerbation during the at-risk period, while those with a CTR of 1 in the baseline period were least likely to experience an exacerbation during the at-risk period (Figure 3).
Outcome Measures
In the unadjusted analyses, the best predictor of severe exacerbation during the at-risk period, was the logistic regression model that included any baseline exacerbation, which demonstrated the highest C-statistic of unadjusted models (0.668); this was followed by the number of rescue units dispensed (0.651), CTR (0.619), and the number of maintenance units dispensed (0.562) (Table 2). Any baseline exacerbation, a higher number of rescue units and higher number of maintenance units, were positively associated with having ≥ 1 severe exacerbation (an OR > 1.0) during the at-risk period. Conversely, baseline CTR was inversely associated (OR < 1.0) with having ≥ 1 severe exacerbation during the at-risk period.
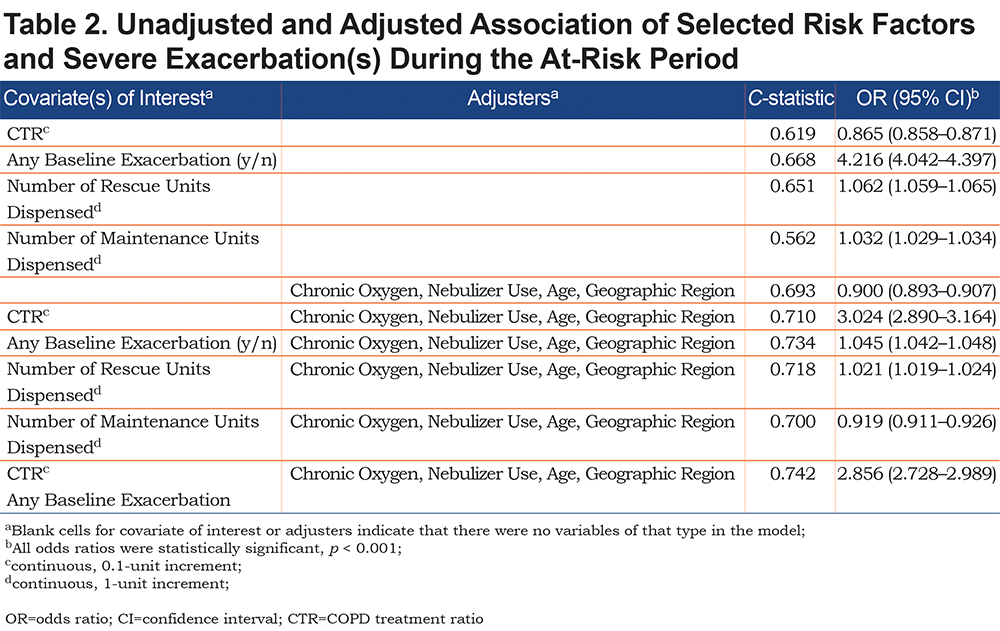
The patterns were similar, but with increased C-statistic values, after adjustment for age, geographic region, chronic oxygen, and nebulizer use, explained below. The adjusted OR of a severe exacerbation was 0.90 (95% CI, 0.89–0.91) per 0.10 change in CTR (C-statistic, 0.710; P<0.001). When any baseline exacerbation was the predictor of interest, the adjusted OR of a severe exacerbation was 3.02 (95% CI, 2.89–3.16; C-statistic, 0.734; P<0.001). The adjustment with inclusion of both any baseline exacerbation and CTR and all previously mentioned predictors resulted in a C-statistic of 0.742, and the OR of a severe exacerbation was 0.92 (95% CI, 0.91–0.93) per 0.10 change in CTR (P<0.001).
Discussion
This retrospective observational study utilized U.S. administrative claims data to validate and compare the CTR, a modifiable measure of COPD exacerbation risk, to other COPD exacerbation predictors in a group of MAPD beneficiaries diagnosed with COPD. The study found that, while previous exacerbation was the strongest predictor for future exacerbation, the CTR was an effective measure for identification of people diagnosed with COPD who are at increased risk of severe exacerbation.
As expected, the mean baseline CTR was lower (i.e., higher rescue medication use relative to maintenance use) among those who experienced a severe exacerbation during the at-risk period, compared with individuals who experienced no exacerbation during the at-risk period (data not shown). The C-statistic from the unadjusted and adjusted models was highest for any baseline exacerbation, corroborating previous studies that found prior exacerbation to be the strongest predictor for a future exacerbation.16,17 In the original CTR validation study,13 the CTR performed well in predicting exacerbation risk at a population level using claims data, reporting C-statistics between 0.714 and 0.761, which compares favorably with other risk models that have utilized clinical information.9-12,18
Previous analyses have included populations that comprise a mix of commercial and Medicare patients.13 The final analysis in this study was limited to those with MAPD coverage with maintenance medication, to further assess the predictive ability of the CTR in the Medicare population.
More recently, several models for predicting exacerbation risk were examined to help identify variables that are important in predicting severe COPD exacerbation risk,19 although no model demonstrated reliable predictors among data available in medical claims. Other studies have examined clinical and biomarker data,20 but results could not be replicated in additional samples. The asthma medication ratio (AMR) is an example of a practical measure for use in community practice to help enhance quality of practice and reduce morbidity, mortality, and health care costs.21,22 The AMR allows health care organizations to use pharmacy data to identify and target people diagnosed with asthma who require intervention to subsequently enhance outcomes (National Committee for Quality Assurance23), and is a beneficial tool for payers, decision makers, and physician groups who want to identify gaps in exacerbation prevention and medical adherence. The CTR is calculated in a similar manner to the AMR and could easily be incorporated as a quality measure alongside the asthma measure for patients diagnosed with COPD.
Compared with other tools for predicting future exacerbation in people diagnosed with COPD, the CTR offers several advantages. Firstly, it utilizes pharmacy claims data, which are readily accessible and can therefore be calculated when other forms of data are unavailable. For example, while prior exacerbation history is the strongest predictor of future exacerbation and many clinical variables may contribute to that risk, these types of data are not always available, especially to national quality-of-care organizations. Secondly, the CTR is a modifiable risk factor. This means that individuals with a low ratio of maintenance medication to rescue medication can be identified and, with appropriate medical intervention and adherence support, can increase the ratio of maintenance to rescue medication. This would allow a program to be developed with the goal of modifying the ratio of maintenance to rescue medication, compared with a program solely focused on the reduction of rescue medication. Finally, the CTR can be used as a valuable guide for organizations making decisions about whether to intervene and dedicate services to exacerbation prevention, specifically allowing those with fewer resources to choose a lower score (e.g., ≤ 0.2) as a cut-off point for identification of high-risk patients, and vice versa.
This study has several limitations that must be considered when interpreting its findings. Exacerbation events were identified according to a definitional algorithm using health insurance claims data; the use of an algorithm with less than 100% sensitivity and specificity will result in misclassification of the outcome. All variables used in the analysis were defined based on the presence of codes on administrative claims, which does not guarantee that the beneficiary received that diagnosis, underwent a procedure, or received a specific medication. While candidate covariates for multivariable analysis in this study were limited to health insurance claims data, disregarding important clinical measures in COPD (e.g., lung function or oxygen saturation), they reflected those used to develop the CTR.13 However, prior research found similar predictive accuracy of the CTR models with and without the Global initiative for chronic Obstructive Lung Disease stage.13 Finally, the results of this analysis are primarily applicable to COPD patients in stable managed care settings and may not be generalizable to the U.S. COPD population as a whole.
Conclusions
This study found that the CTR is an effective tool for identifying people diagnosed with COPD who are at increased risk for severe exacerbation. While the CTR does not predict severe exacerbation as well as the presence of a prior exacerbation, the CTR can measure the risk of exacerbation in patients without a history of exacerbations, and can be assessed without the need for medical history; in addition, it can be used to modify patient management appropriately, particularly in low resource settings. Overall, the CTR is an effective tool for predicting severe exacerbation risk and performed similarly to other pharmacy-based predictors explored in this study. It is suggested that future investigation focuses on determining its predictive value across differing lengths of baseline and at-risk periods, testing the CTR against other pharmacy-based predictors.
Acknowledgments
Study design consultation was provided by Ami Buikema; programming support by Yiyu Fang; and project management by Sharanya Murali and Caroline Jennermann, all of Optum. Editorial support (in the form of writing assistance, collating author comments, assembling tables/figures, grammatical editing and referencing) was provided by Tom Gallagher, PhD, of Gardiner-Caldwell Communications (Macclesfield, UK) and was funded by GlaxoSmithKline plc.
Author Contributions: All authors performed data analysis and/or data interpretation. RHS and LGSB conceived and/or designed the study; SK and LGSB contributed to the acquisition of data; and all authors revised the report for important intellectual content and approved the final article for submission.
Data availability: The data contained in our database contains proprietary elements owned by Optum and, therefore, cannot be broadly disclosed or made publicly available at this time. The disclosure of this data to third-party clients assumes certain data security and privacy protocols are in place, and that the third-party client has executed our standard license agreement which includes restrictive covenants governing the use of the data.
Declaration of Interest
The authors declare the following conflicts of interest during the last 3 years in relation to this article: At the time of the study, RHS was an employee of GlaxoSmithKline plc and holds stock options/shares. At the time of the study, TR was a fellow at UNC. SK, LB, and LGSB are employees of Optum; GlaxoSmithKline plc contracted with Optum to conduct this research.