Running Head: Multiparity, Lung Function and COPD Phenotypes
Funding Support: The COPDGene® study is supported by Award Number U01 HL089897 and Award Number U01 HL089856 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The COPDGene® study is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprised of AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion.
Date of acceptance: January 22, 2020
Abbreviations: chronic obstructive pulmonary disease, COPD; COPD Genetic Epidemiology, COPDGene®; forced expiratory volume in 1 second, FEV1; percentage of predicted, % predicted; forced vital capacity, FVC; computed tomography, CT; square root of the wall area of a hypothetical airway with an internal perimeter of 10 mm, Pi10; National Health and Nutrition Examination Survey, NHANES; beta coefficient, ß; confidence interval, CI; forced expiratory flow at 25%—75% of of the maneuver, FEV25-75; preserved ratio-impaired spirometry, PRISm; low attenuation area, LAA; Hounsfield units, HU; body mass index, BMI; 6-minute walk test distance, 6MWD; pregnancy-associated plasma protein-A, PAPP-A; antigen presenting cells, APCs
Citation: Moll M, Regan EA, Hokanson JE, et al. The association of multiparity with lung function and chronic obstructive pulmonary disease-related phenotypes. Chronic Obstr Pulm Dis. 2020; 7(2): 86-98. doi: http://doi.org/10.15326/jcopdf.7.2.2019.0166
Online Supplemental Material: Read Online Supplemental Material (243KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation, and often related to smoking exposure.1 The pathogenetic mechanisms of COPD remains an active area of investigation, though inflammation, oxidative stress, protease/anti-protease imbalance, autoimmunity, and accelerated aging are implicated.2 COPD was previously thought to be a disease of older males. However, the rise of smoking in females has narrowed the prevalence gap between sexes,3 and the importance of sex-specific differences in COPD susceptibility and pathophysiology has become apparent.4-6 In adolescents, smoking is associated with reduced lung growth in females compared to males.7 A differential decline in lung function is also seen in adult females and males, particularly amongst heavy smokers.8 In a family-based cohort of 84 probands with severe, early-onset COPD (age < 52 years, forced expiratory volume in 1 second [FEV1]< 40 percent predicted[% predicted]), 60(71%) probands were female9,10 despite lower overall smoking history.
A subsequent analysis in 2500 participants from the COPD Genetic Epidemiology (COPDGene®) study reported that 66% of early-onset COPD participants (age < 55 years, FEV1 < 50%) were female. Additionally, maternal smoking, and maternal COPD were associated with early-onset COPD.11 Phenotypically, female smokers are more likely to be hospitalized for COPD exacerbations,12 have more dyspnea, higher mortality risk, and worse functional status.13 These data suggest that female sex, hormonal factors, and age may affect susceptibility to and manifestations of smoking-related lung disease.
Sex-specific hormonal factors may alter COPD susceptibility through several mechanisms.14 Cigarette smoke contains thousands of compounds, many of which are activated to their toxic form by phase I cytochrome P450 enzymes (bioactivation). Detoxification of these metabolites is mediated by Phase II enzymes.15,16 However, in human lungs, estradiol upregulates P450 enzymes without significantly changing expression of Phase II enzymes.17,18 This may lead to increased oxidative stress in the lungs, placing females at higher risk for COPD despite lower overall pack years of smoking.
While there is evidence of sex differences in COPD, and hormonal factors are mechanistically implicated, little is known regarding female hormonal effects in population studies. Recently, menopause has been associated with accelerated FEV1 decline.19 Pregnancy represents a time of high hormonal exposure, and multiparity has been associated with lower lung function during pregnancy. A longitudinal prospective study of 120 pregnant women reported that, in multiparous compared to nulliparous women, the forced expiratory flow in 25% to 75% of the maneuver (FEF25-75) was lower in both the first (3.2 L versus 3.7 L, p=0.0005) and third (3.3 L versus 3.6 L, p = 0.031) trimesters; however, there was no post-partum follow-up of lung function.20 These data provide evidence that endogenous hormones and pregnancy may affect lung function.
We hypothesized that, in smokers, multiparity is associated with reduced lung function and quantitative computed tomography (CT) imaging measures of emphysema and airway disease. We performed an analysis in smokers with and without COPD from the COPDGene® study to understand the effects of number of pregnancies and multiparity on measures of COPD disease severity and progression. As we did not expect to observe these effects in never smokers, we also evaluated associations between multiparity and lung function in never smokers and those with lower smoking exposure from a subset of National Health and Nutrition Examination Survey (NHANES) participants.
Methods
Study Participants
COPD Genetic Epidemiology Study
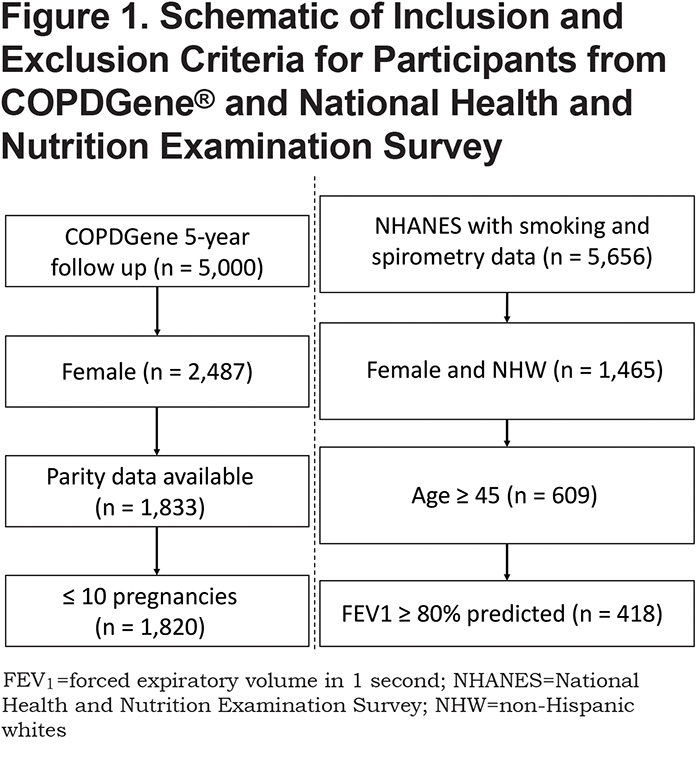
We included female participants from the 5-year follow-up of the COPDGene® study. Details regarding the COPDGene® study design have been previously published.21 Briefly, participants were non-Hispanic whites or African Americans, 45 to 80 years of age at Phase 1 enrollment, with 10 or more pack years of smoking history, recruited from 21 U.S. clinical sites. COPDGene® includes smokers with and without obstruction, including those with normal spirometry (FEV1 ≥ 80% predicted and FEV1/FVC > 0.7), those with preserved ratio-impaired spirometry (FEV1 < 80% predicted and FEV1/FVC > 0.7; aka “PRISm”), and those with Global initiative for chronic Obstructive Lung Disease (GOLD)-defined COPD (FEV1/FVC < 0.7).1 Baseline demographic, clinical, spirometric, and imaging variables were initially collected (Phase 1, n = 10,192), and then re-measured after 5 years (Phase 2, n = 5000). At the 5-year follow up of COPDGene®, questions about pregnancy and menstrual cycles were adapted from standardized and validated questionnaires.22 Inclusion criteria for this current analysis included being a Phase 2 participant (n = 5000), female sex (n = 2487), and having answered the questions regarding parity (n = 1833). We excluded individuals reporting more than 10 pregnancies (n = 13) as grand multiparity is associated with numerous poor outcomes,23 and there is a paucity of studies that included women with this range of numbers of pregnancies. The final number of female participants included is 1820 (Figure 1).
National Health and Nutrition Examination Survey
To assess generalizability in individuals with lower or no smoking exposure, we included non-Hispanic white participants ≥ 45 years of age with spirometry data and similar questions about pregnancy available in the NHANES 2011–2012 dataset. We included weights for individuals in the regression models to account for ascertainment bias due to study design.24 Spirometry values were converted to percent predicted values using Hankinson reference equations.25 We classified participants into GOLD spirometry grades1 based on pre-bronchodilator FEV1 % predicted and FEV1/FVC. We defined a “lower smoking exposure” group as those with ≥ 100 cigarettes in a lifetime, and only included those with no or mild obstruction (FEV1 ≥ 80% predicted); these participants had less than a mean of 10 pack years of smoking history.
Study Design and Analyses
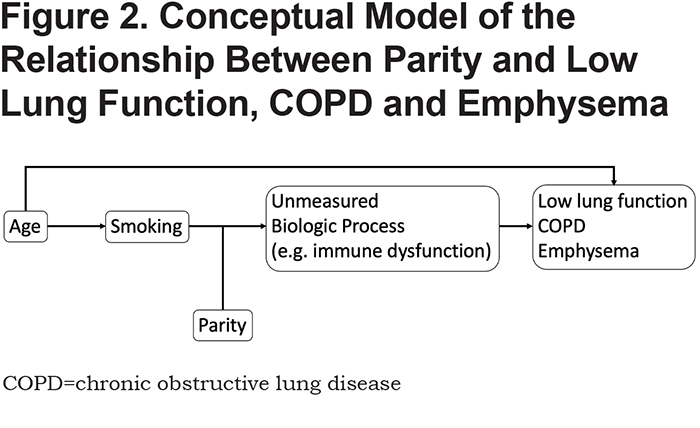
Our conceptual model is shown in Figure 2. Based on this framework, we performed the below epidemiological analyses.
Exposure Variables: We conducted an analysis of the effects of number of pregnancies and multiparity on lung function and quantitative CT imaging variables. Specific questions asked are shown in eTable S1 in the online data supplement. We considered 2 representations for the exposure, parity. (1) The number of times pregnant was transformed into a categorical variable (1 or fewer pregnancies, 2 pregnancies, 3 or more pregnancies), and 1 or fewer pregnancies was used as the reference group since only 13 participants reported zero pregnancies. (2) Multiparity was defined as >1 pregnancy, with 1 or fewer as the reference group.
Outcome Variables: The primary outcome in COPDGene® was the post-bronchodilator FEV1 % predicted. Secondary outcomes included FEV1/FVC ratio, percentage of emphysema on CT imaging (% low attenuation [LAA] < -950 Hounsfield units [HU]),26 and the square root of the wall area of a hypothetical airway with an internal perimeter of 10 mm (Pi10).27 Spirometry measures were obtained from the 5-year follow up visit of the COPDGene® study. In NHANES, the outcomes explored included FEV1 % predicted and FEV1/FVC ratio.
Primary Analyses: To determine the effects of number of pregnancies and multiparity on lung function and quantitative CT imaging variables in the COPDGene® study, we fit linear regression models for each outcome and exposure variables defined above adjusting for age, pack years of smoking, body mass index (BMI), current smoking status, age at which smoking was started, and level of education completed. In addition, we stratified our data into “controls” (no obstruction or GOLD spirometry grade 1), PRISm,28 and GOLD 2–4 (“Cases”), and then repeated our analyses within these groups. In NHANES, we also included never smokers and lower smoking exposure groups to investigate the effect of parity in these populations. For linear regression models in NHANES, we adjusted for age, pack years of smoking, current smoking, BMI, and level of education. Beta coefficients (β) and 95% confidence intervals (CIs) were reported. Variables with a pre-specified threshold p-value of less than 0.05 were considered significant.
Interaction Analyses: We also considered the interaction of multiparity and age, pack years of smoking, or smoking start age on lung function. Each potential interaction was evaluated in a separate linear regression model that included multiparity, the potential interaction variable (i.e. age, pack years of smoking, or smoking start age), and an interaction term. For example, to assess for an interaction between pack years of smoking and multiparity in relation to a given outcome, the regression model would include multiparity, pack years, multiparity x pack years (the interaction term), and relevant covariates. Interaction terms were considered significant if the p-value was less than 0.05.
Results
Characteristics of Participants
Characteristics of COPDGene® participants are shown in Table 1. There were 928 smoker controls (no or mild obstruction), 220 PRISm, and 616 GOLD 2—4 COPD cases enrolled at the 5-year follow-up for COPDGene® that met our selection criteria (Figure 1, total n = 1820). Compared to controls (no or mild obstruction), COPD participants (GOLD 2–4) were slightly older, had more pregnancies, similar number of postmenopausal women, more pack years of smoking history, and lower 6-minute walk test distance (6MWD).
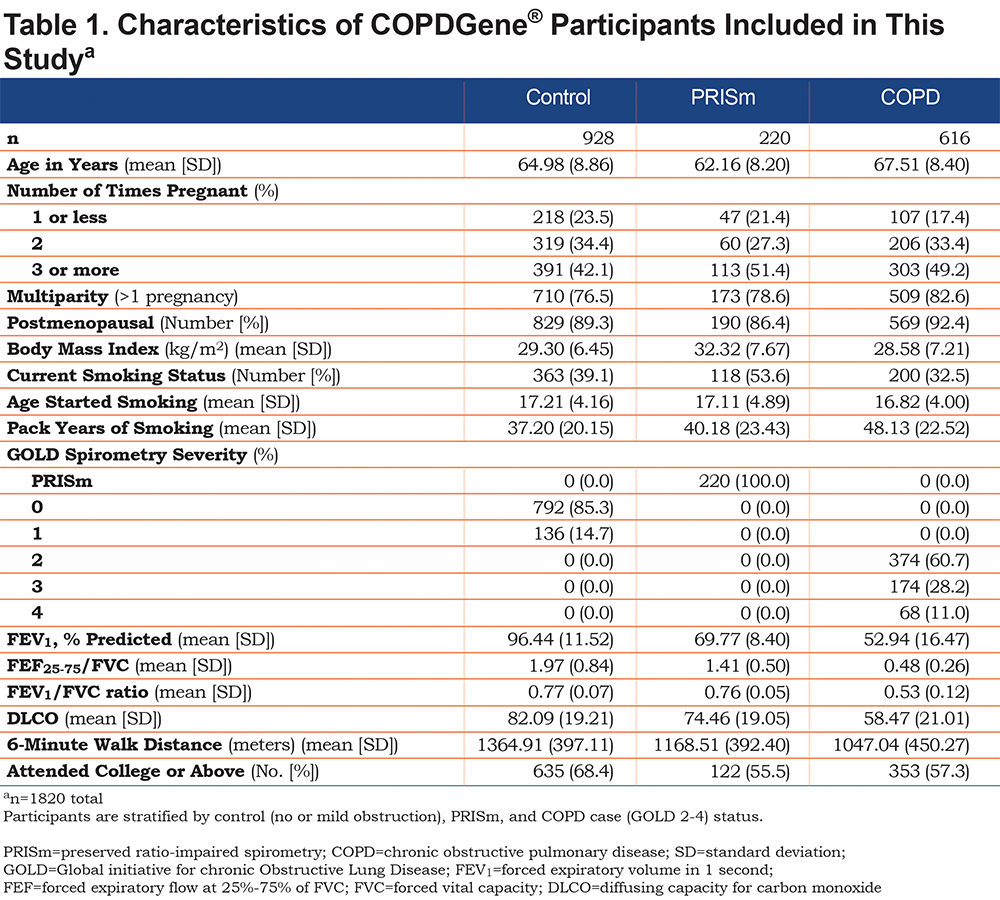
Characteristics of NHANES participants (n=418) are shown in supplemental eTable S2 in the online supplement. There were 321 never smokers and 97 participants in the lower smoking exposure group. Compared to the group with no or mild obstruction in COPDGene®, the NHANES population was slightly younger, and had fewer pack years of smoking (mean 8 versus 37 pack years) and had a fewer number of individuals with 1 or fewer pregnancies.
Association of Parity with Lung Function
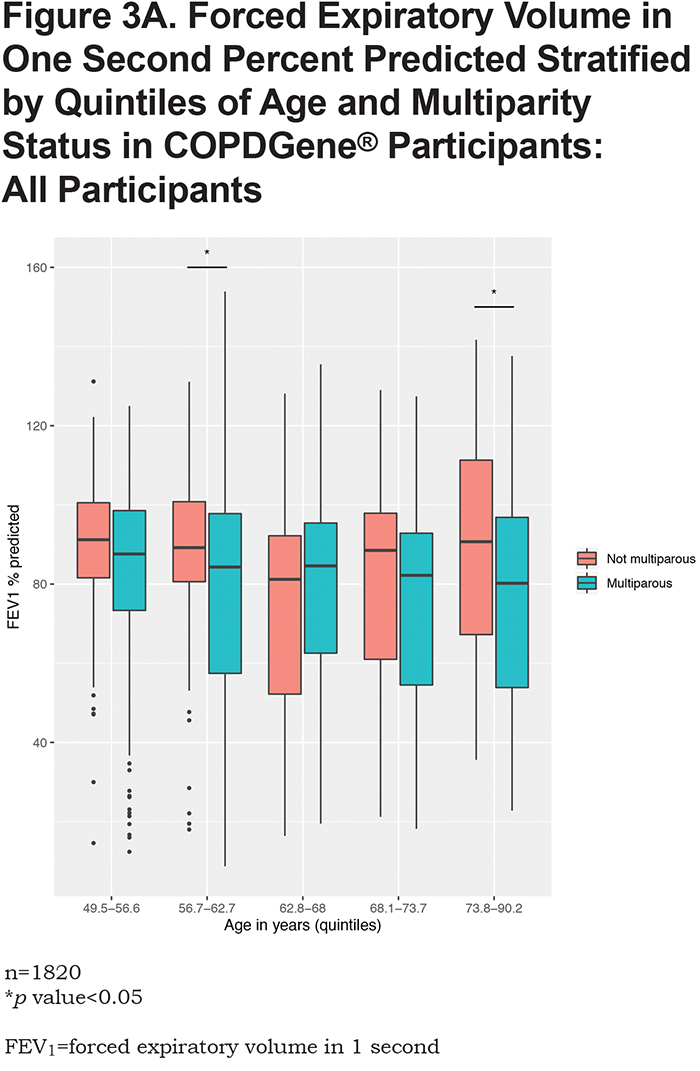
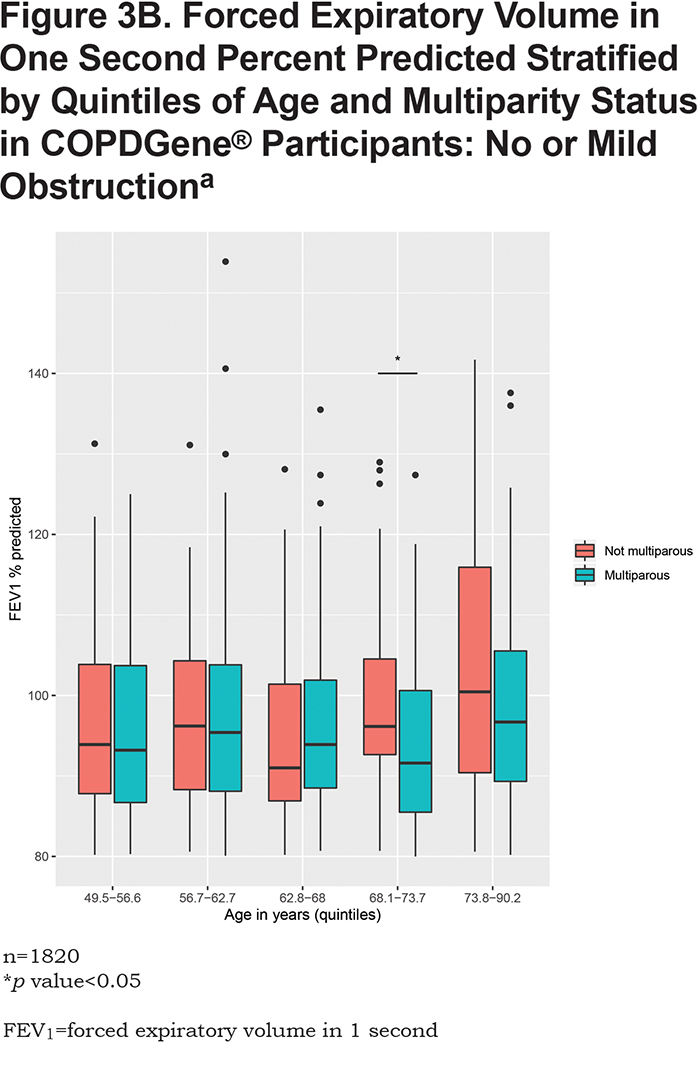
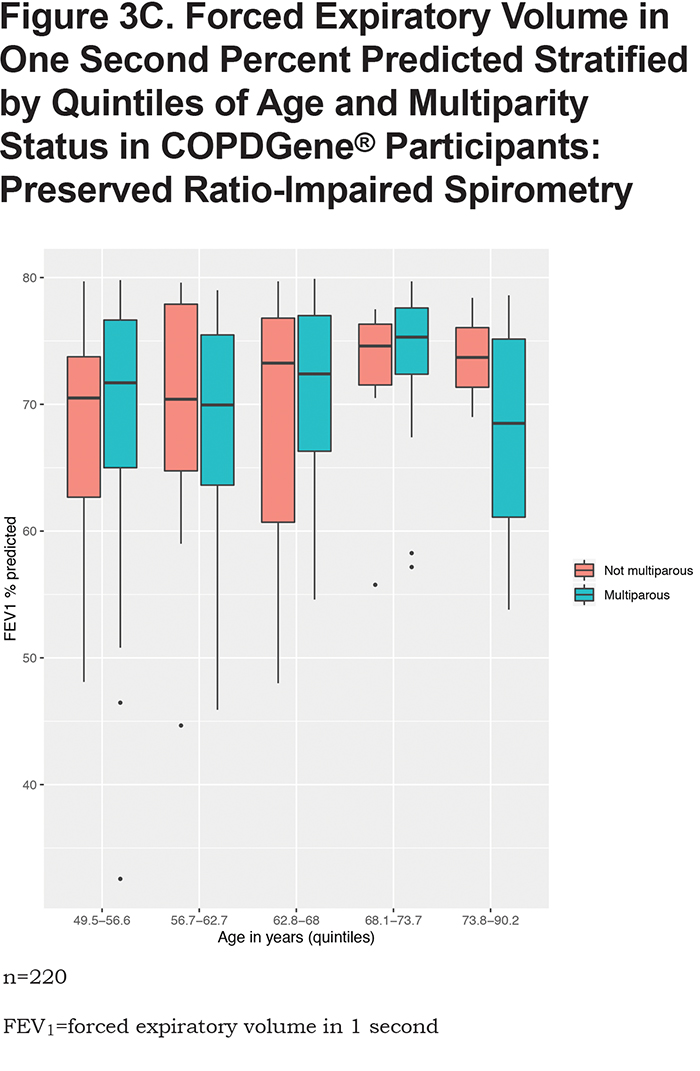
In the overall analysis, including all 1820 COPDGene® participants, having 2 pregnancies (β = -3.1, 95% CI: [-6.1,-0.03], p = 0.048) or 3 or more pregnancies (β = -4.5, 95% CI: [ -7.4,-1.5], p = 0.003) are associated with a lower FEV1 % predicted (Table 2). Multiparity (β = -3.8, 95% CI: [-6.5, -1.1], p = 0.005) is associated with a lower FEV1 % predicted (Table 3). In the stratified analyses, having 2 or 3 or more pregnancies and multiparity are significantly associated with lower FEV1 % predicted in participants with no or mild obstruction (Tables 2 and 3) but not across the more severe GOLD grades. For the interaction analyses, the interaction of multiparity with age on FEV1 % predicted was significant (β = -0.23, 95% CI: [ -0.86,0.4], p = 0.025). Figures 3A, 3B, 3C and 3D, show boxplots of FEV1 % predicted in each age quintile, separated by multiparity status. Multiparity was associated with lower FEV1 % predicted, particularly in lower (57–63 years) and higher (74–90 years) age categories. Interactions of multiparity with smoking or smoking start age on FEV1 % predicted were not significant.
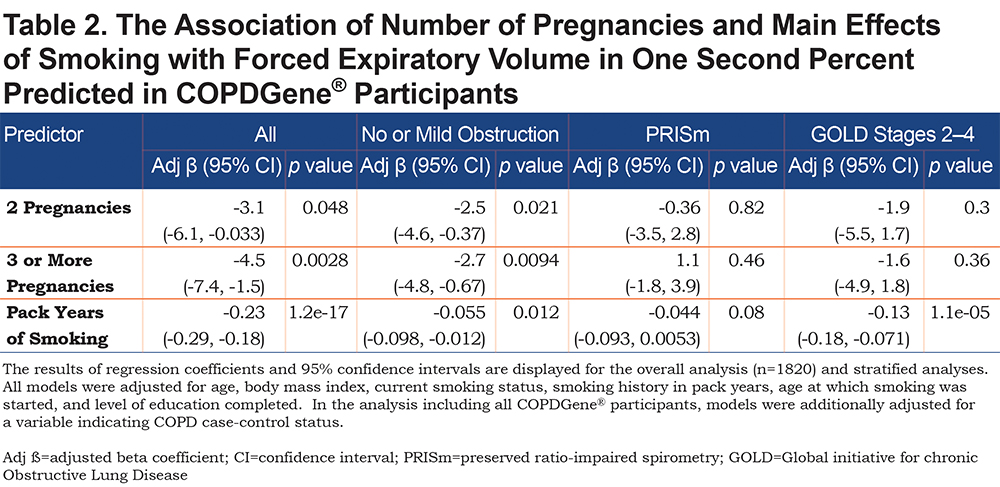
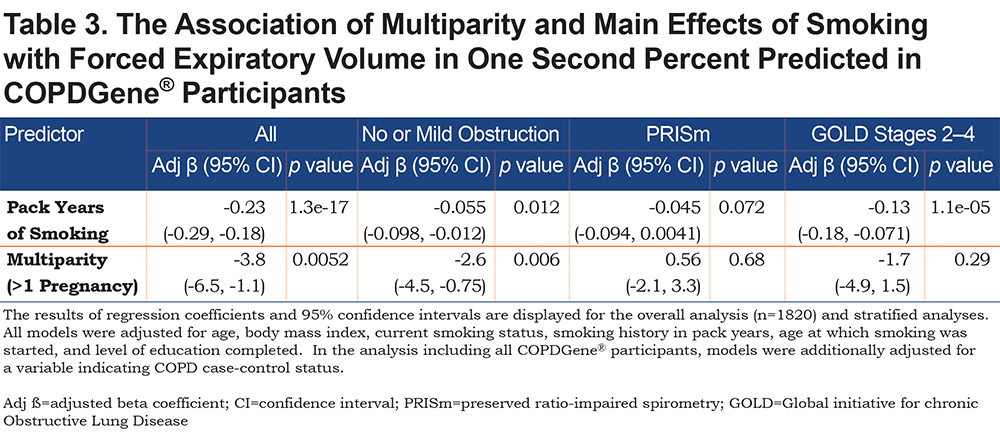
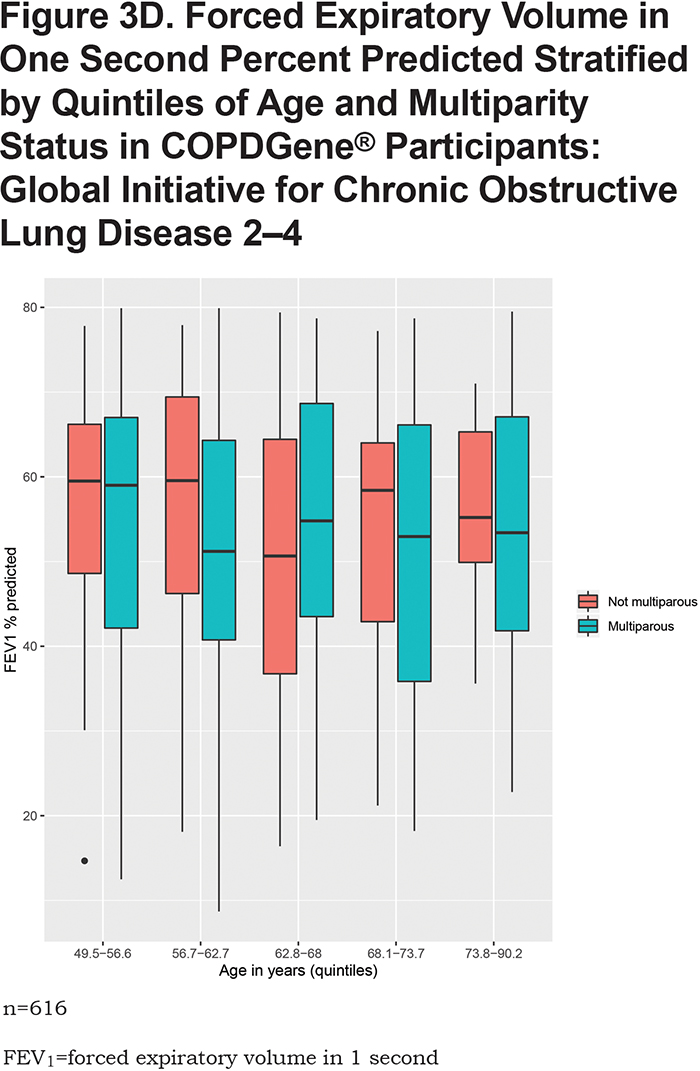
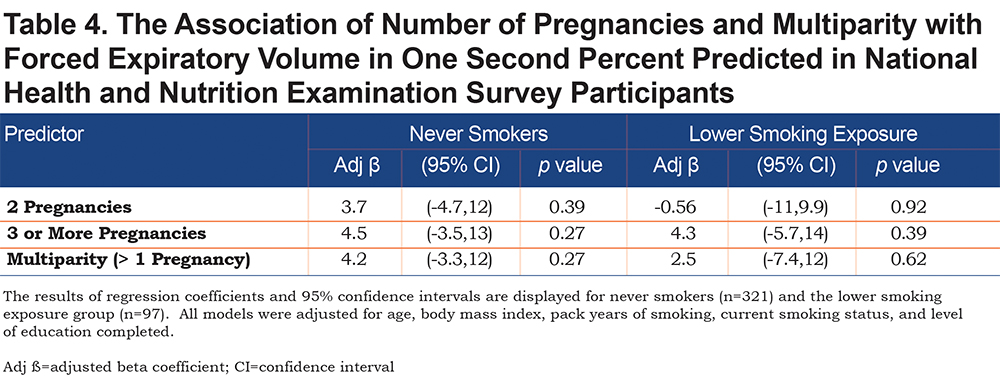
None of the exposure variables were associated with FEV1/FVC ratio (eTable S3 in the online supplement). Multiparity or number of pregnancies were not associated with percent emphysema (% LAA < -950 HU) (eTable S4 in the online supplement) or airway wall measurement (Pi10) (eTable S5 in the online supplement) in overall analyses. In GOLD 2—4 participants, multiparity was associated with higher Pi10 (β = 0.24, 95% CI: [0.05,0.42], p = 0.01). In the never and lower smoking exposure groups from NHANES, neither multiparity or number of pregnancies were associated with FEV1 % predicted or FEV1/FVC ratio (Table 4 and eTable S6 in the online supplement).
Discussion
In this study, we examined the association between parity and lung function within 1820 smokers in the COPDGene® study. We found that, in current and former smoking women from the COPDGene® study with no or mild airflow obstruction, multiparity was associated with lower lung function as measured by FEV1 % predicted. However, we did not observe this effect in 418 individuals from NHANES without obstruction with no or minimal smoking exposure. Finally, we report that multiparity was not associated with quantitative emphysema or airway disease measured on CT scan.
The current study extends prior evidence regarding parity and lung function. A recent longitudinal prospective cohort study of 120 pregnant females compared spirometry in nulliparous and multiparous females during the first and third trimesters of pregnancy; compared to nulliparas, multiparas had lower FEV1, FEF25-75, and FVC in the third trimester 20; however, no post-partum follow-up of lung function was reported. In our study, we compare lung function in multiparas to individuals with 1 or no pregnancies within smoking individuals from the COPDGene® study. When analyzing all COPDGene® participants together, multiparity was associated with lower FEV1 % predicted. After stratifying by controls (no or mild airflow obstruction) and cases (GOLD 2–4), we observe that this relationship holds true only within the stratum of smokers with no or mild obstruction. One potential explanation for this observation is that individuals in GOLD 2–4 have already lost significant lung function, and factors other than parity (e.g., ongoing smoking exposure) may play a more prominent role in the later stages of disease progression. Another explanation may be that GOLD 2–4 participants were older, and age can lead to FEV1 decline independent of smoking and parity (see conceptual model in Figure 2). Thus, we propose that multiparity may be a susceptibility factor for low lung function in smokers with no or mild obstruction.
To assess whether multiparity is a risk factor for lower lung function in persons with no or lower smoking exposure, we evaluated the association between multiparity and FEV1% predicted in NHANES; as expected, we did not observe this association in NHANES never smokers or the lower smoking exposure group. There may be several reasons for this result. There may be a required threshold of smoking exposure to evoke the impact of hormones on lung function; the NHANES sample had an average of 8 pack years of smoking; by contrast, the COPDGene® sample had an average of 37 pack years of smoking history. We did not observe a significant interaction between multiparity and smoking on lung function, but we may not have been sufficiently powered to detect this interaction. Perhaps there was an effect in NHANES participants, but we were not powered to detect it given the small sample size. Finally, there is a possibility that the effect observed in COPDGene® is a false positive, and given the potential implications of such a finding, investigation in a sample with similar smoking history is warranted.
One biologically plausible link to explain an association between multiparity and lower lung function in smokers is autoimmunity. Cigarette smoking has been shown to increase oxidative stress, alter immune function, pre-dispose to infections, and has been implicated in the development of autoimmune diseases.29 There is also growing evidence that autoimmunity may contribute to COPD pathogenesis.30,31 Autoantibodies have been found in sera from individuals with COPD.30 Pregnancy-associated plasma protein-A (PAPP-A), a metalloproteinase secreted by placenta, was found to be elevated in COPD individuals,32 though this was a small study without external replication. Antigen presenting cells (APCs) from patients with emphysema activate T-helper 1 (Th1) and Th17 cells, and transfer of APCs recapitulates emphysema in recipient mice independent of smoking exposure.33 Moreover, a recent network analysis of microarray data showed that activation of B cell pathways is associated with severe COPD.34 While a sex-specific analysis was not performed in this microarray study, it is important to note that females are predisposed to developing many autoimmune disorders,35 and that estrogen appears to regulate B cell antibody production and T cell-mediated inflammation.36 With these data in mind, we propose that multiparity in the presence of cigarette smoke may provide a ‘second hit’ for immune dysregulation. Indeed, multiparity may be a set up for the development of autoimmunity. For example, in cases of maternofetal hemorrhage, maternal exposure to fetal blood positive for Rhesus antigen can predispose to hemolytic reactions in subsequent pregnancies complicated by maternofetal hemorrhage.37 Parity itself has been linked to autoimmunity, which can be influenced by genetic predisposition and epigenetics.38 There are also complex alterations in the immune system during pregnancy.39 Several autoimmune diseases improve in pregnancy (e.g., Graves’ disease 40), while others worsen (e.g., systemic lupus erythematosis41). Development of autoimmune diseases, such as type I autoimmune diabetes, have been reported long after delivery.42 A potential mechanism for this delayed autoimmunity is transplacental transfer of cells between mother and fetus (microchimerism),43 although this has not been specifically studied in relevance to lung disease. Therefore, it is possible that there is an, as yet unidentified, link between female sex, pregnancy, autoimmunity, and COPD susceptibility and disease progression. Future research could focus on unraveling the complexity of this hypothesis.
Many studies have reported health effects associated with multiple pregnancies.44-47 Increased numbers of lifetime pregnancies has also been associated with accelerated markers of aging (including telomere length),48 and oxidative stress.49 Indeed, aging is an important risk factor for lung function decline, and we observed significant interaction between multiparity and age on FEV1 % predicted. We do note that, while the interaction term was significant based on the p-value, the confidence interval overlaps zero; this likely reflects that the interaction between age and multiparity on lung function appear more significant at lower and higher extremes of age in our cohort. Observing this interaction in the lower range of age categories may imply that smoking exposure at a younger age could have longer term impacts on lung function decline. The interactions in the older age categories may represent an acceleration of the lung function decline seen in normal aging. As individuals age, collagen and elastin deteriorate, chest wall compliance decreases, and respiratory muscle strength diminishes, all leading to lower FEV1 and FVC.50 Structurally, the alveolar spaces increase without inflammation or destruction of alveolar walls, and these changes are more pronounced in certain smokers.50,51 This may explain why we are detecting changes in lung function without structural changes on CT imaging; multiparous smokers may experience accelerated aging leading to accelerated lung function decline. Furthermore, we note that, in GOLD 2–4 participants from COPDGene®, multiparity was associated with higher Pi10, a marker of airway wall thickness; this likely represents the more advanced age of this subgroup. Future investigations can explore the link between aging, multiparity, and lung structure and function.
One strength of our study is the novelty of the potential link between multiparity and lung health in current and former smokers and that we tested our hypothesis in a relatively large sample of female smokers with detailed information regarding number of pregnancies. We explored the effects of cigarette smoking exposure on the association between multiparity and lung function and demonstrate in an independent general population sample that this effect is not present in individuals with lower or no smoking exposure. It is important to note that even lower amounts of smoking exposure can have other harmful effects, and that given the small sample size of the lower smoking exposure group, our data further emphasize the need for young females of child-bearing age to abstain from smoking. Physiologically, these data suggest that endogenous hormones and parity may alter COPD disease susceptibility and progression in smokers and we present several speculations as to a role for autoimmunity and accelerated aging as potential drivers of COPD in women.
This study has several limitations. A larger replication sample is needed to support an interaction between smoking and parity. Perhaps a general population sample with similar smoking history may have replicated the association between multiparity and lower FEV1 % predicted. While the COPDGene® study provided a considerably large sample in terms of the availability of exposure data, we are unable to exclude the possibility of selection bias or overfitting. Further investigation in additional cohorts would be ideal. It is also not clear how hormone replacement therapy may alter the relationship between multiparity and lung function, and we were unable to address this important question because of missingness in the dataset. Additionally, we did not have detailed data regarding the timing between pregnancies and lung function assessments, so we were unable to longitudinally model the effects of parity on lung function. Another issue is that hormone-related exposures were obtained by questionnaire, which introduces the potential for recall bias. However, we expect that most females would remember these major life events with high accuracy (e.g., number of pregnancies). Finally, we did not include serum biomarkers (e.g., PAPP-A, measures of autoantibodies); this information may have lent biological insights into the observed associations but represents a direction for future investigation.
In conclusion, multiparity is associated with lower FEV1 in smokers, but not in those with no or lower smoking exposure. The biological mechanisms underlying this association are unclear but may involve mechanisms of accelerated aging or autoimmunity. While these findings need further investigation into the biological underpinnings, our findings further emphasize that it is of utmost importance for women of child-bearing age to abstain from smoking.
Acknowledgements
Author Contributions: Matthew Moll, Elizabeth Regan, Barry Make, and Dawn L. DeMeo contributed to study design. Matthew Moll and Dawn L DeMeo performed statistical analyses. Edwin K. Silverman, Dawn L. DeMeo, and James D. Crapo obtained funding. All authors were responsible for the acquisition, analysis, interpretation of data, and for critical revisions of the manuscript.
Declaration of Interest
MM is supported by National Institutes of Health grant T32HL007427. DLD is supported by P01 HL 114501 and P01 132825. EKS is supported by P01 HL114501, R01 HL133135, R01 HL137927, and R01 HL147148. JDC and EKS are supported by U01 HL089897 and U01 HL089856. SML is supported by National Heart, Lung and Blood Institute grant K01HL125858.
EKS received grant and travel support from GlaxoSmithKline and grant support from Bayer. DLD has received grant support from Bayer.