Running Head: Correct Use of ELLIPTA vs DISKUS plus Handihaler
Funding Support: This study was funded by GlaxoSmithKline plc. (GlaxoSmithKline plc. study 206901). GlaxoSmithKline plc., led the design of the study, collection of data, data analysis and interpretation, and writing of the report. Decisions regarding submission were led by GlaxoSmithKline plc.
Date of Acceptance: February 27, 2020
Abbreviations: chronic obstructive pulmonary disease, COPD; long-acting beta2-agonist, LABA; long-acting muscarinic antagonist, LAMA; inhaled corticosteroids, ICS; dry-powder inhaler, DPI; metered dose inhaler, MDI; health care professional, HCP; single-inhaler triple therapy, SITT; forced expiratory volume in 1 second, FEV1; short-acting beta2-agonist, SABA; instructions for use, IFU; adverse event, AE; serious adverse event, SAE intent-to-treat, ITT; Cochran–Mantel–Haenszel, CMH;; confidence interval, CI; standard deviation, SD; confidence interval, CI; twice daily, BID; once daily, QD
Citation: Kerwin EM, Spangenthal S, Zvarich M, et al. ELLIPTA versus DISKUS plus HANDIHALER in COPD: a randomized, open-label, crossover study in a clinical trial setting. Chronic Obstr Pulm Dis. 2020; 7(2): 118-129. doi: http://doi.org/10.15326/jcopdf.7.2.2019.0153
Online Supplemental Material: Read Online Supplemental Material (329KB)
Introduction
Bronchodilators (long-acting beta2-agonists [LABAs] and long-acting muscarinic antagonists [LAMAs]), used alone or in combination, are key treatments in the management of chronic obstructive pulmonary disease (COPD). The step up to inhaled triple therapy (inhaled corticosteroid [ICS] plus LAMA/LABA) can occur by a number of routes, often by the addition of a LAMA to an ICS/LABA dual therapy,1 and has been shown to improve lung function and reduce exacerbation risk compared with several dual ICS/LABA combinations2-5 in patients with symptomatic COPD and a history of exacerbations.
Triple therapy can be delivered as individual treatments in separate inhalers (such as dry-powder inhalers [DPIs] and metered dose inhalers [MDIs]), which generally require multiple different steps for correct use.6 High error frequencies with both DPIs and MDIs may occur if patients receive minimal training in inhaler technique from health care professionals (HCPs), and could thus, potentially be reduced with training or new drug delivery approaches.7
Single-inhaler triple therapy (SITT) enables the administration of fixed-dose ICS/LAMA/LABA combinations by a single inhaler. The use of a single inhaler is considered an option for simplifying treatment in patients with COPD versus multiple inhalers,8,9 which may reduce overall error frequency and positively influence patient adherence and treatment outcomes.10,11,12
Use of the ELLIPTA DPI in delivering licensed fixed-dose combinations, including SITT with fluticasone furoate/umeclidinium/vilanterol, has been shown to reduce inhaler error frequency compared with commonly used MDI combinations.9 Patient preference for ELLIPTA versus common MDIs and their combinations, related to factors such as a reduced time needed for instruction and ease of use of the inhaler, has also been reported.9,13,14
Our study evaluated the ability of patients with COPD to demonstrate correct use (0 errors) of ELLIPTA compared with the DPI combination of DISKUS plus HandiHaler after 28 days of use. This study was conducted in a formal, clinical trial setting in which participants were instructed by an HCP on the correct use of each inhaler.
Methods
Study Design and Eligibility Criteria
This was a Phase 4, randomized, multicenter, open-label, crossover, placebo study (NCT03227445) conducted at 17 study centers in the United States over 56 days per participant from September 20, 2017 to January 4, 2018.
Participants were at least 40 years of age, with a smoking history of > 10 pack years and a diagnosis and documented COPD history of ≥ 12 months in accordance with American Thoracic Society/European Respiratory Society criteria.15 Details of further inclusion criteria are provided in the online data supplement. Key exclusion criteria included: a diagnosis of asthma; > 1 COPD exacerbation requiring hospitalization in the 12 months prior to randomization; use of the ELLIPTA, DISKUS, or HandiHaler DPI in the 12 months prior to randomization; receipt of current COPD maintenance treatment by any of the study inhalers; and receipt of a short-acting beta2-agonist (SABA) only for COPD maintenance.
This study comprised a total of 4 visits. Screening (Visit 0) and randomization (Visit 1) could both take place on Day 1. At randomization, participants were asked to read the instructions for use (IFU) section of the approved prescribing information for the inhaler(s) they were assigned (ELLIPTA or DISKUS plus HandiHaler DPIs) for the first treatment period. All inhalers contained placebo (lactose monohydrate for DISKUS and HandiHaler DPIs, and one strip each of lactose monohydrate and lactose monohydrate blended with magnesium stearate for ELLIPTA DPI); no active treatment was prescribed, and participants continued to use their own COPD therapy and rescue medications throughout the study. Participants then demonstrated use of the assigned inhaler(s) to an HCP. In the case of inhaler errors, and to optimize inhaler technique, the HCP gave the participant verbal advice to ensure correct technique was understood before leaving the clinic. The participant took the inhaler(s) and IFU home for 28 days. All inhalers were used daily according to the dosing regimen in the IFU: ELLIPTA (1 inhalation once daily); DISKUS (1 inhalation twice daily); and HandiHaler (2 inhalations once daily).
On returning to the clinic at Visit 2 (Day 28), participants were assessed for correct use of their first assigned inhaler(s) and were then given their next assigned inhaler(s) and IFU for Period 2. Participants who received ELLIPTA in Period 1 received DISKUS plus HandiHaler in Period 2, and participants who received DISKUS plus HandiHaler in Period 1 received ELLIPTA in Period 2. Demonstration of correct inhaler use was again required, and HCPs advised if the participant made any errors before taking the appropriate inhaler(s) and IFU home.
Participants returned on Visit 3 (Day 56) for correct use of the second assigned inhaler to be assessed by the HCP, and to answer a preference questionnaire regarding the inhaler(s) they had been assigned throughout the study. Adherence was assessed at Visit 2 and Visit 3 based on the dose counter (ELLIPTA and DISKUS) or remaining capsules (HandiHaler). All HCPs assessing correct inhaler use in this study were trained in the correct use of all 3 inhalers by a licensed respiratory therapist.
The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, 16 the Declaration of Helsinki 2008,17 and applicable local country requirements (the U.S. 21 Code of Federal Regulations 321.3(b) for constitution of independent ethics committees). All participants provided written, informed consent before participation. The ethics and review boards of participating institutions approved the protocol before commencement of the study.
Outcomes and Assessments
The primary endpoint was the proportion of participants demonstrating correct use after a period of 28 days, for ELLIPTA versus DISKUS plus HandiHaler. Correct use (making 0 errors), was evaluated against a protocol-defined checklist of critical and non-critical errors for each inhaler. Further information on inhaler checklists is provided in the online data supplement. Critical errors were defined as errors leading to no or significantly reduced medication being inhaled.
Secondary endpoints, all measured after 28 days of use, were: number of errors by type for each inhaler; number and change in number of errors per participant for each treatment group; number and change in number of errors for each treatment group in participants with at least 1 error; and comparison of percentage of participants with 0 critical errors. Exploratory endpoints and their results are discussed in the online data supplement, excluding patient preference, which is included in the main text.
Inhaler preference based on ease of use, dosing regimen, and number of steps required to take the medication were recorded via patient-preference questionnaires. Two questionnaires were available. Each questionnaire included the same questions, but with responses in an alternative order to avoid response bias. Participants were randomized 1:1 to a questionnaire and inhaler sequence at Visit 1 using an interactive web response system.
Although this was a placebo study with no active medications, safety (recording of adverse events [AEs] and serious AEs [SAEs]) was also monitored.
Statistical Analyses
Full details of the statistical analysis methods used in this study are provided in the online data supplement. Briefly, the error rates observed in a previous study by van der Palen et al(2016)14 including ELLIPTA, DISKUS, and HandiHaler were used to inform sample size calculations in the present study, which used conditional logistic regression and a 2-sided 5% significance level. The error rates for ELLIPTA observed by van der Palen et al(2016) were used as a basis for error rates in our study (Table S1, online data supplement).
Efficacy analyses were performed using the intent-to-treat (ITT) population (all randomized participants excluding those randomized in error). Participants were analyzed in accordance with their randomized treatment sequence. The safety population was the same as the ITT population.
The primary hypothetical estimand estimated the treatment effect in those from the ITT population who completed assessment of both inhaler regimens without intercurrent events (i.e., events occurring after treatment initiation that either preclude observation of an endpoint or affect its interpretation, and which occurred post randomization). Possible intercurrent events were study withdrawal; a change in regular maintenance treatment, or failure to bring an inhaler to a study visit. Statistical analyses were conducted for all endpoints under the primary estimand, excluding the following secondary endpoints for which summary statistics were instead provided: the number of errors by type for each inhaler, the number and change in number of errors per participant for each treatment group, and the number and change in number of errors for each treatment group in participants with at least 1 error, all after 28 days of use. Patient preference was analyzed using the Cochran–Mantel–Haenszel (CMH) test.
Statistical analysis of the primary endpoint used exact conditional regression, and sensitivity analysis of the primary endpoint was performed on discordant cases (participants with correct use of 1 inhaler regimen and errors with the other) using the CMH test. The same analytical approach was also used for the comparison of the percentage of participants with 0 critical errors after 28 days of use (secondary endpoint), and for the percentage of participants with 0 errors after 28 days of use, including the number of prescribed maintenance inhalers as a covariate, and the percentage of participants making 0 errors after reading the IFU (exploratory endpoints).
Results
Baseline Characteristics
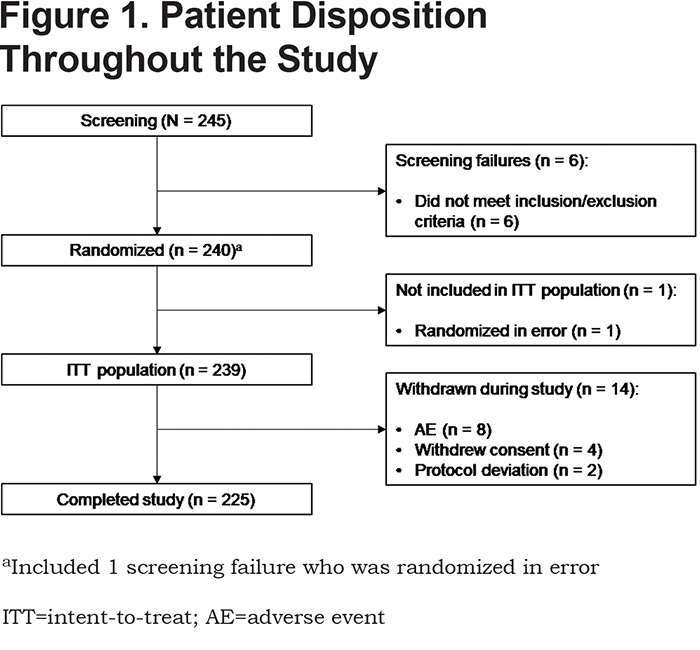
Of the 245 participants screened for this study, 240 were subsequently randomized, 239 comprised the ITT and safety populations, and 225 completed the study (Figure 1). A total of 14 participants were withdrawn during the study due to AEs (n =8), withdrawal of consent (n = 4), or protocol deviation (n = 2).
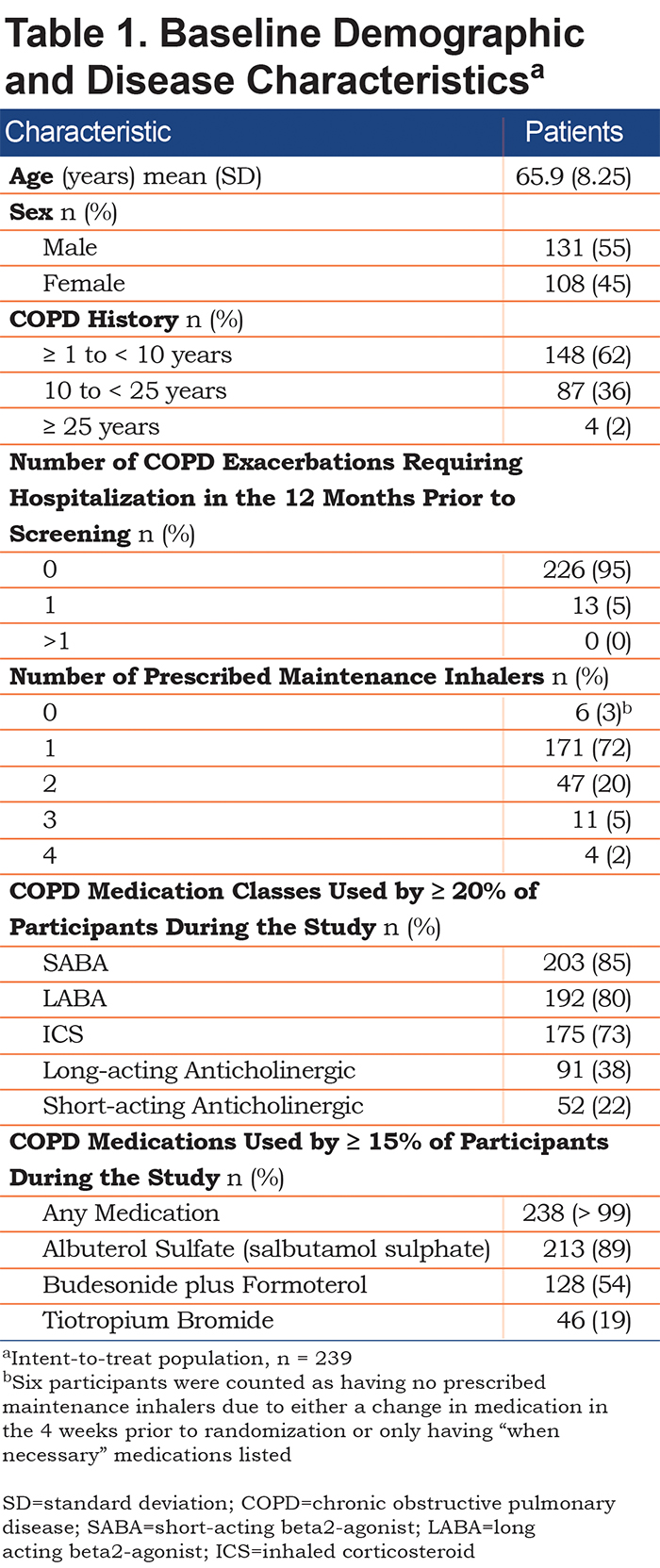
Baseline demographic and disease characteristics for the ITT population are shown in Table 1; mean age was 65.9 years (standard deviation [SD] 8.25) and 55% (n = 131) were male. A COPD history of ≥ 10 years was apparent in 38% (n = 91) of participants. Most participants (n = 171) were prescribed 1 maintenance inhaler (72%). The most common COPD medication class used during the study was a SABA (85%); approximately one-half of participants received a fixed-dose ICS/LABA (budesonide/formoterol) combination.
Intercurrent Events
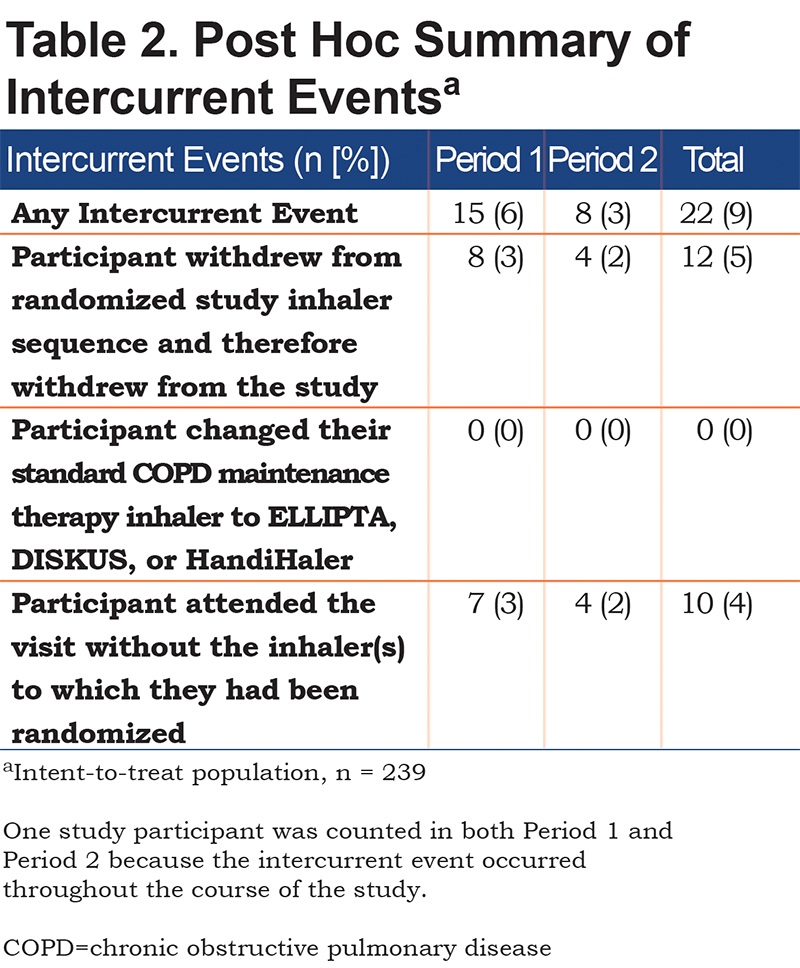
A total of 22 participants (9%) had intercurrent events during the treatment period, consisting of early withdrawal participants (n = 12) and those who did not bring their inhaler to visits (n =10) (Table 2). The primary estimand therefore estimated outcomes in the 217 participants who completed assessment with both inhaler regimens and no intercurrent events.
Correct Inhaler Use After 28 Days (Primary Endpoint)
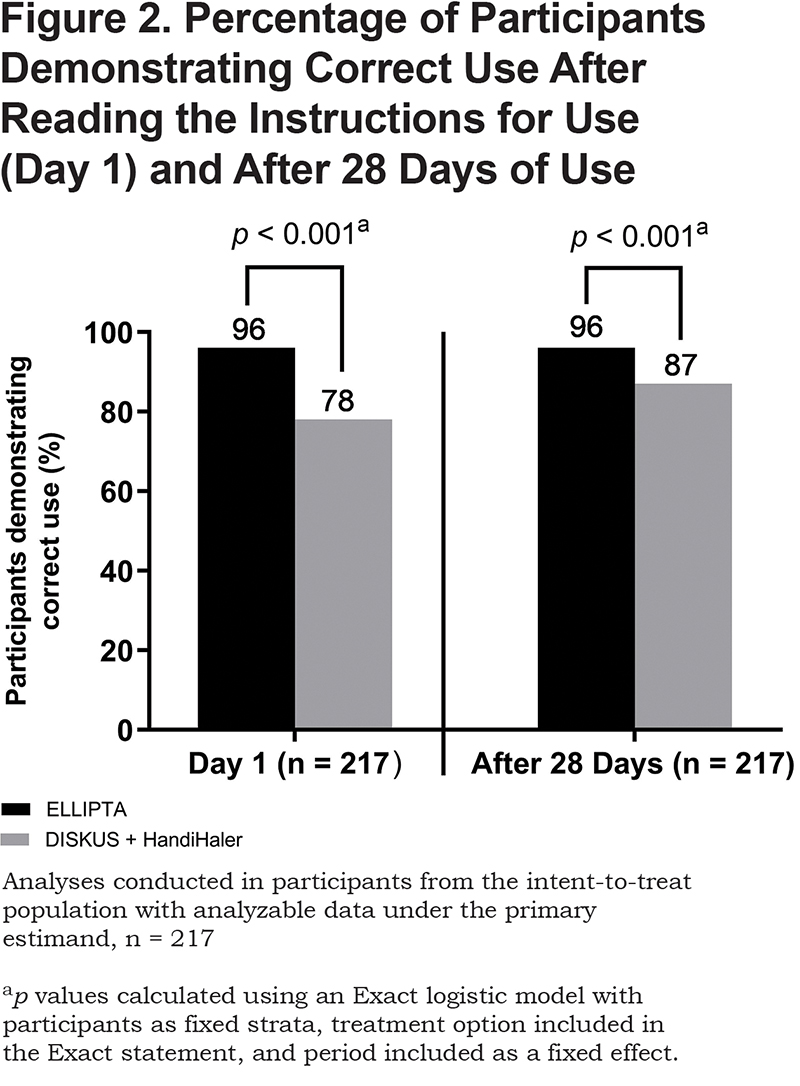
After 28 days of use, a greater proportion of participants demonstrated correct use of ELLIPTA compared with DISKUS plus HandiHaler under the primary estimand (96% versus 87% of n =217, respectively; Figure 2). The odds of demonstrating correct use of ELLIPTA were 6.88 times (95% confidence interval [CI]: 1.97–54.28) higher than the odds of demonstrating correct use of DISKUS plus HandiHaler (p < 0.001). For the few discordant cases observed (n =26), 23 (88%) were cases where participants made at least 1 error with DISKUS plus HandiHaler and 0 errors with ELLIPTA, while 3 (12%) participants made at least 1 error with ELLIPTA but none with DISKUS plus HandiHaler.
Summary of the Number of Errors for Each Inhaler
The correct use checklist for each inhaler is shown in Table 3, alongside the number of participants who made errors on each checklist point for ELLIPTA, DISKUS, and HandiHaler separately. The number of participants demonstrating errors was 10 (4%) for ELLIPTA, 26 (11%) for DISKUS, and 37 (15%) for HandiHaler in the ITT population (n =239) at Day 1. The total number of errors with each inhaler was 14, 31, and 130, respectively. This trend was similar after 28 days of use (primary hypothetical estimand; n = 217); 9 participants (4%) made errors with ELLIPTA, compared with 14 (6%) with DISKUS and 27 (12%) with HandiHaler (Table 3). The most common error with each inhaler was failing to show “participant exhales while holding the inhaler away from their mouth” (6/9 participants; 67%) for ELLIPTA, “participant exhaled away from the mouthpiece of the inhaler” (9/14 participants; 64%) for DISKUS and “participant heard or felt the capsule vibrate/rattle” (14/27 participants; 52%) for HandiHaler.
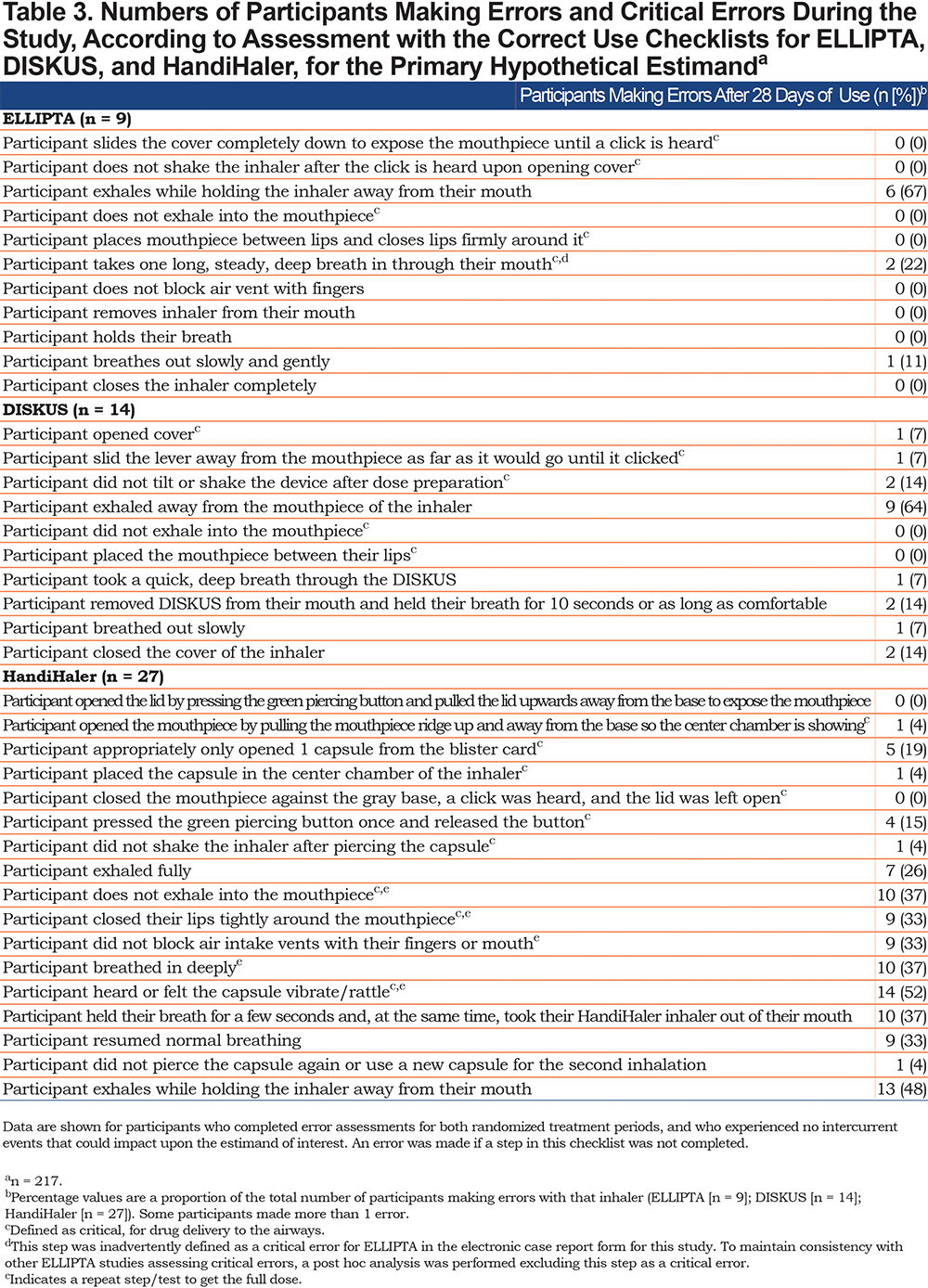
Number and Change in Number of Errors per Participant in Participants with at Least 1 Error
Among participants who made at least 1 error with ELLIPTA on Day 28, the mean number of errors per participant increased from 0.3 on Day 1 to 1.0 on Day 28.For participants using DISKUS plus HandiHaler who made at least 1 error on Day 28, the mean number of errors per participant increased from 1.9 on Day 1 to 4.4 on Day 28.
Proportion of Participants Demonstrating 0 Critical Errors after 28 Days of Use
After 28 days of use, > 99% of participants made 0 critical errors with the ELLIPTA inhaler versus 89% of participants with DISKUS plus HandiHaler. The odds of making 0 critical errors with ELLIPTA were 8.85 (95% CI: 3.45–∞) times higher than the odds of making 0 critical errors with DISKUS plus HandiHaler (p < 0.001). Among discordant cases (n =24, 11%), 23 participants (96%) made 0 critical errors with ELLIPTA and at least 1 critical error with DISKUS plus HandiHaler.
Failure to perform the correct use component, “participant takes one long, steady, deep breath in through their mouth,” with ELLIPTA was initially categorized in the protocol as a critical error, but was later re-categorized as an overall error to maintain consistency with previous studies. Subsequent post hoc analysis of the proportion of participants demonstrating 0 critical errors after 28 days of use showed that 100% of participants demonstrated correct use of ELLIPTA, compared with 89% (n =193) with DISKUS plus HandiHaler.
Patient Preference
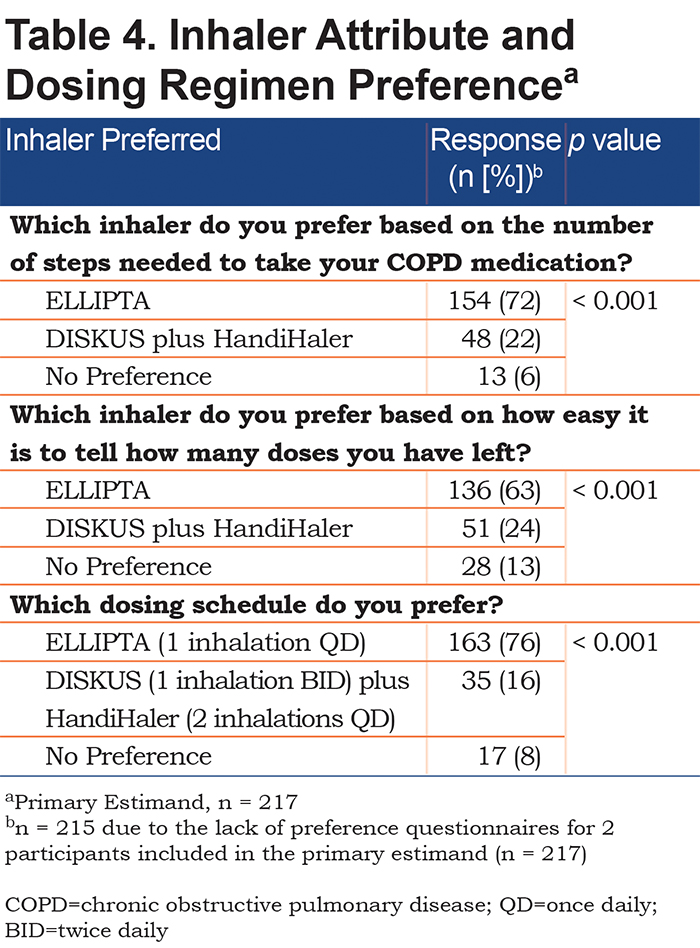
Patient preference was evaluated via questionnaire in 215 patients; 2 patients were not included under the primary estimand due to study withdrawal. More patients preferred ELLIPTA to DISKUS plus HandiHaler in terms of number of steps required to take medications (72% versus 22%, respectively), ease of telling the number of remaining doses (63% vs. 24%, respectively), and dosing schedule (76% versus 16%, respectively) (Table 4). Small numbers of patients reported no preference between the 2 inhalers in terms of number of steps required to take medication (6%), number of doses left (13%), and dosing schedule (8%).
Safety
A total of 59 participants (25%) experienced an AE during or after treatment. On-treatment AEs were reported for 57 participants (24%), 5 (2%) of which led to withdrawal. Per preferred term, the on-treatment AEs reported in ≥ 2% of participants were pneumonia (5 participants, 2%), cough, headache, nasopharyngitis, and urinary tract infection (4 participants, 2% each). A total of 4 (2%) on-treatment AEs were considered to be drug-related which, in this case, referred to maintenance therapy since placebo inhalers containing no active treatment were used.
SAEs were reported for 10 participants (4%). Five participants (2%) experienced a COPD exacerbation that was recorded as an SAE. All other SAEs occurred in < 1% of participants. No SAEs were fatal or considered drug related. There were no deaths during the study.
A total of 26 participants (11%) experienced 30 on-treatment moderate or severe COPD exacerbations; 4 participants experienced 2 exacerbations each. Of the 30 moderate or severe exacerbations, 27 (90%) were treated with oral/systemic corticosteroids, 23 (77%) were treated with antibiotics, and 4 (13%) led to hospitalization. Most exacerbations (93%) were resolved by the end of the study.
Discussion
Correct inhaler use is essential to maximize the benefits of COPD maintenance therapy by ensuring effective drug delivery throughout the lungs.18 Nevertheless, inhaler errors remain common among patients. A recent meta-analysis suggested that the overall error frequency (95% CI) is as high as 86.8% (79.4–91.1) with MDIs and 60.9% (39.4–79.0) with DPIs.19 Several factors, including lack of education regarding inhaled medications and the correct use of inhalers used to deliver them, could contribute to these high error rates. 6,18
In this study of patients with COPD, conducted in an optimized technique setting where HCPs advised participants on correct inhaler use during each study period if required, a significantly greater proportion of participants demonstrated correct use of ELLIPTA compared with DISKUS plus HandiHaler, both on Day 1 after reading the IFU (96% versus 78%; exploratory endpoint, see online data supplement) and after 28 days of use (96% versus 87%).
A previous study of inhaler preference by van der Palen et al (2018),9 conducted throughout European Union countries in a single visit, reported that after reading the IFU and receiving guidance from an HCP if required, significantly fewer patients with COPD made critical errors with the ELLIPTA inhaler compared with 2 DPI combinations (DISKUS plus HandiHaler and Turbuhaler plus HandiHaler; p < 0.001 for both comparisons). Another single-visit study by van der Palen et al (2016)14 also demonstrated an increased rate of correct use of the ELLIPTA inhaler versus 5 commonly used inhalers (DISKUS, MDI, Turbuhaler, HandiHaler, and Breezhaler) when used alone. This suggested that the differences in inhaler use were not simply due to the additional instruction required to use 2 inhalers rather than 1, but rather the design of the inhaler. Consistent with these findings, our study demonstrated correct inhaler use over an extended study period (28 days) among patients in the United States after initial instruction from an HCP. Our results add to the available evidence that SITT via the ELLIPTA dry-powder inhaler can help reduce error frequency among patients with COPD when compared to multiple-inhaler triple therapy. Improved rates of correct use with ELLIPTA have previously been attributed to its intuitive design, with relatively few steps required to administer medications,14 which could help to improve treatment adherence and, ultimately, effectiveness in patients with COPD who use the device.
The ease of use of ELLIPTA is reiterated in the outcomes of the current study regarding the proportion of participants demonstrating correct use after reading the IFU on Day 1 (exploratory endpoint; see information in online data supplement). In total, 96% of participants made 0 errors with ELLIPTA after reading the IFU compared with 78% with DISKUS plus HandiHaler, and only 2 of the 42 discordant cases on this endpoint comprised errors with ELLIPTA but not DISKUS plus HandiHaler. This result demonstrates that, even without HCP training, almost all patients would be likely to receive their expected ICS/LAMA/LABA dosage via ELLIPTA after reading the IFU.
The observation of high correct use rates after reading the IFU, when considered alongside the fact that HCP training on inhaler technique may sometimes be suboptimal,20 highlights that use of the ELLIPTA inhaler may contribute to an overall improvement in COPD medication delivery versus DISKUS plus HandiHaler outside the trial environment. At present, specialist HCP knowledge and time-related factors, from both patient and HCP perspectives, act as barriers to the receipt of specialized inhaler training.21 If an easy-to-use inhaler such as ELLIPTA is made available, it could increase the receipt of inhaler training in clinics as it would reduce the associated time investment compared with a more complex device.
The higher rate of correct use observed with ELLIPTA versus DISKUS plus HandiHaler is important not just in terms of receiving COPD medications, but also because perceived mastery of inhaler technique is an important factor in therapeutic adherence,18 alongside patient preference regarding factors such as ease of use.22 In the current study, patient preference for the ELLIPTA inhaler versus DISKUS plus HandiHaler was demonstrated with regards to the number of steps required to take the medication, the ease of telling how many doses remain, and the dosing regimen itself. Improved patient adherence may also contribute to a reduction in COPD exacerbation rates, an association that has been demonstrated previously.23
A strength of this study was the crossover design, which allowed participants to act as their own control regarding their performance on, and preference for, each inhaler regimen. In addition, enrolled participants had not used the inhalers that they were allocated in the prior 12 months. The inhalers used in the study contained placebo, allowing for a focus on inhaler attributes and effectively removing the possibility of bias due to treatment effects. Furthermore, the study was conducted over a 28-day period for each inhaler regimen, so the retention of inhaler knowledge over a time period aligned with the number of doses provided in an inhaler prior to refill was assessed. The inclusion of participant training by HCPs on inhaler use in our protocol allowed observation of the potential effects of optimized inhaler training in a clinical trial setting, as was also the case in the recent van der Palen et al (2018) study.9
Perhaps the most notable limitation of this study was the unavoidable element of subjectivity involved in the assessment of inhaler errors, despite the implementation of standardized checklists. Moreover, the participants enrolled in such trials may be considered highly motivated and more able to follow instructions than is the case in the broader COPD population; as such, it is likely that the differences identified in this study are an underestimation of the true incidence in routine clinical practice. The optimization of inhaler technique through the receipt of training from HCPs in the clinical setting of this study may have further reduced the applicability of the results to true clinical practice as such training is often not provided in clinics. Further potential limitations include the difficulty of enrolling truly inhaler-naïve patients; the non-assessment of participant health literacy, which would have a clear impact on participant understanding of the IFU; and inability to capture long-term inhaler technique in a 28-day study.
Conclusions
In this study, a significantly higher number of participants demonstrated correct use of the ELLIPTA DPI compared with DISKUS plus HandiHaler after 28 days of use. The ease of use of ELLIPTA is reflected not only in this outcome, but also in the observation of lower overall numbers of errors with ELLIPTA versus the other tested device combination, and also in the minimal number of patients demonstrating errors after reading the IFU alone.
Acknowledgments
KC and MZ were involved in the literature search. KC, MZ, and RS were involved in study design. EMK and SS were involved in data collection. VM performed the data analysis, and VM, RJ, KC, MZ, RS, and EMK were involved in data interpretation. All authors participated in the writing and review of the manuscript and approved it for publication.
Declaration of Interest
EMK has participated in consulting, advisory boards, speaker panels, or received travel reimbursement from Amphastar, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline plc., Mylan, Novartis, Oriel, Pearl, Sunovion, Teva, and Theravance. He has conducted multicenter clinical research trials for approximately 40 pharmaceutical companies. SS has been a principal investigator for 20 years and has conducted clinical research trials for a large number of pharmaceutical companies. He has also participated as a consultant and on advisory boards and speaker panels for pharmaceutical companies including AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline plc., Mylan, Menlo, Novartis, Pearl, Sunovion, Teva, and Theravance. MZ, VM, RJ, KC, and RS are employees of, and own shares in, GlaxoSmithKline plc. Editorial support (in the form of writing assistance, collating author comments, assembling tables and/or figures, grammatical editing, fact checking, and referencing) was provided by Matthew Hallam, MSc(Res) and Joanna Wilson, PhD, of Gardine—Caldwell Communications (Macclesfield, UK) and was funded by GlaxoSmithKline plc. Trademarks are the property of their respective owners.