Running Head: Author Response
Abbreviations: chronic obstructive pulmonary disease, COPD; preserved ratio-impaired spirometry, PRISm; Global initiative for chronic Obstructive Lung Disease, GOLD; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; interquartile range, IQR; COPDGene Phase 1, P1; COPDGene Phase 2, five-year follow up, P2
Citation: Lowe KE, Hokanson JE, Make BJ, et al. Letter to editor: response by authors. Chronic Obstr Pulm Dis. 2020; 7(2): 82-85. doi: http://doi.org/10.15326/jcopdf.7.2.2020.0152
Response:
The authors thank Drs. Miller and Brown for their insight and critical questions.1 The primary goal of our article, “COPDGene 2019: Redefining the Diagnosis of Chronic Obstructive Pulmonary Disease,”2 was to propose a critical next step in expanding the diagnosis of chronic obstructive pulmonary disease (COPD) to include participants with early expression of the disease and participants whose pathway of disease progression follows a different pattern through preserved ratio-impaired spirometry (PRISm) physiology rather than through classically defined airflow obstruction of the Global initiative for chronic Obstructive Lung Disease (GOLD) stages 1, 2, 3 and 4.3 This was accomplished by considering symptoms and computed tomography structural changes as inherent components of the diagnosis of COPD. The exciting finding was that we identified many participants who had been excluded by classic spirometric criteria and who yet had a risk of disease progression or death. This included both early disease (GOLD 0) and PRISm participants, some of whom had a significant loss of forced expiratory volume in 1 second (FEV1) at baseline. PRISm participants were shown in a companion paper4 to progress to classic GOLD stages 2, 3, and 4 disease.5 We recognize that there are important benefits of smoking cessation. Current and former smoking was a covariate controlled for in the models underlying the existing manuscript. Additional analyses are ongoing to identify benefits of smoking cessation on COPD progression within the clinical categories described in this article. This will be the subject of a future publication.
Miller and Brown point out that there was no evidence of increased odds of progression (defined as > 350 ml loss in FEV1) for those with only abnormal spirometry or those with both abnormal spirometry and symptoms. This is not an artifact of classification, but rather an extremely important component of our recommendation for a change in the diagnostic criteria for COPD. The assessment of COPD progression using only spirometry is flawed and does not adequately identify risk of progression in a large fraction of patients. Categories D and F include participants with a diverse pathogenesis. Greater than 50% of the participants in both categories D and F have a substantially lower FEV1 at baseline while sustaining a roughly equivalent loss of forced vital capacity (FVC) and thus exhibit PRISm physiology. These participants are not identified as having COPD by current GOLD criteria.3 A smaller fraction of participants in Categories D and F have abnormal physiology that would place them in a more traditional pathway of GOLD 1, 2 and 3. The characteristics of participants in Categories D and F are shown in Table 1 and the spirometric distribution of participants in Categories D and F are shown in Figures 1a and 1b.
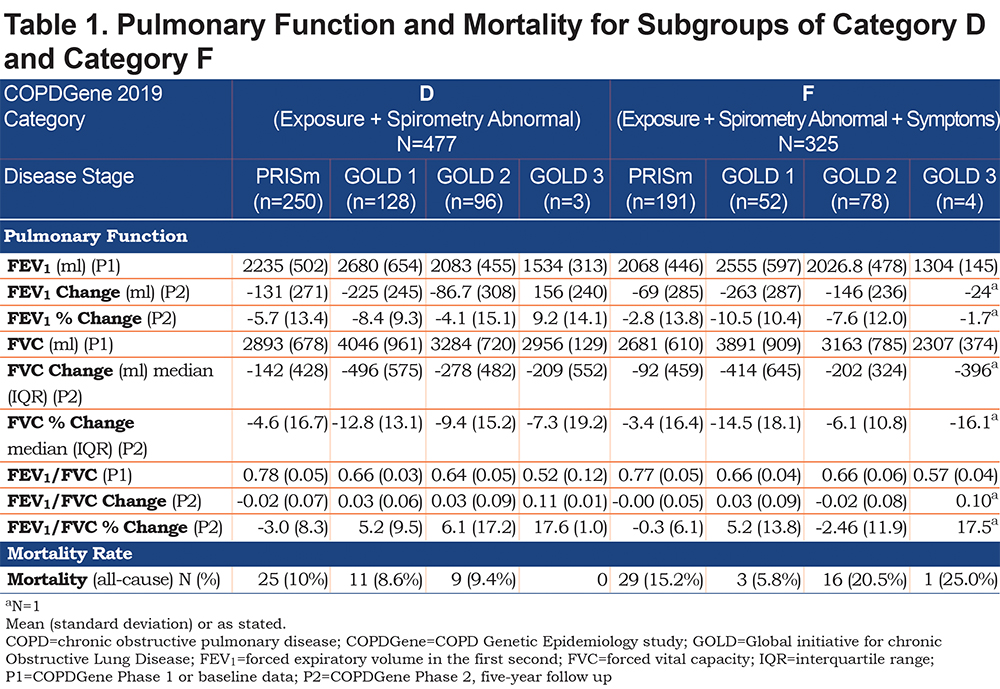
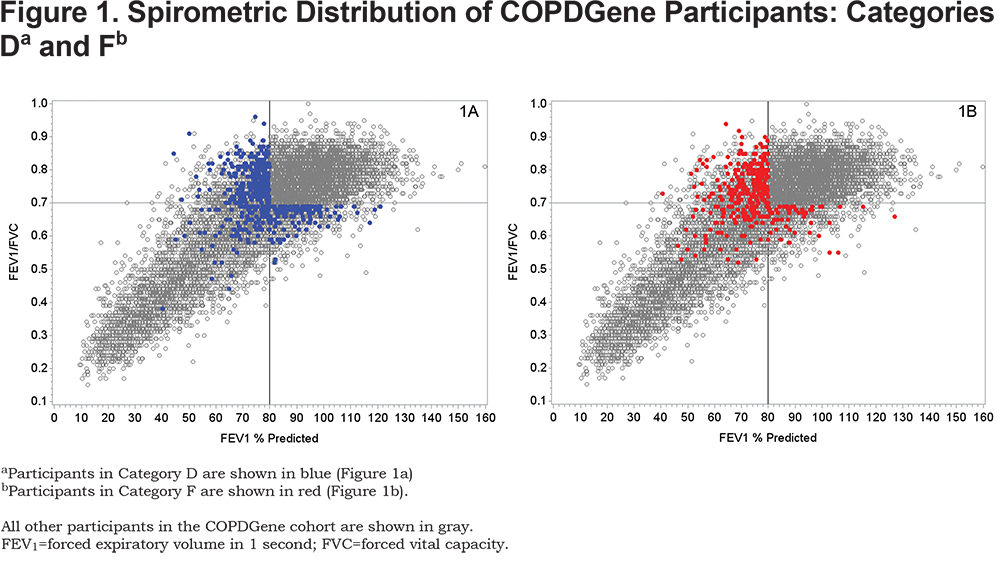
Note that participants in Category D who had PRISm physiology had low FEV1 values compared to those with GOLD 1 physiology (2235 ml versus 2680 ml) and had markedly lower FVC values (2893 versus 4046 ml). The pattern of disease progression is quite different for PRISm individuals who show concurrent loss of both FEV1 and FVC, thus preserving a normal FEV1/FVC ratio. When COPD symptoms are added to spirometric abnormality (Category F) the baseline FEV1 and FVC are even lower. Many of these participants were found to have lost 40%-50% or more of their predicted FEV1 at baseline. These individuals should not be expected to show COPD progression by further large losses in FEV1. Figures 1a and 1b show that few COPDGene participants were observed with an FEV1% predicted < 40% and an FEV1/FVC > 0.7, delineating for heavy smokers a potential physiological limit to the amount of FEV1 that can be lost before there is a sufficiently low FEV1/FVC to move the participant into GOLD stages 3 or 4.
Failure to achieve a statistically significant increased odds of FEV1 loss > 350 ml does not mean that participants in categories D and F did not experience significant disease progression over the 5-year observational period. The hazard ratio for all-cause mortality was significantly elevated for both Category D and Category F. When symptoms of COPD are added to spirometry (Category F), there is an extraordinary increase in mortality. The finding of positive symptoms of COPD consistently increased the hazard ratio for all-cause mortality as noted for Categories C, E, F and H. We acknowledge that all-cause mortality is an imperfect metric for COPD progression.
Evidence of COPD progression must be assessed by parameters other than loss of FEV1. This is particularly true for individuals with PRISm physiology where substantial loss of FEV1 is present at baseline. Participants with PRISm physiology had the highest all-cause mortality in this subanalysis of Categories D and F (Table 1). While there are not enough participants in the subdivisions of Categories D and F to achieve statistical significance, the finding is consistent with prior analysis of individuals at risk for COPD who develop PRISm physiology.5 Young et al4 reported that patients with PRISm physiology can progress directly to advanced COPD (GOLD stages 2–4) and carry an extremely high mortality risk.6
1. Miller A, Brown LK. Comments on “COPDGene 2019: redefining the diagnosis of chronic obstructive pulmonary disease.” Chronic Obstr Pulm Dis. 2020;7(2):79-81. doi: https://doi.org/10.15326/jcopdf.7.2.2020.0134
2. Lowe KE, Regan EA, Anzueto A, et al. COPDGene 2019: redefining the diagnosis of chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2019;6(5):384-399. doi: http://doi.org/10.15326/jcopdf.6.5.2019.0149
3. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557-582. doi: https://doi.org/10.1164/rccm.201701-0218pp
4. Young KA, Strand MJ, Ragland MF, et al for the COPDGene® Investigators. Pulmonary subtypes exhibit differential Global Initiative for Chronic Obstructive Lung Disease spirometry stage progression: the COPDGene study. Chronic Obstr Pulm Dis. 2019;6(5):414-429. doi: https://doi.org/10.15326/jcopdf.6.5.2019.0155
5. Kinney GL, Santorico SA, Young KA, et al. Identification of chronic obstructive pulmonary disease axes that predict allcause mortality: the COPDGene study. Am J Epidemiol. 2018; 187(10):2109-2116. doi: https://doi.org/10.1093/aje/kwy087
6. Young KA, Regan EA, Han MK, et al and the COPDGene Investigators. Subtypes of COPD have unique distributions and differential risk of mortality. Chronic Obstr Pulm Dis. 2019;6(5):400-413. doi: https://doi.org/10.15326/jcopdf.6.5.2019.0150