Running Head: Liver Disease Assessment and Management in Alpha-1
Funding Support: This work was supported by the EASL registry grant on alpha-1 antitrypsin-related liver disease, the Deutsche Forschungsgemeinschaft (DFG) consortium SFB/TRR57 “Liver fibrosis”, the Interdisciplinary Center for Clinical Research (IZKF) within the medical faculty at RWTH Aachen University, the Else Kroener Excellence Fellowship, (all to P.S.) as well as the START program within the medical faculty at RWTH Aachen University, the German Liver Foundation, and the German Gastroenterological Association (all to K.H.).
Date of Acceptance: February 13, 2020
Abbreviations: alpha-1 antitrypsin deficiency, AATD; liver stiffness measurement, LSM; vibration controlled transient elastography, VCTE; AST-to-platelet ratio, APRI; fibrosis-4, FIB4; enhanced liver fibrosis, ELF; non-alcoholic fatty liver disease, NAFLD; body mass index, BMI; acoustic radiation force impulse imaging, ARFI; 2D-shear wave elastography, 2D-SWE; controlled attenuation parameter, CAP; magnetic-resonance-based elastography, MRE; proton density fat fraction, PDFF; area under the receiver operating curve, AUROC; gamma-glutamyl transferase, GGT; hepatocellular carcinoma, HCC; alcohol fatty liver disease, ALD
Citation: Hamesch K, Strnad P. Non-invasive assessment and management of liver involvement in adults with alpha-1 antitrypsin deficiency. Chronic Obstr Pulm Dis. 2020; 7(3): 260-271. doi: http://doi.org/10.15326/jcopdf.7.3.2019.0161
What is Liver Fibrosis and What Are Its Consequences?
Chronic liver disease of any etiology may result in liver scarring (liver fibrosis) as the uniform response to ongoing liver damage. Cirrhosis is the end-stage of such a process.1 Mechanistically, the persistent hepatic insult leads to loss of hepatocytes and release of proinflammatory mediators that result in chronic inflammation and activation of hepatic stellate cells.2 The latter are transformed into myofibroblasts and represent major producers of extracellular matrix.2 Importantly, the presence of liver fibrosis, but not other histological features such as liver steatosis (fatty liver) or chronic inflammation, is associated with increased hepatic morbidity and mortality.3,4 Because of that, a proper assessment of liver fibrosis is essential to understand the severity of liver disease as well as the prognosis in order to guide further clinical management.5
Patients with chronic hepatitis or early liver fibrosis are usually asymptomatic or present with unspecific symptoms, hence, they typically remain undiagnosed for many years. This is facilitated by the fact, that detailed medical history and physical examination are often not sufficient for detecting advanced liver fibrosis.6 Indeed, patients with fibrosis or even cirrhosis may present with normal serum liver enzymes.6 While patients with early stages of so-called compensated cirrhosis might still be asymptomatic, their decompensation is of great clinical relevance since it may lead to multiple life-threatening sequelae. Notably, the diagnosis of compensated cirrhosis results in a 4.7x increased risk of death compared to the general population while decompensated cirrhosis is associated with a nearly 10x higher risk.7 The average life expectancy of patients with compensated cirrhosis is 10 to 13 years, but drops to 2 years when a decompensation occurs.8 As a consequence, cirrhosis is the 13th leading cause of death globally.1 Moreover, hepatocellular carcinoma, which almost exclusively occurs in the cirrhotic liver, is the 4th leading cause of cancer-related death and its incidence continues to grow.9
The main complications seen in individuals with decompensated cirrhosis are typically related to an elevated blood pressure in the portal venous system.1,10 This can result in ascites, esophageal varices, hepatorenal syndrome, and/or hepatic encephalopathy. While ascites may be refractory and greatly restrict life quality, spontaneous bacterial peritonitis may be fatal due to progression to multi-organ failure if not treated early. Esophageal varices may result in severe bleeding and hepatorenal syndrome in persistent renal failure, both reducing life expectancy. Finally, hepatic encephalopathy may be highly debilitating. Additional organs such as the lungs (portopulmonary hypertension or hepatopulmonary syndrome) or the heart (cirrhotic cardiomyopathy) can also become affected due to cirrhosis and the resulting alterations.
The presented diagnostic challenges as well as the deadly consequences of cirrhosis, especially if diagnosed at the late stage when a patient becomes symptomatic, highlight the importance of detecting liver fibrosis as early as possible to prevent further progression and/or to discuss the possibility of liver transplantation. This is particularly true in conditions known to associate with liver involvement such as alpha-1 antitrypsin deficiency.
How to Assess Liver Fibrosis?
For decades, liver biopsy was the key diagnostic procedure in hepatology and was used to evaluate the cause and stage of liver disease. Consequently, it was performed to facilitate the treatment decisions and to determine the prognosis.5 While biopsy is still considered the gold standard, the clinical approach to patients with chronic liver disease has undergone a transformation with an increased use of non-invasive tools to assess the extent of liver fibrosis.5 Notwithstanding its importance to assess the nature of a distinct liver disease,11 liver biopsy has several disadvantages that limit its use as a first-line tool to assess the stage of fibrosis. A major concern is its invasiveness with complications such as pain, bleeding, injury to the bile ducts, infection, or rarely, even death.5 Other important issues are the so-called sampling bias arising from the fact that the disease is often not uniformly distributed throughout the liver12-14 and an observer bias caused by the subjective assessment of pathologists regarding both severity and etiology of disease.12 Additionally, sampling size is not well standardized and may greatly affect the pathologist’s interpretation.15 Therefore, a single biopsy might have limited predictive value14 and the necessity to perform serial biopsies to confirm the etiology and to monitor the course of disease further potentiates the risk of complications. Because of all of these issues, liver biopsy constitutes a significant burden for the health care system (i.e., the need of an expert gastroenterologist [or radiologist] and pathologist as well as the required periprocedural monitoring) and the patient (i.e., inability to work for a significant amount time).
In the past 3 decades, several non-invasive tools for assessing liver fibrosis have been developed and validated for use in the clinical routine. While these methods typically cannot accurately differentiate the stages of fibrosis, they are useful in ruling-out the presence of clinically significant liver fibrosis (i.e., fibrosis stage of 2 or higher on a 0–4 scale) and in ruling-in the presence of advanced liver fibrosis (i.e., fibrosis stage of 3 or 4).16 From the less to the more technically complicated alternatives, these non-invasive fibrosis tests include: blood-based tests, ultrasound-based elastography, and magnetic-resonance-based elastography (Table 1).5,17
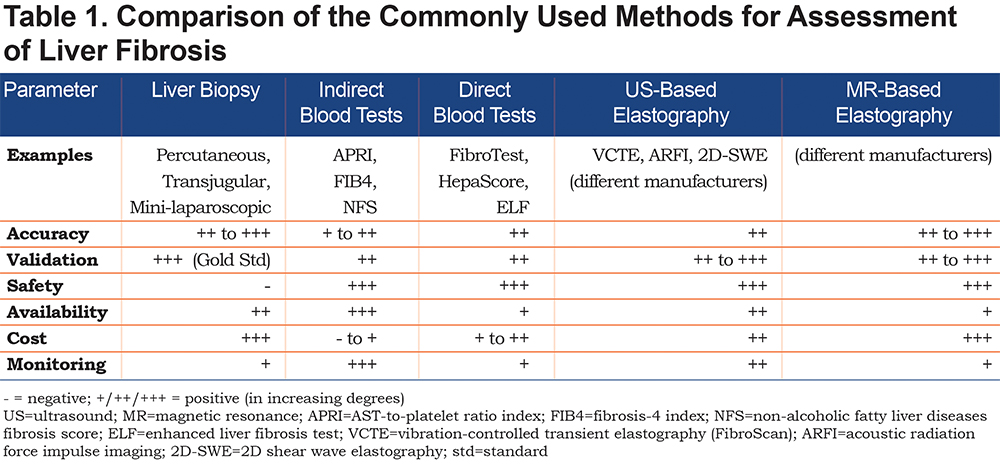
Blood tests include the ubiquitously available indirect tests, that are based on routine laboratory parameters (e.g., AST-to-platelet ratio index[APRI]) and fibrosis-4 [FIB4]) as well as the direct tests, using more sophisticated parameters and protected formulas (e.g., enhanced liver fibrosis [ELF]®, HepaScore®, and FibroTest®).18 The indirect blood tests generally reflect the amount of liver injury and/or the alterations occurring during progressive liver scaring and thereby mirror the extent of disease. For instance, thrombocytopenia is regarded as the earliest indicator of cirrhosis among routine blood tests, capturing multiple processes such as diminished liver function (reduced thrombopoietin production) and portal hypertension (splenic blood sequestration).5,6,19 However, some tests depend on the etiology of chronic liver disease such as the “non-alcoholic liver disease (NAFLD) fibrosis score” that includes the body mass index (BMI) and the presence of diabetes mellitus. Direct blood tests try to circumvent the low specificity of parameters employed in indirect test scores and instead of that, take advantage of parameters of fibrogenesis and/or fibrinolysis. However, even this more direct approach may lead to false positive/negative results. Moreover, these assays are less widely available and costlier than indirect tests.5,18
Since conventional liver imaging fails to reliably detect early stages of liver fibrosis, elastographic methods measuring the elasticity of the liver tissue emerged as tools to fill this diagnostic gap. These take advantage of a shear wave, that is sent from the probe through the chest wall into the liver. Subsequently, the velocity of wave propagation is evaluated by a receiver in the probe. Given that the velocity is related to the elasticity of the tissues, it is converted into a measurement of liver stiffness.5,17 In most entities of chronic liver disease, liver stiffness robustly correlates with the extent of liver fibrosis.5,17 Likely because of that, liver stiffness is incrementally predictive of adverse outcomes of chronic liver disease.5,20,21 For the various entities, specific liver stiffness cut-offs for ruling-out significant liver fibrosis and ruling-in cirrhosis have been proposed.17 However, the values of liver stiffness (i.e., kPa or m/s) determined by different elastographic tools are not interchangeable. Hence, cut-offs have still to be seen in the context of the underlying etiology and the used method and still carry a certain degree of uncertainty. However, as elastography is increasingly used as a first line tool to assess the extent of liver fibrosis, some authors proposed etiology-unspecific cut-offs.17
When used properly, elastography is excellent in ruling-out significant liver fibrosis (i.e., excellent negative likelihood ratios for F ≤ 1) and ruling-in advanced liver fibrosis (i.e., excellent positive likelihood ratios for F ≥ 3). However, several potential confounders must be considered when elevated liver stiffness values are measured to make sure that these really reflect an advanced scaring. Among others, non-fasting (i.e., the postprandially increased blood flow through the liver), severe inflammation (i.e., liver enzymes > 5x the upper limit of normal), or passive liver congestion (i.e., heart failure) may result in high stiffness values that potentially overestimate the amount of liver fibrosis.5,17,22
The most widely available ultrasound-based elastography methods are: vibration-controlled transient elastography (VCTE; FibroScan®; Echosens, France), acoustic radiation force impulse imaging (ARFI; Siemens, Germany), and 2D-shear wave elastography (2D-SWE; offered by different companies [e.g. ,Toshiba, Japan]).17 All methods are used to measure liver stiffness of the right liver lobe and the measurement is performed via an intercostal access. Of these methods, VCTE is the most widely used one and is backed up by the largest amount of scientific evidence.5,17,23 In addition, VCTE offers a simultaneous measurement of controlled attenuation parameter (CAP), that is used as a surrogate of hepatic steatosis.24 On the other hand, ARFI and 2D-SWE allow a simultaneous visualization of the area in which liver stiffness is measured, whereas VTCE is performed in a “blind” manner.17
Magnetic-resonance-based elastography (MRE) is another attractive method for assessment of liver stiffness. It is regarded to be at least equivalent to VCTE in distinguishing between advanced and non-advanced liver fibrosis.25,26 Similar to VCTE, it can also quantify hepatic steatosis using proton density fat fraction (PDFF). Notably, MRE appears to perform better than VCTE in patients with severe obesity.5 Another advantage of MRE is the fact that it analyzes the liver as a whole while all ultrasound-based elastography methods assess only a limited region of the liver. Still, basically the same confounders of liver stiffness measurement apply to MRE as to the ultrasound-based techniques. In conclusion, MRE is equal or possibly even superior to VCTE, especially in difficult cases, but is less widely available and remains to be standardized between health care centers and different vendors.27
As non-invasive risk stratification is emerging as the new standard of initial liver disease evaluation, risk assessment has shifted from histological fibrosis staging to the dichotomization of fibrosis as advanced versus non-advanced.3-5 Thus, the current strategy is to triage the patients into different risk categories. Consequently, liver biopsy is employed for individuals with inconclusive non-invasive results as well as for obtaining additional diagnostic clues. This approach greatly reduced the number of performed liver biopsies. A commonly used strategy is to use an indirect blood-based test (e.g., APRI or FIB-4) and an elastographic method (e.g., VCTE).5 If both tests concordantly indicate that advanced liver fibrosis is not present, a more or less stringent follow-up is recommended. If both tests concordantly indicate the presence of advanced liver fibrosis, the patient should be managed accordingly (e.g., monitoring for complications of cirrhosis). If both tests are discordant (and if potential confounders of both blood-based and elastography-based tests had previously been excluded), liver biopsy should be discussed, especially if it affects further management.
Taken together, non-invasive measures of liver fibrosis assessment are increasingly available, have no/minimal procedure-related risks and are less costly than biopsy. Therefore, they are well suited for a high-throughput monitoring of liver disease progression. This is particularly relevant given the high burden of NAFLD affecting up to 30% of the population. Despite the reduced need for liver biopsy, histology remains an important tool in specific indications, such as in individuals with an inconclusive result of non-invasive assessment or in patients, where further information about the etiology and/or activity of liver injury are needed.
What is the Liver Disease Burden in Adults With the Pi*ZZ and Pi*MZ Genotype of Alpha-1 Antitrypsin Deficiency?
Alpha-1 antitrypsin deficiency (AATD) is one of the most common, potentially lethal genetic diseases worldwide. While over 100 mutations of the SERPINA1 gene are known, the “Pi*Z” variant (Glu342Lys) is the most relevant mutation. Heterozygous Pi*Z carriage is termed as “Pi*MZ” genotype and occurs in about 1:30 whites. Homozygosity of the Pi*Z variant is termed as the “Pi*ZZ” genotype and is seen in about 1:3000 whites. AATD is a multi-systemic disease mainly affecting the lungs (due to deficiency of AAT in the circulation) and the liver (due to intrahepatic accumulation of misfolded AAT).28-30 While AATD-related lung disease is the most common disease manifestation, liver disease is the second most common organ involvement. It displays a biphasic pattern in that it typically occurs either in early childhood or late adulthood.31 Notably, the relative proportion of AATD-related liver disease is higher in never-smokers compared to smokers.32,33 An increasing prevalence of AATD-related liver disease is to be expected due to: (1) the decreasing proportion of smokers in the population and consequently a decreasing proportion of AATD-related lung disease; (2) the current epidemic of obesity that constitutes an important co-factor in liver disease development;34,35 (3) the increased awareness of AATD-related liver disease among patients and physicians; and (4) the growing number of therapeutic studies targeting the liver.36
While diagnosis of adult liver disease in general is difficult, early diagnosis of liver involvement in patients with AATD – whether AATD itself is diagnosed or not – is particularly challenging.18 A major reason is that in homozygous Pi*ZZ adults, liver enzymes are frequently not elevated despite significant liver fibrosis.37,38 Additionally, likely due to low awareness about the importance of liver disease burden, liver enzymes are not determined regularly.38To change that, several recent studies evaluated the liver phenotype of Pi*ZZ individuals. First, a biopsy study evaluated the amount of liver fibrosis in 94 non-cirrhotic Pi*ZZ individuals and observed significant liver fibrosis in 35% of them.39 This endeavor also validated the usefulness of several blood- and elastography-based fibrosis scores to predict the extent of histological liver fibrosis.39 APRI and FIB-4 both predicted the presence of clinically significant fibrosis on liver histology (i.e., fibrosis stage of at least 2 on a 0–4 METAVIR scale). The areas under the receiver operating curve (AUROC) were 0.69 and 0.66 for APRI and FIB-4, respectively. VCTE was also validated in this cohort and had an AUROC of 0.70 for significant liver fibrosis. Interestingly, the gamma-glutamyl transferase (GGT) test was numerically better for recognizing the presence of significant fibrosis (i.e., F ≥ 2) with an AUROC of 0.77 compared to indirect blood tests as well as VCTE. However, VCTE was better than all blood-based parameters to distinguish advanced liver fibrosis (i.e., F ≥ 3 compared to F ≤ 2; AUROC 0.92) and this finding was confirmed in an independent study assessing Pi*ZZ adults with advanced liver fibrosis.40 This is reminiscent of the situation in other chronic liver diseases as liver stiffness is known to be useful to detect advanced liver fibrosis but is less well suited to demarcate mild or significant liver fibrosis stages (i.e., F ≤ 2).17
VCTE was also used in a large multinational cohort comprising 554 Pi*ZZ adults and proved to be an easy-to-use tool which was cross-validated against the aforementioned APRI score (indirect blood test) as well as the patented HepaScore (direct blood test). This multinational effort confirmed the high prevalence of significant liver fibrosis in Pi*ZZ individuals. Depending on the method, significant fibrosis was seen in 20%-36% of them and advanced liver fibrosis was 9–20x more frequent in Pi*ZZ individuals compared to individuals without AAT mutation.38 These data are in line with other observations. In particular, Pi*ZZ individuals are about 20x more likely to require liver transplantation than non-carriers (Table 2).38,41,42
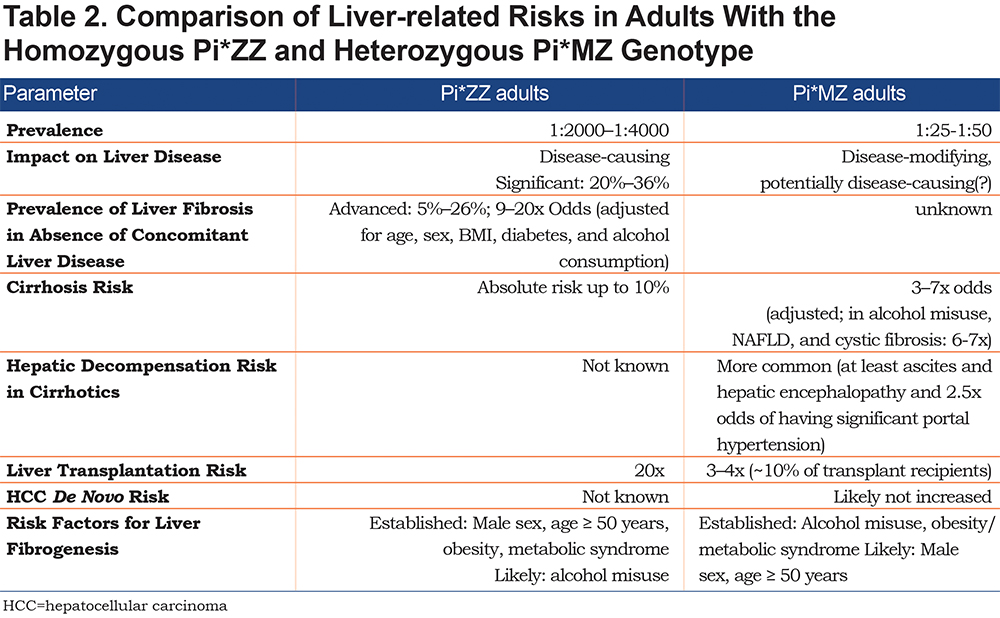
The above described, VCTE-based study38 offers an unprecedented insight into a large population of Pi*ZZ individuals and allows an assessment of disease modifying factors. This is particularly important, since Pi*ZZ-related liver disease is highly heterogeneous.43 This heterogeneity is likely due to both additional genetic factors as well as second hits, that presumably result in accelerated liver fibrogenesis. While these modifiers remain largely unknown, male sex, age ≥ 50 years, obesity, and presence of diabetes and/or metabolic syndrome play an important role.38,39,43,44 While alcohol misuse – as one of the leading causes of liver disease worldwide – was excluded from studies with Pi*ZZ adults, it is likely that alcohol misuse is also a relevant risk factor for liver fibrogenesis. This is also supported by the strong impact of heterozygous Pi*Z carriage on cirrhosis development in adults with alcohol misuse.45 Notably, the above described parameters have all been associated with accelerated liver disease progression in other liver disorders. Importantly, the severity of lung and liver disease do not seem to correlate, which is well in line with the fact that they are pathomechanistically different.38
Several smaller studies tested other modalities of non-invasive liver fibrosis assessment in Pi*ZZ adults: 2 studies employed MRE46,47 with one of them validating its usefulness in a small cohort of 11 biopsied Pi*ZZ adults.43 This very limited evidence suggests that MRE might be suitable to exclude the presence of significant liver fibrosis. However, the current data need to be confirmed in larger investigations. While MRE has the advantage of assessing the whole liver with high accuracy, its main disadvantage is the low availability and the high costs which limits its applicability for the routine evaluation of Pi*ZZ individuals.
Two other studies evaluated other ultrasound-based elastography methods (ARFI47,48 and 2D-SWE47). However, ARFI and 2D-SWE approaches were not validated against liver histology and these methods are, even for other disease entities, not as well-validated as VCTE.17 Notwithstanding this limitation, in a small Pi*ZZ cohort, ARFI, 2D-SWE, and MRE correlated strongly with each other.47 Therefore, ARFI, and 2D-SWE might be useful in conjunction with a blood-based fibrosis score to assess whether advanced liver fibrosis is present. As for other liver diseases,5 the combination of a blood-based fibrosis test (with APRI being the most validated one) and an elastography method (with VCTE being the most established one) offers the best, currently available diagnostic accuracy and inconclusive results should foster liver biopsy. A combination of different methods might be especially helpful for patients with intermediate liver stiffness values (i.e., 5-7 kPa).
In addition to liver fibrosis assessment, the recently published studies revealed other interesting features of Pi*ZZ-related liver disease. For example, 44% of Pi*ZZ individuals displayed histological liver steatosis39 and these data meshed well with non-invasive evaluations using CAP as a surrogate of liver steatosis.24 In particular, CAP ≥ 280 dB/m, suggesting severe steatosis (grade 3), was detected in 39% of Pi*ZZ homozygotes versus 31% of non-carriers while CAP ≥ 248 dB/m, suggesting the presence of steatosis grade ≥ 1, was present in 61% of Pi*ZZ individuals.38 Another small study using CAP detected steatosis grade ≥ 1 in 65% of Pi*ZZ/Pi*SZ adults and grade ≥ 2 in 52% of individuals.49 However, the accuracy of CAP for predicting histological steatosis has not been histologically validated in AATD yet. As a potential underlying mechanism, Pi*ZZ individuals displayed lower serum triglyceride, VLDL, and LDL cholesterol concentrations than Pi*Z non-carriers which points to the hypothesis that Pi*ZZ individuals may have impaired hepatic lipid secretion. These findings were supported by the observations in transgenic mice overexpressing the Pi*Z mutation, that also harboured mild liver steatosis.38
While the above described studies allowed a solid insight into the likelihood of Pi*ZZ individuals to suffer from cirrhosis, their risk to develop hepatocellular carcinoma (HCC) has not been systematically analyzed. While early studies suggested a high risk, several others imply a lower likelihood and suggest that HCC develops almost exclusively in cirrhotic Pi*ZZ individuals.50,51 Therefore, additional studies are needed to clarify this discrepancy. Likewise, longitudinal studies assessing the exact risk of hepatic decompensation in Pi*ZZ adults need to be performed. These studies are crucial for development of a hepatic surveillance plan.
In contrast to the Pi*ZZ genotype that is disease-causing and therefore may lead to cirrhosis development on its own, heterozygous Pi*Z carriage (Pi*MZ genotype) is considered disease-modifying. With regard to the latter, Pi*MZ individuals have an increased risk of developing cirrhosis when chronic liver injury of another etiology co-exists. This has been particularly convincingly demonstrated in children with cystic fibrosis-related liver disease52 as well as in adults with NAFLD and alcoholic fatty liver disease (ALD).51,45 In all of these studies, heterozygous Pi*Z carriage conferred a strong risk for development of cirrhosis with an odds ratio as high as 5-7. Moreover, in a large genome-wide association study, the Pi*Z variant was the single-nucleotide polymorphism conferring the strongest odds ratio to develop ALD-/NAFLD-related cirrhosis.51 Notably, the heterozygous Pi*Z presence may not only affect the risk to suffer from cirrhosis, but may also lead to faster hepatic decompensation53 and thereby result in increased morbidity and mortality. While the risk of de novo HCC development in Pi*MZ heterozygotes has not been systematically studied,50 Pi*MZ adults with cirrhosis due to ALD or NAFLD do not seem to have an increased HCC risk compared to cirrhosis patients without Pi*Z carriage.51 As heterozygous Pi*Z carriage is quite common (2%–4% of the white population) it appears useful to screen for Pi*Z carriage in patients with chronic liver disease and inform them about their risk of rapid progression (e.g., more pronounced portal hypertension49) as well as to counsel them to avoid hepatologic risk factors (e.g., alcohol misuse, obesity, and metabolic syndrome). Such a genotyping might also be useful to prioritize them in listing for liver transplantation.53 Finally, a recent study investigating the natural history of liver disease in Pi*MZ individuals without concomitant liver disease showed that heterozygous Pi*Z carriage is not only a disease modifier of cirrhosis development, but also associated with a distinct liver phenotype.54
In conclusion, the available evidence demonstrates that Pi*ZZ homozygotes are 10–20x more likely to develop advanced liver fibrosis and that they more commonly have liver steatosis with metabolic alterations. Heterozygous Pi*Z carriage (Pi*MZ genotype) is a strong risk factor for cirrhosis development, especially in the context of alcoholic misuse or non-alcoholic fatty liver disease. Further work is needed to determine the hepatic consequences of less prominent AAT variants including the compound heterozygous Pi*SZ genotype.
How to Approach Liver Disease in Adults with Alpha-1 Antitrypsin Deficiency?
For the clinical routine, there are 3 major questions:
· Which AATD individuals should be assessed for the presence of liver disease?
· How should these individuals be monitored?
· How should AATD patients with signs of liver disease be managed?
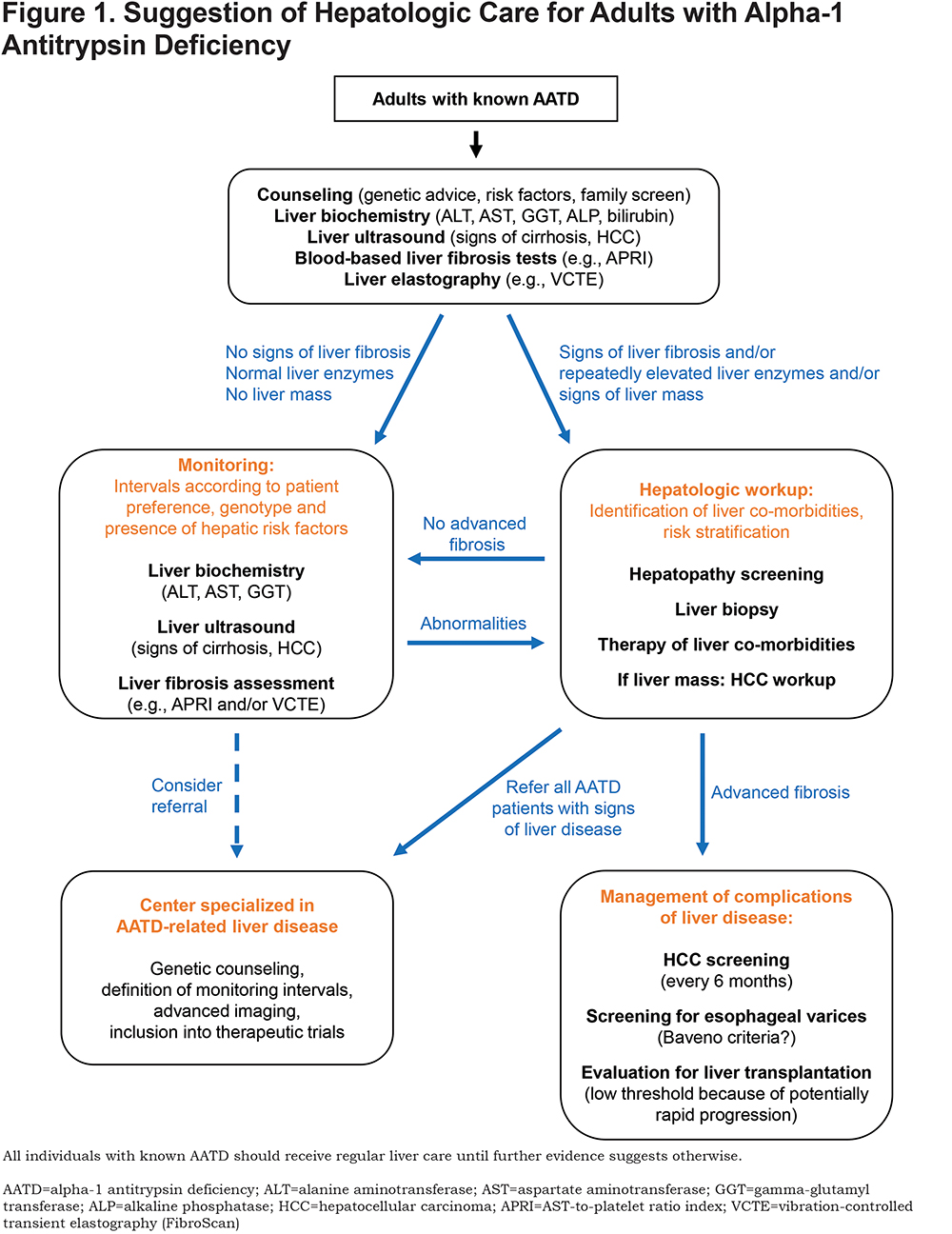
While longitudinal studies systematically assessing the occurrence of liver disease in Pi*ZZ and Pi*MZ adults are missing, the current data suggests that health care providers should be aware of the relatively high risk of liver disease in these individuals and to, at least, determine serum liver enzymes regularly (i.e., alanine aminotransferase, aspartate aminotransferase, GGT, alkaline phosphatase, and bilirubin). Surprisingly, in a large multinational cohort of 554 adult Pi*ZZ homozygotes, 55% did not receive regular liver monitoring by serial measurements of liver enzymes,38 despite the fact that this surveillance can be offered easily and at low cost.
The hepatologic monitoring of Pi*ZZ adults is particularly challenging because of the striking heterogeneity of liver disease and because most Pi*ZZ individuals display normal serum liver enzymes and in spite of that, some of them may already have advanced liver fibrosis.38,39 We recommend at least an initial, non-invasive assessment of liver disease in Pi*ZZ individuals with elastography as well as with liver ultrasound. Alternatively, a non-invasive evaluation with an indirect fibrosis test can be carried out and individuals with intermediate or higher risk should be further assessed.
While there is no evidence-based hepatologic care plan for individuals with AATD-related liver fibrosis yet, based on the above mentioned findings,38,39 we recommend to approach these individuals in the same manner as patients with other liver disease entities. As detailed above, liver diseases should be evaluated by blood-based parameters, ultrasonography, and an invasive or non-invasive assessment of liver fibrosis and the management should depend on the determined amount of fibrosis. In particular, individuals with end-stage liver disease and preserved lung function constitute ideal candidates for liver transplantation55 and patients with both Pi*ZZ-related lung and liver disease can be evaluated for combined lung and liver transplantation. Cirrhotics should be also subjected to ultrasound-based HCC surveillance every 6 months and screened for the potential presence of esophageal varices.
Individuals with less advanced liver fibrosis likely require a less stringent monitoring and the exact frequency of monitoring should be determined individually, based on the patient genotype, preferences, expected amount of fibrosis, as well as the presumed disease activity. The clinical context including age, sex, presence of comorbidities (such as obesity, diabetes mellitus, metabolic syndrome, or alcohol misuse) should also be taken into consideration. Moreover, AATD individuals should be counseled about the established risk factors and to maintain an active, healthy lifestyle, normal BMI, and to abstain from risky alcohol consumption. Individuals with recurrently elevated liver enzymes should be screened for presence of comorbidities and once these are excluded, suggested to perform liver biopsy to rule-out presence of concomitant liver disease.
As no evidence-based hepatologic care plan exists, it is recommended to refer Pi*ZZ individuals– especially those with signs of liver disease–to a health care center specializing in AATD-related liver disease. Moreover, these centers can evaluate whether an individual is suitable for one of the therapeutic trials that are currently being initiated. In our center, we recommend to control liver enzymes at least every 6–12 months and to perform an assessment of liver fibrosis every 1–2 years. Given that the risk of HCC has not been clearly determined, we tend to perform liver ultrasounds once a year in non-cirrhotic individuals. (Figure 1)
Acknowledgements
We thank the national patient organizations (i.e., Alpha-1 Deutschland: Marion Wilkens and Gabi Niethammer; Alpha-1 Austria: Ella Geiblinger; Alpha-1 Belgium: Frank Willersinn; Alpha-1 Netherlands: Heleen Groen; Alpha-1 Denmark: Gunhil Norhave, as well as Alpha-1 Spain, Alpha-1 Italy, Alpha-1 Poland, and Alpha-1 Portugal for their help with the execution of our study. We also thank all patients for their participation in our ongoing Europe-wide registry.
Declaration of Interest
PS has received speaker or consulting fees from Grifols, CSL Behring, Alnylam, and Arrowhead Pharmaceuticals. KH has received speaker fees from CSL Behring.