Running Head: Impact of GLY by Metabolic Syndrome Status
Funding Support: The GOLDEN studies were supported by funding from Sunovion Pharmaceuticals, Inc. Medical writing support was funded by Sunovion Pharmaceuticals, Inc.
Date of Acceptance: April 22, 2020
Abbreviations: chronic obstructive pulmonary disease, COPD; metabolic syndrome, MetS; nebulized glycopyrrolate, GLY; patient-reported outcome, PRO; body mass index, BMI; cardiovascular disease, CV; twice daily, BID; closed system, CS; long-acting muscarinic antagonist, LAMA; U.S. Food and Drug Administration, FDA; long-acting beta2-agonist, LABA; Global initiative for chronic Obstructive Lung Disease, GOLD; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; St George’s Respiratory Questionnaire, SGRQ; Evaluating Respiratory Symptoms in COPD tool, E-RS: COPD®; adverse event, AE; serious adverse event, SAE; major adverse cardiovascular event, MACE; myocardial infarction, MI; intent-to-treat, ITT; inhaled corticosteroid, ICS; minimum, min.; treatment, tx; standard deviation, SD; least squares, LS; standard error, SE; confidence interval, CI; odds ratio, OR
Citation: Carlin B, Ferguson GT, Ozol-Godfrey A, Goodin T, Sanjar S. The effect of metabolic syndrome status on lung function and patient-reported outcomes in patients with COPD receiving nebulized glycopyrrolate. Chronic Obstr Pulm Dis. 2020; 7(4): 315-326. doi: http://doi.org/10.15326/jcopdf.7.4.2020.0145
Introduction
Note: The abstract of this paper was presented at the American Thoracic Society 2019 International Conference as a poster with interim findings.
Chronic obstructive pulmonary disease (COPD) is a progressive disease characterized by persistent respiratory symptoms and airflow limitation.1 Patients with COPD commonly present with coexisting comorbidities that impact quality of life and morbidity, such as cardiovascular (CV) disease, hypertension, and diabetes.2-4
Metabolic syndrome (MetS) is defined as a clustering of ≥ 3 CV risk factors including hypertension, obesity, hyperlipidemia, and type 2 diabetes.5-7 There is a strong correlation between MetS and diminished airflow8 that has been shown to increase the risk of CV disease in patients with airway inflammation, impaired lung function9 and may worsen COPD progression.10 The reported prevalence of MetS in the general population, and in patients with COPD, varies by region. Global MetS prevalence ranges between approximately 20% and 50%,8,11-14 while prevalence of MetS in patients with COPD is similar or slightly higher compared with the general population, ranging between approximately 20% and 60%.8,10,13-22 Symptoms, such as shortness of breath (i.e., dyspnea), occur in patients with either COPD or CV disease, and may overlap in patients who have respiratory disease and CV comorbidities4,9 that could confound a diagnosis and initiation of appropriate therapy.4 This highlights the importance of the differential diagnosis and active management of COPD and concurrent comorbidities.18
Nebulized glycopyrrolate (GLY; LONHALA® 25 mcg twice daily [BID]), delivered using the eFlow® Closed System (CS) Nebulizer (MAGNAIR®; PARI Pharma GmbH; Starnberg, Germany) as a twice-daily, nebulized, long-acting muscarinic antagonist (LAMA) is approved by the U.S. Food and Drug Administration (FDA) for the long-term maintenance treatment of airflow obstruction in patients with COPD.23 This approval was based, in part, on the results from two 12-week, randomized, placebo-controlled, Phase 3 studies (GOLDEN 3 [NCT02347761] and GOLDEN 4 [NCT02347774]), which demonstrated that nebulized GLY treatment was well-tolerated and led to significant, clinically important improvements in lung function and patient-reported outcomes (PROs).24
A post-hoc analysis of pooled data from GOLDEN 3 and GOLDEN 4 was conducted to evaluate the effects of nebulized GLY 25 mcg BID on lung function, PROs and safety in COPD patients with and without MetS.
Methods
Study Design and Treatment
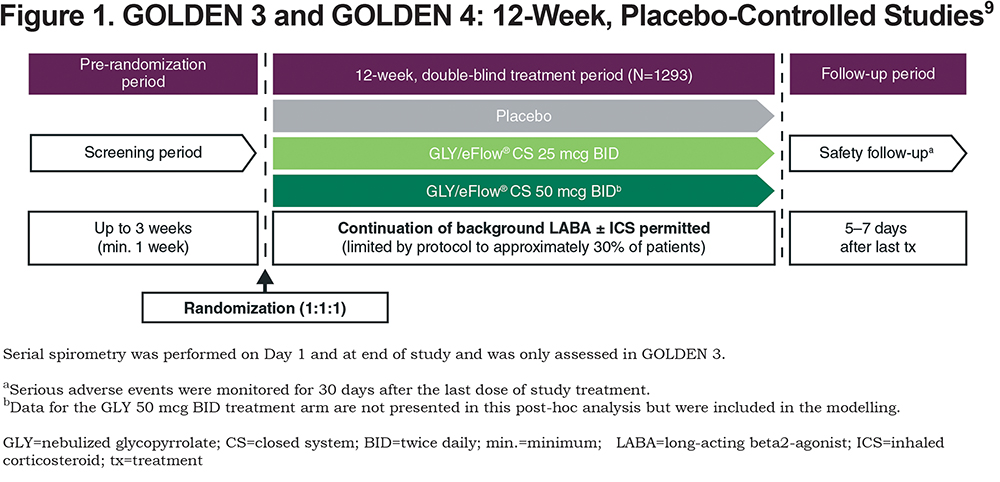
Study designs of GOLDEN 3 and GOLDEN 4 have been described previously24 and are shown in Figure 1. In brief, patients were randomized 1:1:1 and received placebo or GLY (25 or 50 mcg BID), administered via the eFlow® CS Nebulizer. Randomization was stratified by CV risk (high/low) and background long-acting beta2-agonist (LABA) use (yes/no). The proportion of patients who continued background LABA use (with or without concomitant inhaled corticosteroids) during the treatment period was limited by protocol to approximately 30%. Ipratropium bromide, as supplemental medication, and albuterol (salbutamol), as rescue medication, were permitted.
Patients
Detailed study entry criteria have been reported previously.24 Eligible patients were current or ex-smokers, ≥ 40 years of age with ≥ 10 pack-year smoking history, with a clinical diagnosis of moderate-to-very-severe COPD (defined using Global initiative for chronic Obstructive Lung Disease [GOLD] 2014 Report criteria),1 and with qualifying post-bronchodilator (ipratropium 68 mcg) spirometry, including forced expiratory volume in 1 second (FEV1) ≤ 80% of predicted normal, FEV1 > 0.7 L, and FEV1/forced vital capacity (FVC) ratio < 0.70. Patients with severe comorbidities, history of or current unstable CV disease and/or long QT syndrome were excluded.
Study protocols were approved (GOLDEN 3: project approval number 28481; GOLDEN 4: project approval number 28482) by Quorum Review Institutional Review Board, North American (U.S. and Canadian) Board (Panel II) prior to patient enrollment, and were conducted in accordance with the protocols, International Council for Harmonization Good Clinical Practice guidelines, and Declaration of Helsinki. All patients provided written informed consent.
Statistical Analyses
The placebo, GLY 25 and 50 mcg BID data from GOLDEN 3 and 4 were pooled (N=1293), and data for both GLY doses were included in all statistical models. Inclusion of the 50 mcg BID data in the modelling does not confound the interpretation of the GLY 25 mcg BID dose. The data and results presented are for the FDA-approved GLY dose of 25 mcg BID.
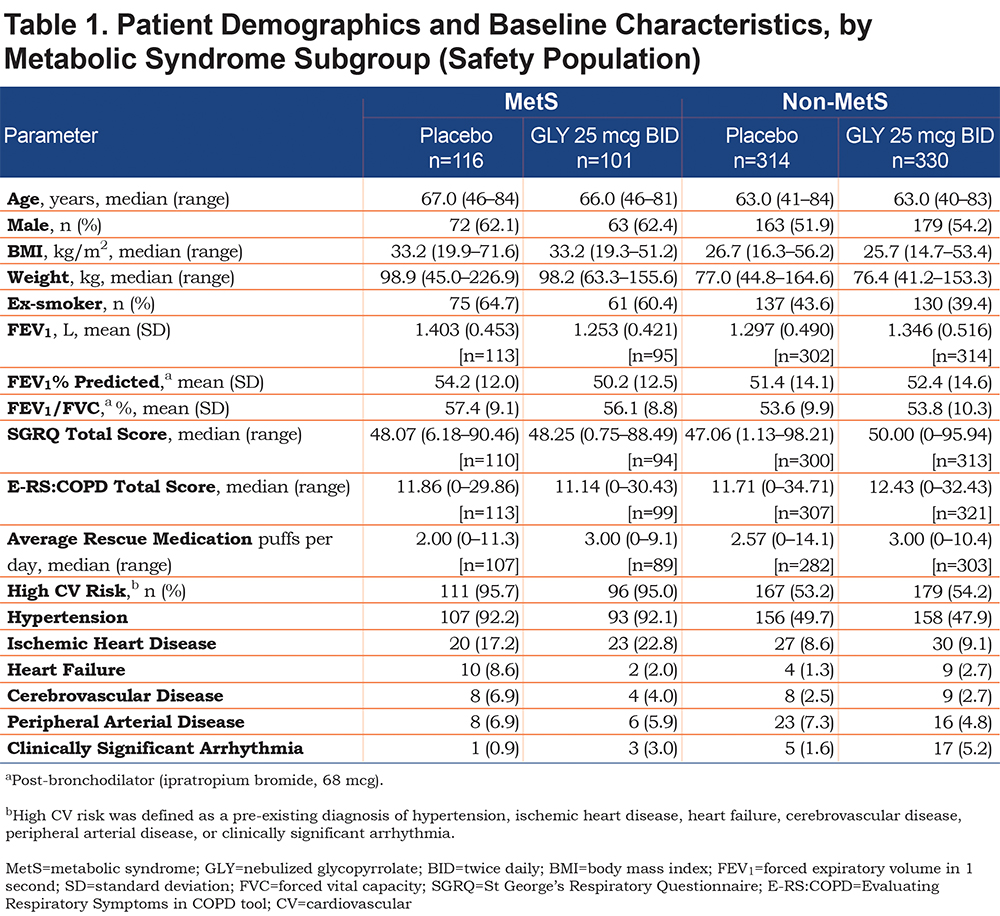
Patients were grouped according to MetS status (MetS or non-MetS). MetS was defined as having at least 3 of the following: hypertension, hyperlipidemia, diabetes, or body mass index (BMI) > 30 kg/m2. According to the protocol, high CV risk was determined based on a history of 1 or more of the following pre-specified disorders: ischemic heart disease, cerebrovascular disease, peripheral arterial disease, clinically significant arrhythmia (defined as any arrhythmia for which the patient was receiving or had received medication, or an interventional procedure, or identified by Holter monitoring at screening), heart failure, or hypertension.
Lung function and PRO endpoints included: change from baseline in trough FEV1, St George’s Respiratory Questionnaire (SGRQ; including responders) total score, Evaluating Respiratory Symptoms in COPD tool (E-RS:COPD®; including responders) total score, and rescue medication use.
Changes from baseline in trough FEV1 and E-RS:COPD total score were analyzed using a mixed-model for repeated measures, and changes from baseline in SGRQ total score and rescue medication were analyzed by analysis of covariance. Minimum clinically important differences for the different measures were defined as: reduction in SGRQ25 total score ≥ 4; reduction in E-RS:COPD26 total score ≥ 2. SGRQ responders were analyzed using logistic regression and E-RS:COPD responders were analyzed using a longitudinal logistic regression. All models included covariates for the baseline level of the appropriate outcome measure, CV risk, and background LABA use. All statistical procedures were performed using SAS® v9.2 or higher (SAS Institute Inc., Cary, North Carolina). Only data that were measured while on randomized blinded study treatment (i.e., on-treatment data) were analyzed. No adjustments were made for the post-hoc multiple comparisons. All p-value interpretations are made at the 5% significance level.
Safety outcomes included adverse events (AEs), serious AEs (SAEs), and major adverse CV events (MACEs; as determined by a blinded, independent committee, including CV death, non-fatal myocardial infarction [MI], and non-fatal stroke). Safety data were analyzed using descriptive statistics. AEs and SAEs were coded according to MedDRA v15.1.
Safety analyses were conducted using the safety population, and efficacy analyses using the intent-to-treat (ITT) population, both consisting of all patients randomized to treatment who received ≥ 1 dose of study drug.
Results
Patient Demographics and Baseline Characteristics
Data for the GLY 25 mcg BID and placebo treated patients (N=861) were reported by MetS (n=217 [25.2%]; placebo: n=116; GLY 25 mcg BID: n=101) and non-MetS (n=644 [74.8%]; placebo: n=314; GLY 25 mcg BID: n=330) subgroups. The MetS subgroup included more males, ex-smokers, and patients with higher median BMI and weight than the non-MetS subgroup (Table 1). Baseline lung function, PRO total scores and rescue medication use were generally similar across the treatment groups and MetS/non-MetS subgroups. Most patients with MetS were classified as having high CV risk, based on a medical history of per protocol, pre-specified CV risk factors. Specifically, a substantially higher incidence of CV risk factors including hypertension and ischemia were present in MetS patients, whereas peripheral arterial disease and arrhythmias appeared to a similar extent in both MetS subgroups.
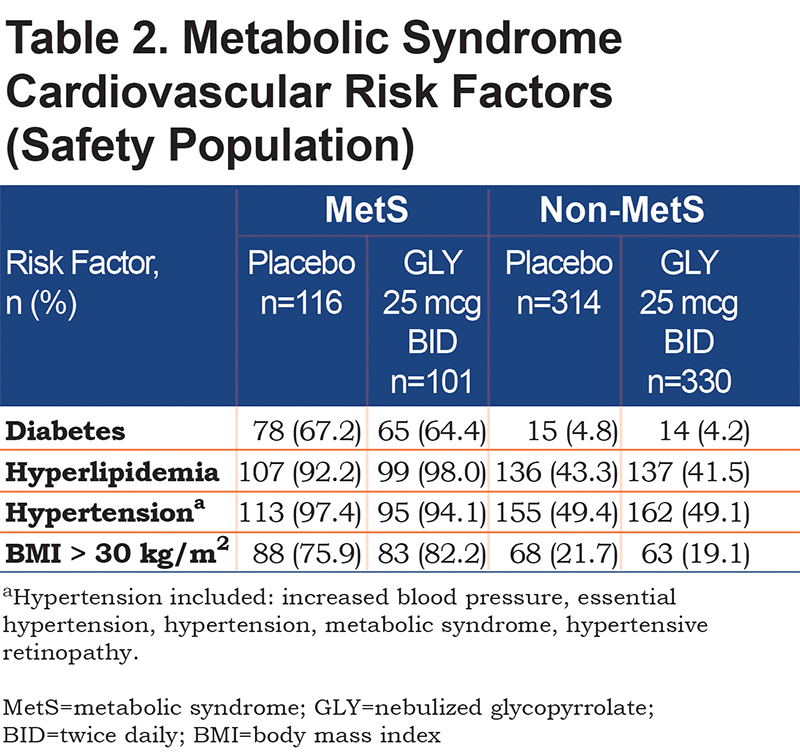
The incidence of MetS risk factors in the MetS versus non-MetS subgroup ranged between 2‑ (hypertension) and approximately14‑times higher (diabetes) (Table 2). Over 90% of patients in the MetS subgroup had hypertension and/or hyperlipidemia, while less than 5% of patients in the non-MetS subgroup had diabetes.
Efficacy
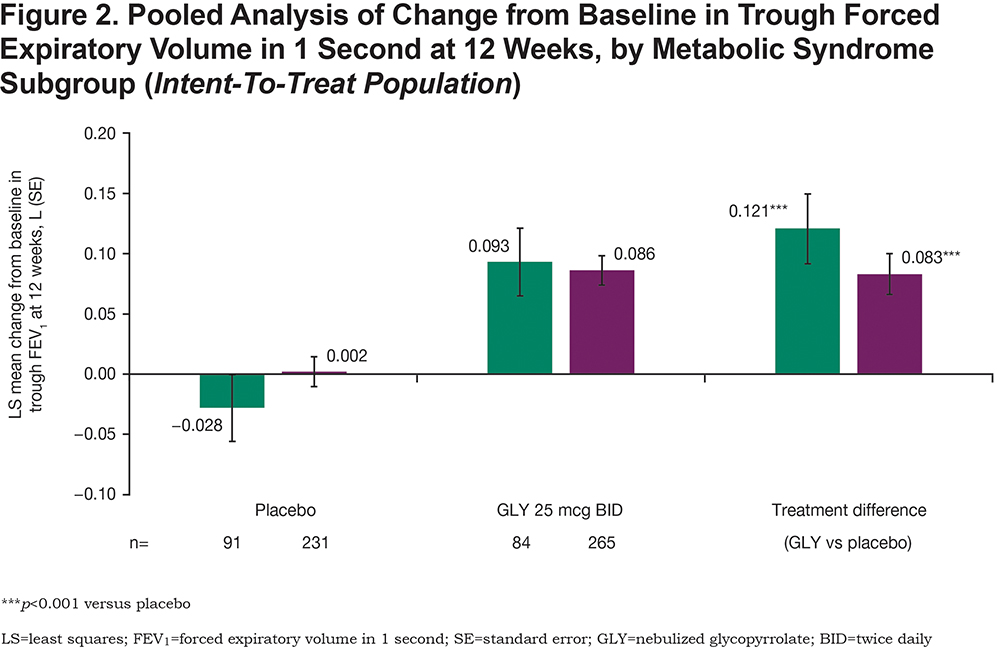
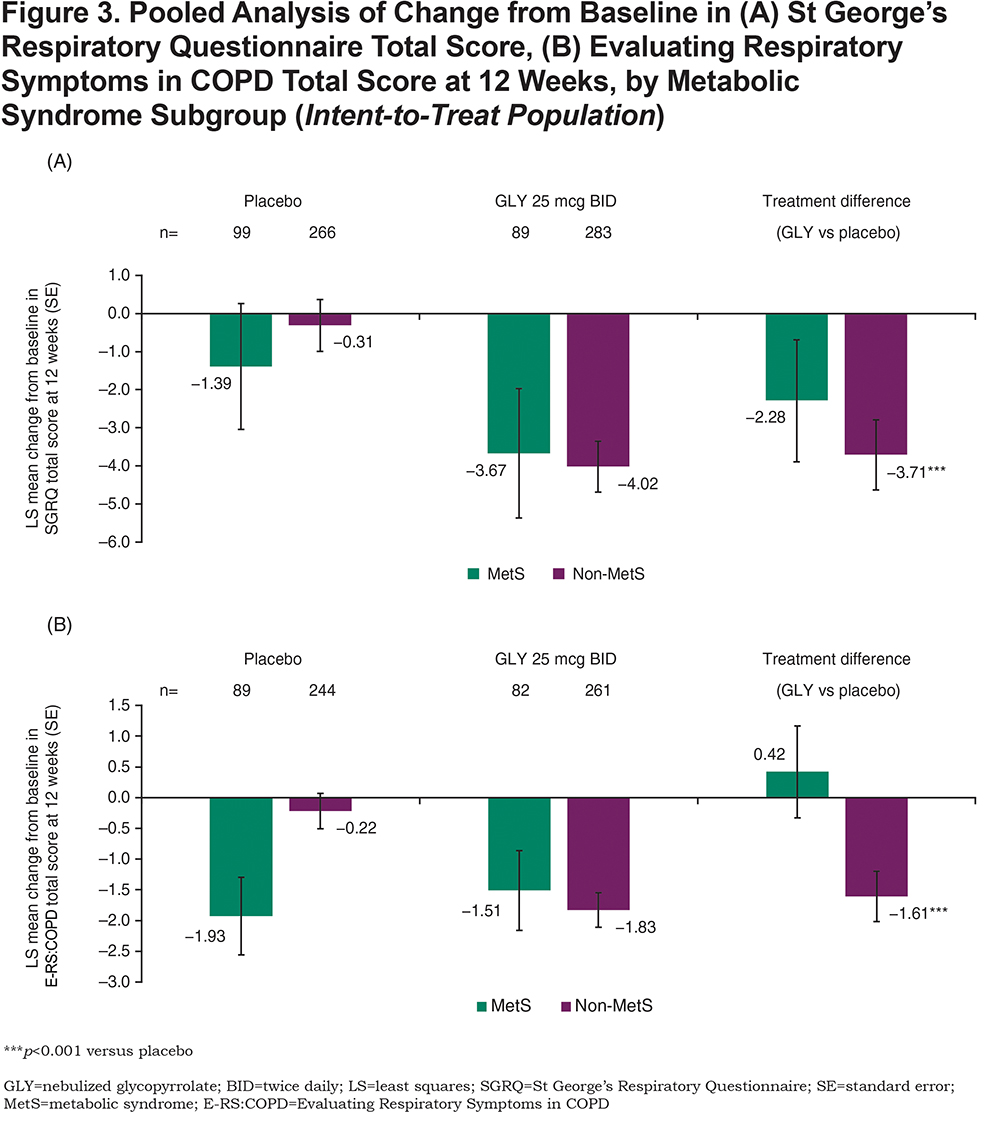
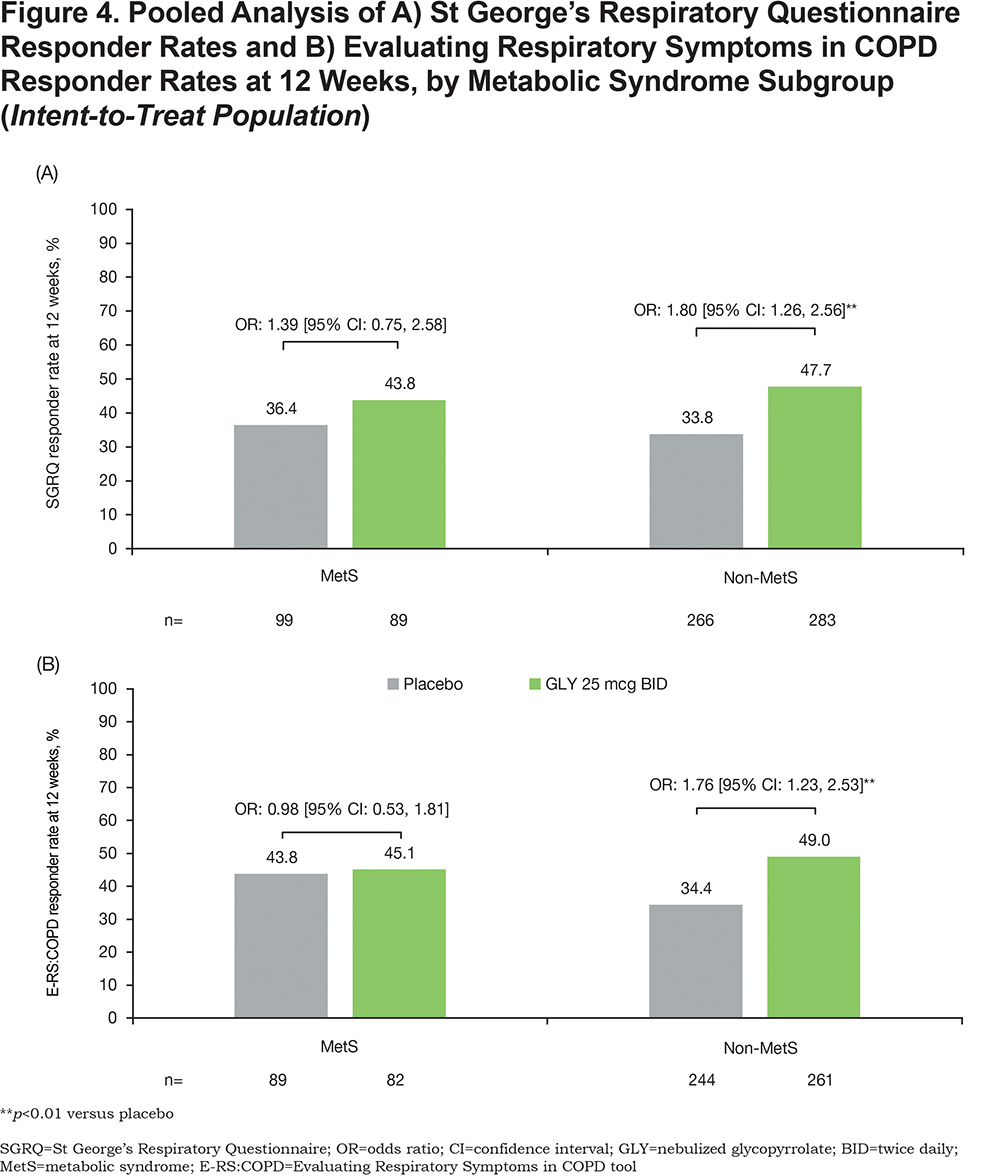
Treatment with GLY 25 mcg BID resulted in significant (p<0.001), clinically important improvements in placebo-adjusted trough FEV1 regardless of MetS status at week 12 (Figure 2). Additionally, GLY treatment resulted in significant placebo-adjusted improvements from baseline in SGRQ total score (MetS: p=0.157; non-MetS: p<0.001; Figure 3A) and E-RS:COPD total score (MetS: p=0.574; non-MetS: p<0.001; Figure 3B) among non-MetS patients. The odds of being an SGRQ responder (≥ 4-unit reduction in total score; Figure 4A) or an E-RS:COPD responder (≥ 2-unit reduction in total score; Figure 4B) in the GLY 25 mcg BID treatment group were significant (p<0.01) in the non-MetS subgroup.
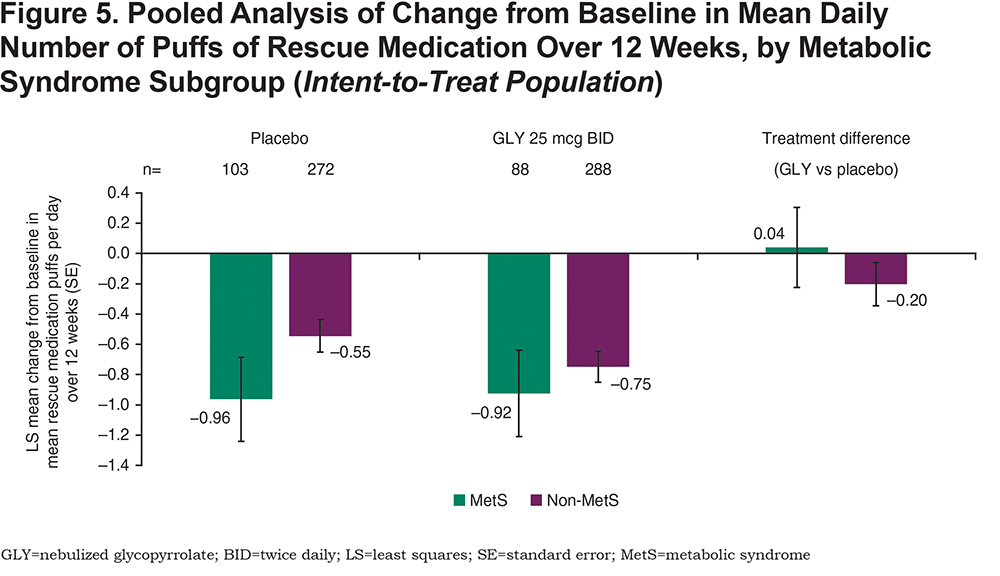
Placebo-adjusted change from baseline in mean daily number of rescue medication puffs over 12 weeks was not significant for either subgroup (MetS: p=0.881; non-MetS: p=0.154; Figure 5).
Safety
Overall, GLY 25 mcg BID was well-tolerated in both MetS and non-MetS subgroups. A lower proportion of patients treated with GLY 25 mcg BID experienced AEs compared with placebo regardless of MetS status, and the incidences of any AE in MetS and non-MetS patients were similar (Table 3). The most common AEs across treatment groups were cough, worsening of COPD, and dyspnea, and were generally similar in MetS and non-MetS subgroups. Patients treated with GLY 25 mcg BID in both the MetS and non-MetS subgroups experienced fewer SAEs than patients taking placebo.
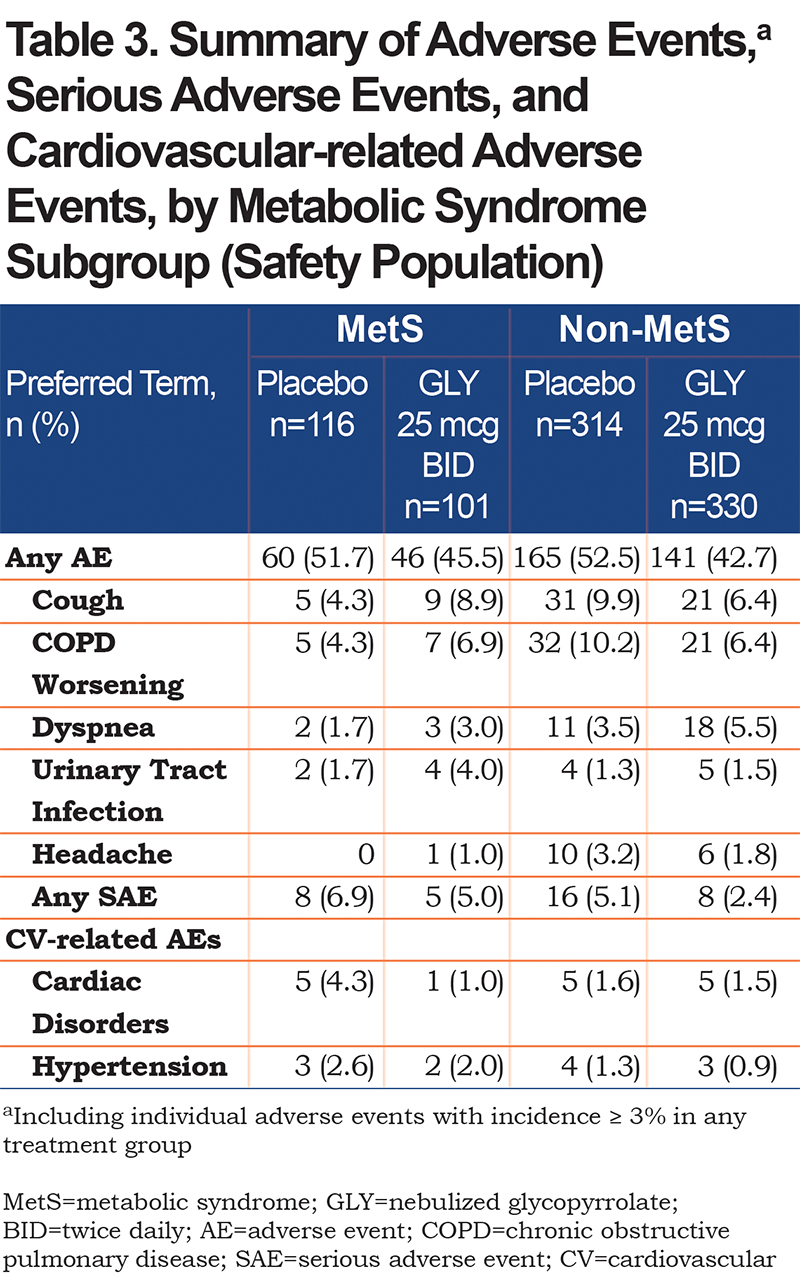
The incidences of AEs related to cardiac disorders or hypertension in patients treated with GLY 25 mcg BID were similar between MetS and non-MetS subgroups and were lower compared with the corresponding placebo treatment groups. A MACE occurred only in 2 patients, both in the MetS subgroup taking placebo (both non-fatal MIs; incidence rate per thousand person-years = 16.4).
Discussion
Clinically, patients with concurrent COPD and MetS present important challenges in disease management with bronchodilators based on overlapping symptomology.Despite the marked prevalence of comorbidities among patients with COPD, there have been limited studies assessing the impact of comorbidities on bronchodilator therapy. The results showed lung function improvements with GLY 25 mcg BID were consistent and significantly greater than placebo in both MetS and non-MetS subgroups; however, PROs were only significantly improved with GLY 25 mcg BID among patients in the non-MetS subgroup. Importantly, GLY 25 mcg BID was well-tolerated with similar AE incidence across MetS subgroups. While the GOLDEN 3 and 4 studies were not prospectively designed to assess the effect of MetS on COPD, the results of this post-hoc analysis provides valuable insights for clinicians to help guide the individualized care of complex COPD patients.
In GOLDEN 3 and GOLDEN 4, baseline lung function and SGRQ total scores were comparable to values reported in the literature for COPD patients with MetS. For patients with COPD and MetS, mean post-bronchodilator baseline FEV1 % predicted in GOLDEN 3 and 4 (50.2%−54.2%) was similar to that reported across 5 studies (in a systematic review of 19 studies) by Cebron Lipovec et al15 (54% [n=414]) and in a prospective cohort analysis by Breyer et al16 (58% [n=101]). Mean baseline FEV1/FVC in COPD patients with MetS from GOLDEN 3 and 4 (56.1%−57.4%) was similar to mean values reported by Piazzolla et al17 (60% [n=60]) and Park et al13 (56% [n=54]), although Breyer et al16 reported a somewhat lower ratio of 44.6% (n=101). Breyer et al16also reported a mean baseline SGRQ total score of 52.7 for COPD patients with MetS, which is consistent with the median baseline values in GOLDEN 3 and 4 (48.1–48.3).
Variability of placebo response, particularly in patients with MetS in the placebo treatment group, may have contributed to the effect sizes of outcomes in this post-hoc analysis. This could be due to the difference in patient numbers, as the non-MetS subgroup was approximately 3-times larger than the MetS subgroup, or due to the comorbidities, which may have affected the appearance and resolution of symptoms among patients receiving placebo. It is also possible that the variability in trough FEV1 results could be due to the relatively high baseline FEV1 in the placebo MetS subgroup (1.403 L) compared with other subgroups (1.253–1.346 L), however, differences in SGRQ and E-RS:COPD results cannot be similarly explained, as baseline values were comparable across MetS status and treatments.
Significant differences in placebo-adjusted PROs were noted only in the non-MetS subgroup. This may be due to patients without MetS having better PRO responses, as they do not experience overlapping symptoms of MetS and COPD (e.g., shortness of breath), thereby making it easier to identify COPD-related symptom improvements following bronchodilator therapy.
GLY 25 mcg BID was well-tolerated regardless of MetS status, with a lower incidence of AEs, SAEs, CV-related AEs and MACEs compared with placebo. CV risk factors are more common in patients with MetS, but there was no increase in the incidence of CV-related AEs in patients treated with nebulized GLY in these studies, including those with MetS. These results support the overall and CV safety of GLY 25 mcg BID in patients with COPD, independent of MetS status.
Limitations to the current analysis include the posthoc patient classification, with MetS not included as a pre-specified sub-population involving the categorization of risk factors at screening but determined after study completion. Although patients were stratified by CV risk, high risk only required one CV risk factor, rather than multiple factors as per MetS. This is evident in the large variation in the number of patients within each subgroup, even though the prevalence of MetS in COPD patients in this pooled population (25%) is consistent with the lower end of reported prevalence (approximately 20%−60%),8,10,13-22 albeit the range of reported values is wide, across different geographic regions, and with varying population sizes.
Conclusions
Patients with COPD and concurrent MetS represent an important clinical phenotype with overlapping symptomology who may respond differentially to bronchodilator therapy. Treatment with GLY 25 mcg BID produced lung function improvements compared with placebo, regardless of MetS status, whereas significant differences in placebo-adjusted PROs were noted only in the non-MetS subgroup. The incidence of AEs and SAEs were lower in patients treated with nebulized GLY compared with placebo in patients with and without MetS, including CV‑related AEs. These results suggest that nebulized GLY 25 mcg BID is a treatment option for patients with COPD, with or without concurrent MetS and highlights the importance of comorbidities on physiological and symptomatic responses to bronchodilators in COPD patients.
Acknowledgments
The authors would like to thank Shane Hornibrook from Sarepta Therapeutics, Inc., Diane Hall from Sunovion Pharmaceuticals, Inc., and Rajeshwari Sammishetty from Sage Therapeutics, Inc., for support with statistical analyses performed. Medical writing support was provided by Linda Townsend, PhD, and Hashem Dbouk, PhD, of Ashfield Healthcare, part of UDG Healthcare plc, and funded by Sunovion Pharmaceuticals, Inc.
Author Contributions: All authors were involved in data analysis and interpretation, were involved at all stages of manuscript development, writing and revision, approved the final manuscript and agree to be held accountable for all aspects of the work. Gary T. Ferguson was involved in data acquisition.
Data Sharing Statement: Sunovion Pharmaceuticals, Inc., is part of a clinical trial data sharing consortium that facilitates access for qualified researchers to selected anonymized clinical trial data. For up-to-date information on data availability please visit https://www.clinicalstudydatarequest.com/Study-Sponsors.aspx and click on Sunovion.
Declaration of Interest
Dr. Brian Carlin has served on speakers’ bureaus for Sunovion Pharmaceuticals Inc., and GlaxoSmithKline and is an advisory board member for Sunovion Pharmaceuticals Inc., GlaxoSmithKline, and Theravance. Dr. Gary T. Ferguson reports grants, personal fees, and non-financial support from Sunovion Pharmaceuticals Inc., during the conduct of the study; Dr Ferguson has also received grants, personal fees and non-financial support from AstraZeneca, Boehringer Ingelheim, Novartis, Pearl Therapeutics, Sunovion, Theravance, and GlaxoSmithKline; grants and personal fees from Verona and Sanofi; grants from Altavant; and personal fees from Mylan, Innoviva and Circassia. Dr. Ayca Ozol-Godfrey, Dr. Thomas Goodin, and Dr. Shahin Sanjar are employees of Sunovion Pharmaceuticals, Inc.