Running Head: Daily Symptoms and Health Measures in COPD
Funding support: National Institutes of Environmental Health Sciences K23 ES026204; National Heart, Lung, and Blood Institute F32 HL143819
Date of acceptance: July 1, 2020
Abbreviations: chronic obstructive pulmonary disease, COPD; Global initiative for chronic Obstructive Lung Disease, GOLD; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; Research Electronic Data Capture, REDCap
Citation: Sun WY, Zhang C, Synn AJ, Nurhussien L, Coull BA, Rice MB. Change in inhaler use, lung function and oxygenation in association with symptoms in COPD. Chronic Obstr Pulm Dis. 2020; 7(4): 404-412. doi: http://doi.org/10.15326/jcopdf.7.4.2020.0138
Online Supplemental Material: Read Online Supplemental Material (479KB)
Note: These findings have been previously presented in the form of an abstract at the 2019 American Thoracic Society International Conference in Dallas, Texas.
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the United States1 and became the third leading cause of death in the world in 2016.2 Numerous studies have established that exacerbations, defined by acute periods of worsened symptoms, are a major driver of the morbidity and mortality of this disease.3-6 As a result, there is growing interest in the role of self-management in COPD to prompt early initiation of pharmacological treatment of exacerbation symptoms.7
A number of COPD self-management action plans have been evaluated.8-11 While rescue treatment via short-acting inhalers has been extensively evaluated as the initial step for self-treatment of day-to-day fluctuations of asthma symptoms and is a key component of any asthma action plan, 12-18 the focus of self-treatment action plans for COPD has been on the use of oral corticosteroids and antibiotics to self-treat moderate-to-severe exacerbations. Nonetheless, the 2019 Global initiative for chronic Obstructive Lung Disease (GOLD) document recommends the use of short-acting beta2-agonists as the initial step for immediate symptom relief and treatment of an acute exacerbation of COPD.19 This recommendation is based on a limited number of studies that have found that short-acting bronchodilators can relieve COPD symptoms in most patients.20,21
Short-acting inhalers and nebulizers are commonly prescribed for COPD in accordance with the GOLD document.22 However, it is not well understood how COPD patients use or integrate as-needed bronchodilators in the management of their daily symptoms and milder exacerbations. The aim of this study was to evaluate self-treatment of COPD symptoms and exacerbations with short-acting inhaler/nebulizer medication in the outpatient setting in a cohort of 26 participants with moderate-to-severe COPD, from whom we collected daily data on symptoms, oxygenation, lung function, and medication use for up to 4 months each. We investigated if changes in self-reported COPD symptoms and exacerbations by validated daily questionnaire were associated with daily differences in as-needed inhaler/nebulizer use or objective measures of COPD disease activity, including oxygenation and lung function.
Materials and Methods
Study Population
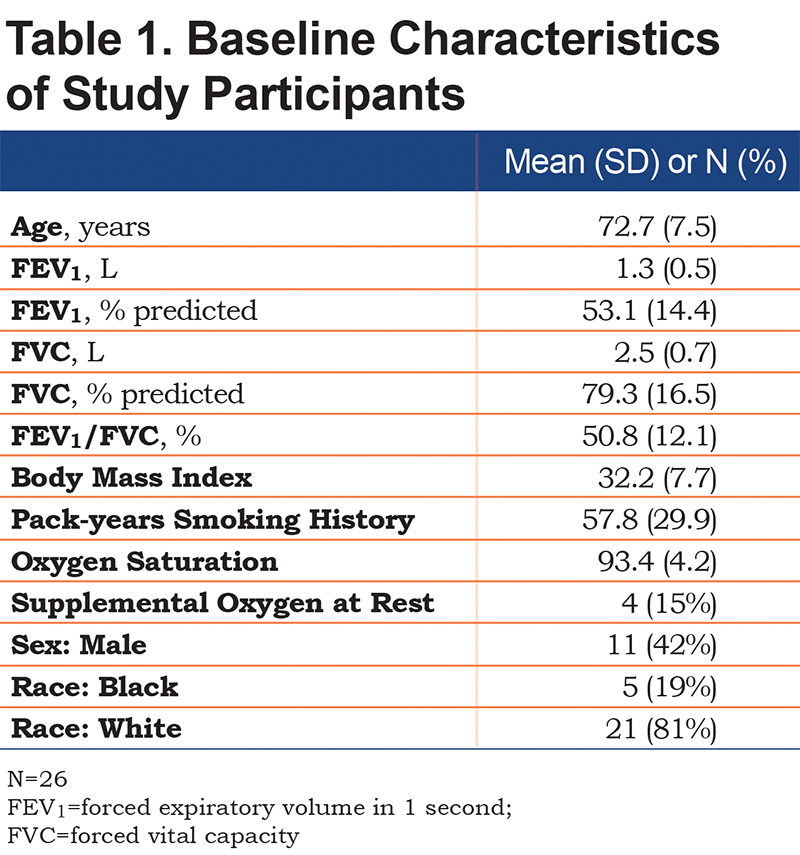
The study population consisted of 26 former smokers with COPD who were recruited as part of the Study of Air Pollution and COPD Exacerbation at Beth Israel Deaconess Medical Center in Boston, Massachusetts. To be eligible, study participants were required to be former smokers with a ≥10 pack-year smoking history, and to have a clinical diagnosis of COPD with at least moderate GOLD Stage 2 airflow obstruction on spirometry (defined as forced expiratory volume in 1 second [FEV1]/forced vital capacity [FVC]<70% and FEV1<80% predicted). Participants with a history of lung cancer, interstitial lung disease, or bronchiectasis were ineligible to take part in the study. The study was approved by the authorized institutional review board of the Beth Israel Deaconess Medical Center (Protocol # 2015P000336/03). Written informed consent was obtained from all study participants.
Data Collection
Participants entered the study between February 24, 2017 and January 17, 2019. At study entry, demographic information, height, weight, past medical history, medication history, and baseline measures of lung function and oxygen saturation were obtained. Study participants were instructed on how to use a portable spirometer and oximeter and were shown how to complete a daily electronic questionnaire (see the online supplement to view the full questionnaire). Participants were instructed to measure their oxygen saturation at a consistent time in the morning. Those on supplemental oxygen at rest were instructed to use their usual supplemental oxygen when measuring their oxygen saturation.
Study participants were then observed for up to 4 non-consecutive 30-day periods in different seasons over 12 months, up until February 20, 2019. During observation periods, participants recorded daily changes in respiratory symptoms and treatment by daily questionnaire, accessed electronically through Research Electronic Data Capture (REDCap), a secure web application developed by Vanderbilt University.23 This questionnaire was developed and validated for the purpose of identifying COPD exacerbations from self-reported worsening respiratory symptoms.10,24-27 The questionnaire consisted of the 2 questions below:
- Please record any worsening of symptoms above your usual daily level in the previous 24 hours. Any symptom that is at your “normal” level should be left blank. [Response options: No change; increased breathlessness; increased sputum color; increased sputum amount; a cold (such as a runny or blocked nose); increased wheeze or chest tightness; sore throat; increased cough; fever]
- Please record any change to your usual treatment in the previous 24 hours for as many days as it applies. [Response options: No change; I am taking: more than my usual inhaler, more than my usual nebulizer, steroid (prednisone) tablets, and/or an antibiotic]
In addition, participants recorded and submitted their daily oxygen saturation (SpO2) via the REDCap questionnaire, measured using a Nonin GO2 LED Achieve 9571 Finger Pulse Oximeter (Plymouth, Minnesota). The Nonin GO2 device uses the same PureSAT® SoO2 technology as the medical grade oximeters and has a declared oxygen saturation accuracy range of 70%-100% SpO2 + 2 digits. Participants also performed spirometry every morning before taking their morning medication, with a portable EasyOneTM Plus Diagnostic Spirometer (ndd, Switzerland). This device meets American Thoracic Society guidelines and has built-in quality assurance and incentive software.28,29
Statistical Analysis
Respiratory symptom days and COPD exacerbations were defined per the survey tool.10,24-27 Dyspnea, sputum purulence, and sputum amount were classified as major symptoms. Nasal discharge/congestion, wheeze, sore throat, cough, and fever were classified as minor symptoms.
Observation-days were classified as follows, in accordance with the survey tool:
- Mild-to-moderate: increase in at least 1 minor symptom, but no major symptoms, in the previous 24 hours compared to baseline.
- Moderate-to-severe: increase in at least 1 major symptom in the previous 24 hours compared to baseline.
- Exacerbation: increase in 2 or more symptoms (at least 1 major symptom) in the previous 24 hours compared to baseline on 2 or more consecutive days.
We constructed multi-level linear mixed effects models to assess for associations between the presence of self-reported respiratory symptoms, using the 3 classifications of “symptom day” as described above, and oxygenation and FEV1:
Yimt=b0 + b1 sympimt + b2 cimt + wi +eimt
where Yimt is any continuous outcome (oxygenation or FEV1) for participant i at observation month m on day t; sympimt indicates "symptom day,” the main predictor of interest, and cimt represents a vector of potential confounders (age, sex, race, height, weight, and season). The term wi denotes a participant-specific random effect and was used to account for intra-individual correlations between repeated measurements among the same person. The residuals eimt are normally distributed errors, assumed to follow a first order autoregressive process modeling the serial correlation among the daily time series within the same person’s observation month.
Season was categorized as winter, spring, summer, and fall, based on the calendar start dates of each observation month. We excluded 25 outlier observations with oxygen saturation values >100% or <80% that were likely entered erroneously by the study participant. We also performed a sensitivity analysis examining differences in oxygen saturation on symptoms days in which we excluded all participants who reported use of supplemental oxygen at rest (N=4). In addition, we evaluated the frequency of as-needed inhaler and nebulizer use on symptom days for each of the 3 classifications of “symptom day.” Finally, we examined the association between the total number of reported symptoms per day (minor or major) and differences in oxygenation and lung function. In this analysis, we used the same mixed model structure as in our primary analyses.
Results
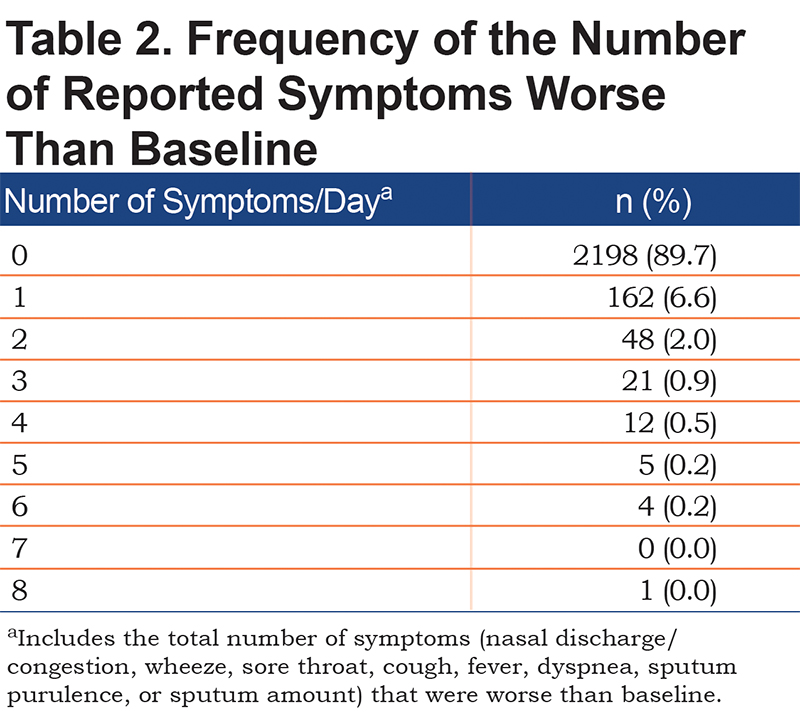
Baseline measures of the 26 study participants that were collected at the time of their respective study entry visit are presented in Table 1. There was a total of 2451 observations (days) with complete records of both spirometry and questionnaire data, with a mean of 94.3 observations per study participant. The distribution of the number of symptoms per day is displayed in Table 2. On 89.7% of observation days, there were no changes in baseline symptoms. On 6.6% of days, participants reported 1 respiratory symptom (nasal discharge/congestion, wheeze, sore throat, cough, fever, dyspnea, sputum purulence, or sputum amount) that was worse than baseline. On 2.0% of observation days, participants reported 2 of these symptoms were worse than baseline.
Associations between symptom days and oxygenation and lung function are shown in Table 3. There were 130 mild-to-moderate symptom days (5.3% of 2451), and 123 moderate-to-severe symptom days (5.0% of 2451). There was a total of 47 COPD exacerbation days (1.9% of 2451).
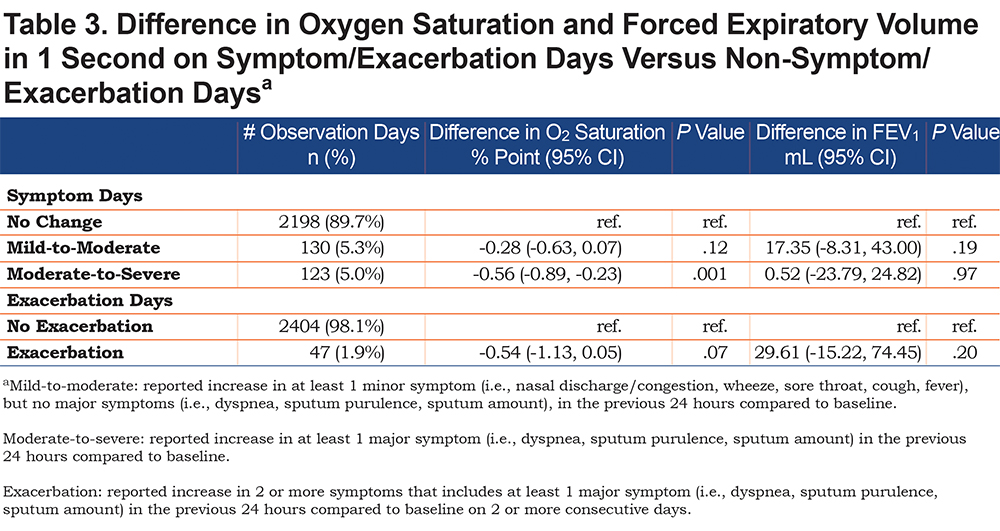
Of the 26 total participants, 9 reported any use of supplemental oxygen, and of these 9, a total of 4 used oxygen at rest. In fully adjusted linear mixed models using all participant data, mild-to-moderate symptom days were associated with a 0.28 percentage point lower oxygen saturation (95% CI -0.63 to 0.07, p=0.012) compared to non-symptom days. Moderate-to-severe symptom days were associated with a 0.56 percentage point lower oxygen saturation (95% CI -0.89 to -0.23, p=0.001) compared to non-symptom days. Exacerbation days were associated with a 0.54 percentage point lower oxygen saturation (95% CI -1.13 to 0.05, p=0.07) compared to days without a COPD exacerbation (Table 3). In the sensitivity analysis excluding the 4 participants on supplemental oxygen at rest, associations between symptoms and oxygenation were similar and slightly greater in magnitude than in the full cohort: oxygen saturation was 0.68 percentage points lower (95% CI -1.03 to -0.34) on moderate-to-severe symptom days compared to baseline (p=0.0001), and each respiratory symptom reported worse than baseline was associated with a 0.22 percentage point (95% CI -0.22 to -0.10) lower daily oxygen saturation (p = 0.0003).
We found no associations between self-reported worsening symptoms and FEV1 for all 3 classifications of symptom days (Table 3). In our analysis examining the association between the number of symptoms per day and differences in oxygenation and lung function, each additional respiratory symptom reported to be worse than baseline was associated with a 0.19 percentage point lower oxygen saturation (95% CI -0.31 to -0.07, p=0.002) per additional symptom. There was no association between the number of symptoms per day and lung function.
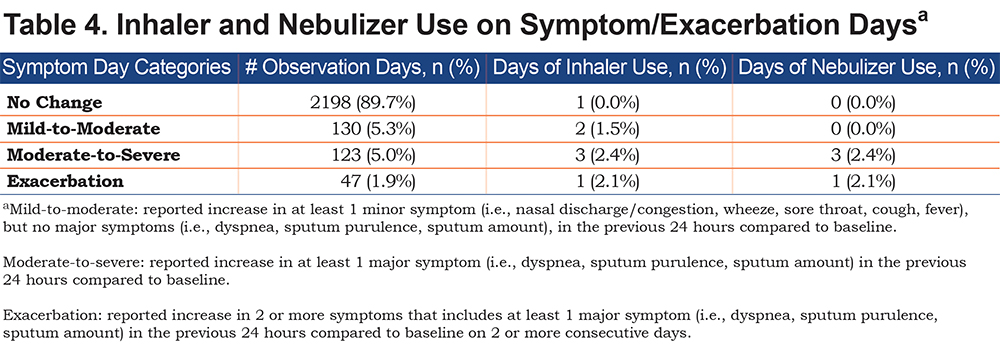
Table 4 displays the use of additional inhaler and/or nebulizer medication on self-reported symptom days. Despite the fact that all 26 (100%) of the study participants held an active prescription for rescue medication (i.e., short-acting beta agonist and/or short-acting muscarinic antagonist, via inhaler or nebulizer) at the time of enrollment, there were very few reports of increased inhaler or nebulizer use, with only 6 reports of increased inhaler use and 3 reports of increased nebulizer use out of 2451 observation-days. Eight of these 9 reported increases in inhaler or nebulizer use fell on symptom days. There were also few reports of oral prednisone and antibiotic use to treat symptom changes, with only 3 reports of each across the study period.
Discussion
This is one of few studies to examine daily changes in respiratory symptoms, oxygenation, lung function, and rescue inhaler/nebulizer use in moderate-to-severe COPD participants. We found that increases in self-reported respiratory symptoms were common and aligned with an objective measure of COPD disease activity (i.e., oxygenation). However, there was an extremely low (< 4%) utilization rate of rescue inhaler and nebulizer medication to self-treat these worsening symptoms.
To our knowledge, no previous studies have evaluated the relationship between worsening daily symptoms and changes in oxygenation in COPD patients. We found that each additional symptom was associated with a 0.19 percentage point lower oxygen saturation. Self-report of at least 1 moderate-to-severe symptom (defined as worsening dyspnea, sputum purulence or amount) was associated with a 0.56 percentage point lower average oxygen saturation, compared to days without any symptom increases. We observed a similar difference in oxygenation on exacerbation days compared to non-exacerbation days; however, this relationship had a wider confidence interval, likely due to limited power (there were only 47 observations meeting the questionnaire definition of a COPD exacerbation). We found a statistically robust relationship of lower average oxygen saturation and days with increased respiratory symptoms. The magnitude of this association (0.6% lower pulse oximetry on major symptom days, and 0.2% lower pulse oximetry per self-reported symptom) is modest, and unlikely to be clinically meaningful as it falls within the normal variation of oximeter. Rather, our findings provide evidence that subjective worsening of patient-reported respiratory symptoms are associated with an objective measure of worsened gas exchange and COPD disease activity. Our findings are consistent with other studies that have also found patient-reported COPD symptoms to be associated with objective health measures, such as FEV1% predicted, peak expiratory flow, and increased risk of clinically confirmed exacerbation.30-33
We found no association between worsening self-reported symptoms and lung function measured by FEV1. In contrast, previous studies using the same symptom survey tool observed declines in lung function as measured by peak expiratory flow rate, FEV1, and FVC after onset of COPD exacerbation (defined by consecutive days of minor and major symptoms), but did not evaluate associations with milder respiratory symptoms.25,27 Although there is evidence linking exacerbation frequency and moderate-to-severe exacerbation episodes to the rate of long-term decline in lung function in COPD,26,34,35 the literature on acute changes in lung function in association with COPD symptoms or exacerbations is limited.36-38
Perhaps the most striking finding of our study is that despite the common occurrence of worsening COPD symptoms, and the association of these symptoms with worsened oxygenation, study participants rarely increased their use of inhalers or nebulizers on symptom days. There is only limited evidence supporting the use of short-acting bronchodilators to improve lung function and symptoms (e.g.,breathlessness) for COPD,21 and these results have informed recommendations for initial pharmacological treatment in the GOLD document.19 While all of our study participants had access to short-acting bronchodilators for relief of acute respiratory symptoms, our results suggest that adherence to these recommendations in the outpatient setting is poor.
One possible explanation for this poor adherence issue could be a perception of little to no therapeutic benefit. It has been reported that COPD patients do not often feel their medication alleviates symptoms.39 Another possibility is that these short-acting inhalers are being overused at baseline and not as a step-up therapy to alleviate worsening symptoms. This has been observed in other studies. For example, a 2016 study of 32 participants with moderate-to-severe COPD found that almost half of participants overused short-acting beta2-agonists during clinically stable days.22 It is also possible that participants did not accurately report incremental inhaler or nebulizer use, or that they under-reported rescue treatment because the quantitative threshold for reporting increased use of rescue medication “above your usual treatment” was not well-defined. For example, a patient who usually reaches for her rescue inhaler 2 days per week may not report the days that she used her rescue inhaler as “above your usual treatment” because twice weekly use still falls within her usual pattern. Future work is needed to explore factors associated with over- and under-use of rescue medications in patients with COPD.
Our study has a number of limitations. Our findings are based on a small sample size of only 26 former smokers with COPD living in the Boston area who are followed at a tertiary academic institution and adherent to a complex study protocol involving daily lung function and electronic surveys. Therefore, our findings may not be generalizable to other COPD populations, including current smokers, followed in the community. Additionally, we relied on self-reported daily respiratory symptoms and treatment changes through an electronic questionnaire, and there is potential for reporting error by these elderly participants. Our work did not assess the effectiveness of rescue inhaler/nebulizer medication on treating daily COPD symptoms, reducing risk of exacerbation, or improving overall health outcomes. There are also some important strengths of our study. Our study is one of few to collect months of daily health measures in former smokers with moderate-to-severe COPD. Our repeated measures study design allowed us to uniquely evaluate day-to-day changes in respiratory symptoms, oxygenation, lung function, and self-treatment over many months among clinically stable COPD participants.
In conclusion, this study confirms that self-reported COPD symptoms are associated with worsening oxygenation, but also finds that symptoms do not prompt increased utilization of as-needed inhaler or nebulizer medication for symptom relief. Future research evaluating patient utilization, clinical effectiveness, and patient perception of rescue short-acting bronchodilators may identify the reasons for the apparent low utilization of rescue inhaler/nebulizer among patients with COPD.
Acknowledgements
Role of the sponsors: The funding source did not contribute to the study design, data analysis, or preparation of the final manuscript.
Data availability statement: Analytical plans and requests for study data can be sent to Dr. Mary Rice
Author contributions: WYS, CZ, and MBR designed the project. WYS was primarily responsible for data collection, analysis and interpretation, and drafting the manuscript. MBR provided supervision for the project, including data collection, analysis, interpretation, and writing of the manuscript. CZ, LAN and AJS contributed to the analysis, interpretation, and presentation of the data. BAC provided expertise in statistical methodology and development of the analytical plan. All authors contributed to the critical revision of the manuscript and approved of the final submitted version.
Other contributions: We would like to thank all the study participants for volunteering their time to research, and the clinical staff and nurses at Beth Israel Deaconess Medical Center who assisted in this research study.
Declaration of Interest
Dr. Rice is supported by the National Institute for Environmental Health Sciences (grant K23 ES026204). Dr. Coull is supported by the National Institute for Environmental Health Sciences (grant ES000002). Dr. Synn is supported by the National Heart, Lung, And Blood Institute (grant F32 HL143819). All other authors have no disclosures.