Running Head: Expanding Readmission Metrics in COPD
Funding Support: This work was funded by The Permanente Medical Group (Oakland, California). Dr. Camargo was supported by grant R01 HS-023305 from the Agency for Healthcare Research and Quality (Rockville, Maryland).
Date of Acceptance: August 14, 2020 ǀ Published Online: November 6, 2020
Abbreviations: chronic obstructive pulmonary disease, COPD; Hospital Readmissions Reduction Program, HRRP; Centers for Medicare and Medicaid Services, CMS; Comorbidity Point Score, version 2, COPS2; Laboratory-based Acute Physiology Score, version 2, LAPS2; skilled nursing facility, SNF
Citation: Myers LC, Camargo C, Escobar G, Liu VX. Expanding post-discharge readmission metrics in patients with chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2021; 8(1): 54-59. doi: http://doi.org/10.15326/jcopdf.2020.0160
Background
Chronic obstructive pulmonary disease (COPD) causes 700,000 hospitalizations per year at an annual cost of $18 billion.1,2 Approximately 20% of these hospitalizations result in rehospitalizations within 30 days.3,4 Under the Hospital Readmissions Reduction Program (HRRP),5 hospitals experience financial penalties for excess rehospitalizations occurring after index hospitalizations for various target conditions, including COPD.
Many have criticized how the Center for Medicare and Medicaid Services (CMS) counts rehospitalizations under the HRRP. For example, CMS does not penalize hospitalizations with “observation status” under the HRRP. Additionally, CMS does not treat death as a competing risk. In other words, patients who die before being rehospitalized are not counted in the metric. It is unclear whether adding either outcome would impact the raw counts of COPD patients counted in the quality metric.
We sought to determine how broadening the HRRP outcome definition would alter the numbers of patients being counted if patients were included who (1) were rehospitalized under observation status (not admission status) and/or (2) died in the 30-day post-discharge window.
Methods
Study Design and Data Source
We performed a retrospective cohort study of patients hospitalized for COPD and examined the events occurring in the 30-day post-discharge window. The goal was to determine if including events, such as observation hospitalizations and death, might alter the number of patients counted in the HRRP quality metric for COPD. We included adults (age≥18 years) hospitalized between July 1, 2010 and December 31, 2017 in 21 hospitals in the Kaiser Permanente Northern California system, an integrated health care system serving 4.4 million members with Medicare Advantage, Medi-Cal and/or the Kaiser Foundation Health Plan. Kaiser Permanente’s institutional review board approved this work (CN-17-2876_02). They provided a waiver of informed consent.
Cohort Formation
Index hospitalizations had COPD as the primary discharge diagnosis based on the Agency for Healthcare Research and Quality’s Clinical Classification Software (Hypergroup 4, Subcategory 127).6 We excluded patients with bronchiectasis codes (J470/J471/J479/494/4940/4941). Index hospitalizations could be inpatient or observation hospitalizations according to our group’s previous work.7 Given that observation stays and death are rare events, we allowed rehospitalizations to be index hospitalizations as well as outcome events, i.e., patients could contribute multiple index hospitalizations if the inclusion and exclusion criteria were met. This approach has been done previously and maximizes power.7
We required that patients survive index hospitalizations. We also required that patients either (1) be a health plan member for 30 days after discharge from index hospitalizations or (2) have terminal events (death) in the 30-day post period. These criteria ensured that patients were eligible for the outcomes described below and were not lost to follow-up. We did not require continuous membership in the year prior to the hospitalization in order to maximize sample size, and because turnover within the Kaiser Permanente Northern California health care system is limited.8 Patients transported into Kaiser hospitals for rehospitalizations were excluded because decisions to rehospitalize were made by outside institutions; these were rare (<6%).
Outcome Groups
We classified encounters into the following outcome groups based on data from the 30-day post-discharge follow-up period:
Group (1) non-elective, all-cause inpatient rehospitalizations, which is the current metric used in the HRRP3,5,9;
Group (2) composite outcome comprised of non-elective, all-cause inpatient rehospitalization or all-cause mortality;
Group (3) composite outcome comprised of non-elective, all-cause inpatient rehospitalization or non-elective all-cause observation rehospitalization.
We defined rehospitalizations as “non-elective” according to federal definitions put forth by CMS, which we have also used in previous work.7 Rehospitalizations that began as observation rehospitalizations and converted to inpatient rehospitalizations were considered inpatient rehospitalizations.
Statistical Methods
In order to find the best fitting curve of the cumulative distribution lines as a function of days, we used the Box-Cox method to find the transformation of the cumulative curves that resulted in the smallest mean standard error.10 This analysis showed that the best fitting curves were not linear when mortality or observation stays were included as an outcome. However, since the relationships between the transformed curve and days were linear, we used the slope of the transformed curve against days to test for significant differences between pairs of cumulative density curves for the various measures. We show the curves with 95% confidence intervals. Because we had separate hypotheses for adding mortality and observation stays, we did not adjust for multiple comparisons.
We performed the analysis at the encounter-level in order to understand the impact of expanding the definition of encounter-level outcome events, not hospital-level rates. We examined counts because we wanted to understand if the raw counts would change. We stratified the results before and after 2015, which was when COPD became a target condition in the HRRP.
We used the transreg procedure in SAS (version 9.4, Cary, North Carolina).11
Results
Of 1,384,025 hospitalizations, 11,304 encounters from 8097 patients met criteria to be index hospitalizations. Most index hospitalizations were inpatient hospitalizations (74.8%) versus observation hospitalizations (25.2%). We report the baseline characteristics of the cohort (Table 1). The mean age of patients was 73.5 years (95% CI 70.7–76.8). The cohort consisted of 19.8% current smokers and 66.9% previous smokers. The median Charlson score was 3 (interquartile range 3-4). The severity of illness at the time of hospitalization was Laboratory Acute Physiology Score, version 212,13 of 80.3 (95% CI 72.6–85.9). The mean length of stay was 4.1 days (95% CI 3.5–5.2 days). Most patients had advanced care directives of full code at the time of discharge (68.6%). Most patients were discharged home (74.6%) with only 3.6% referred to hospice.
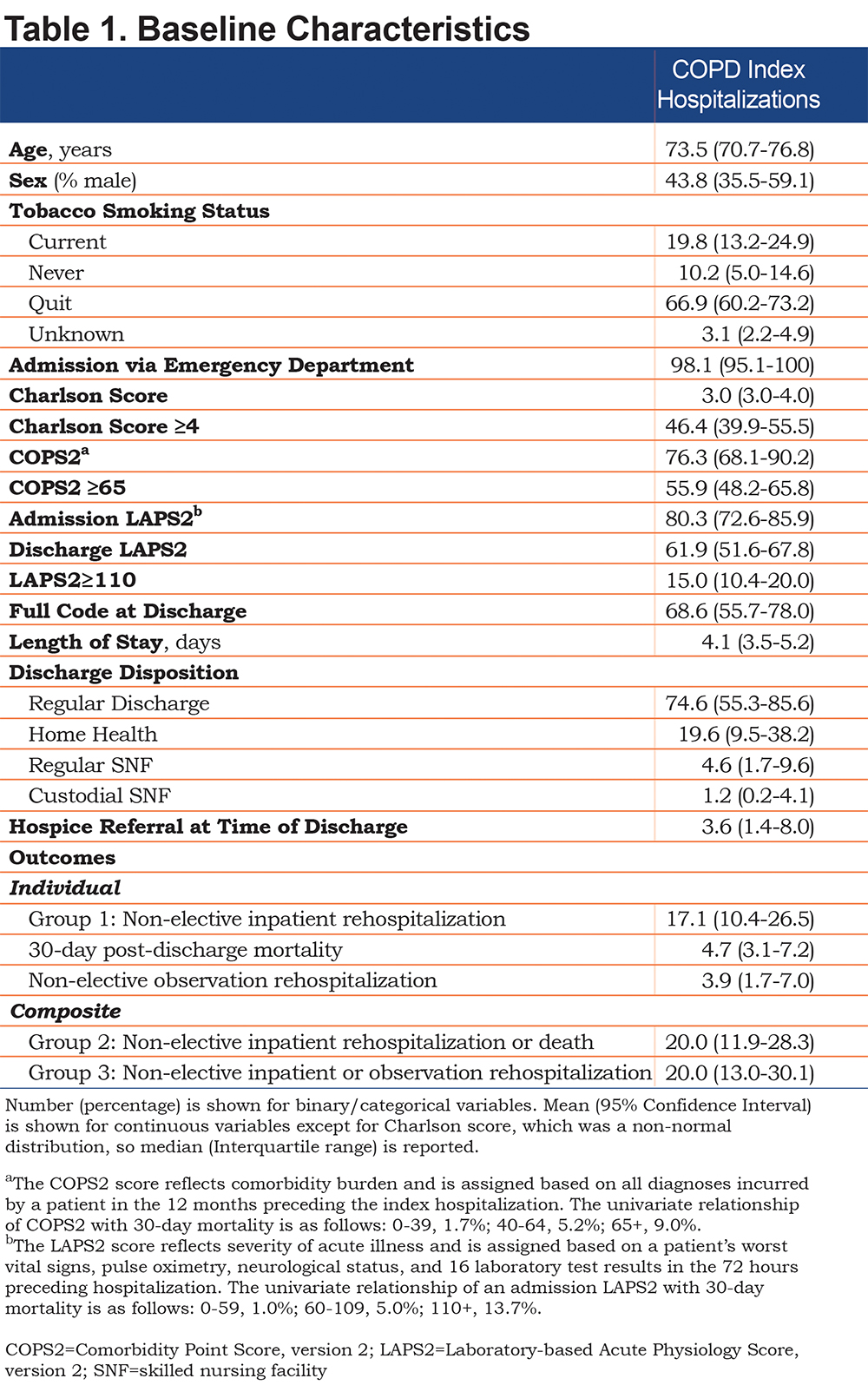
The individual and composite outcome rates are listed in Table 1. The event rate for non-elective, all-cause inpatient rehospitalizations (Group 1) was 17.1% (95% CI 10.4–26.5). The event rate for all-cause mortality was 4.7% (95% CI 3.1–7.7). The event rate for non-elective observation rehospitalizations was 3.9% (95% CI 1.7–7.0). The outcome rate in Group 2 was 20.0% (95% CI 11.9–28.3). The outcome rate in Group 3 was 20.0% (95% CI 13.0–30.1).
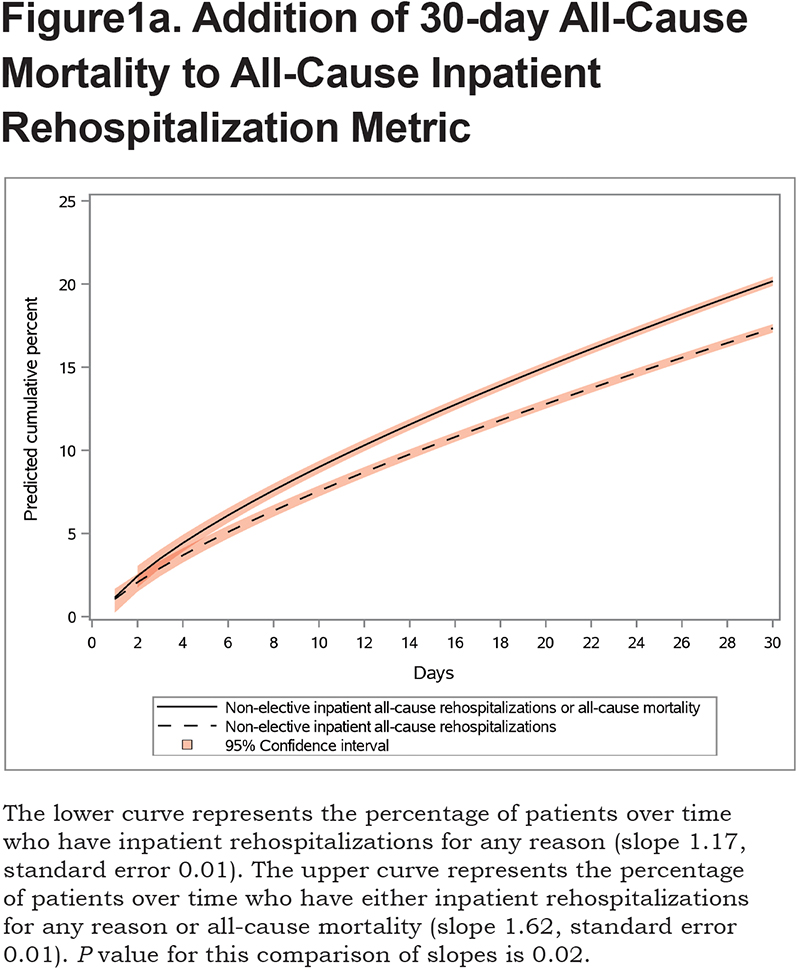
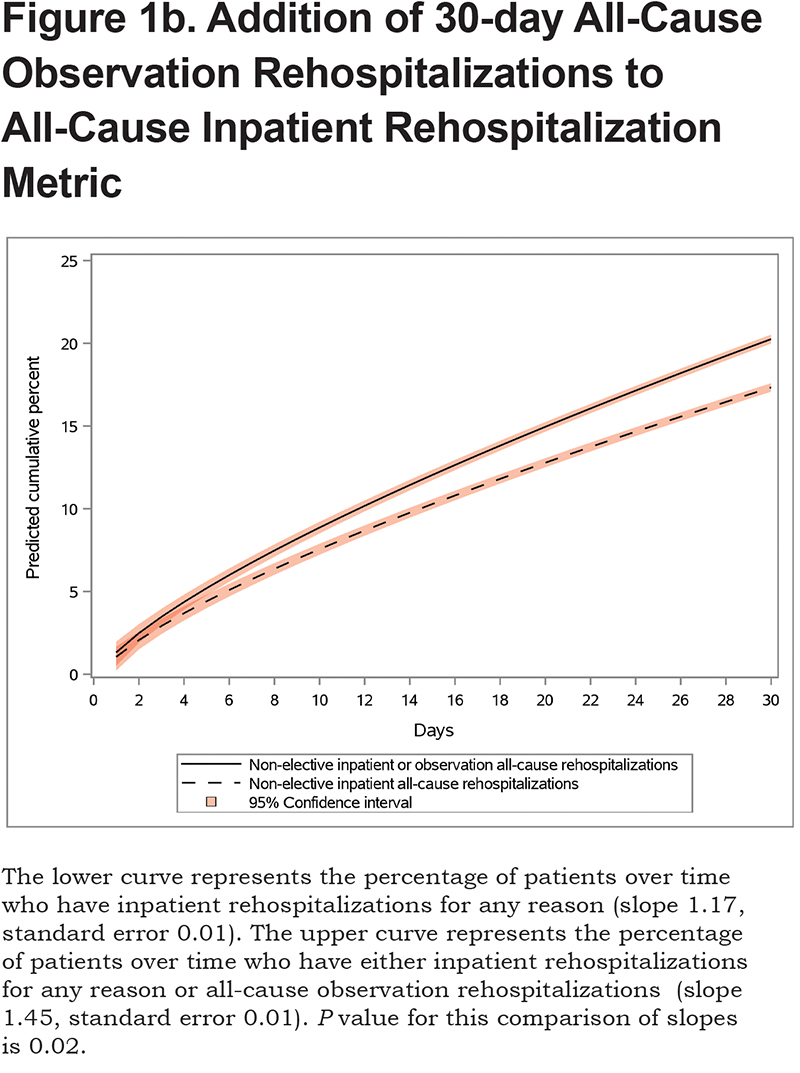
We show the differences between groups over the 30-day post-discharge period.First, we show the difference in groups depending on whether mortality was included in the outcome. The slope and standard error for Group 1 were 1.17 and 0.01, respectively, while the slope and standard error for Group 2 were 1.62 and 0.01, respectively (P=0.02 comparing Groups 1 and 2, Figure 1a). Next, weshow the difference in groups depending on whether observation rehospitalizations were included in the outcome. The slope and standard error for Group 3 were 1.45 and 0.01, respectively (P=0.02 comparing Groups 1 and 3, Figure 1b). The sensitivity analysis showed that these findings were consistent before and after 2015.
Discussion
We show that adding outcomes such as mortality and observation rehospitalizations would change the counts of patients meeting criteria for the HRRP metric for COPD. We believe this is an important finding for multiple reasons. First, the rates of mortality and observation rehospitalizations were low (<5%) yet including them would change the counts of patients flagged by the metric. Second, quality metrics used in programs such as the HRRP induce behavior change by incentivizing hospitals and providers. Knowing the effect of these incentives and whether changing the definitions of the outcomes might incentivize different behavior is critical for developing effective reimbursement policies. Including mortality or observation stays in the outcome of interest could incentivize hospitals and providers to focus on preventing these events too.
As previously mentioned, CMS does not penalize hospitalizations recorded as observation status under the HRRP. There is controversy as to whether observation hospitalizations have changed in response to the policy for any of the target conditions. Some evidence suggests that they did increase regardless of condition treated in the index stay,7 while other evidence using Medicare data shows the opposite.14 Additionally, CMS does not treat death as a competing risk. In other words, patients who die before being rehospitalized are not counted in the metric. There is controversy about whether this adversely incentivizes hospitals not to readmit patients, even if they are at high risk of death. Some evidence actually suggests an association between increased mortality and HRRP for patients with heart failure and pneumonia,15 but another study shows that death rates are low for heart failure and pneumonia and randomly distributed among hospital types.16 Neither outcome has been assessed in COPD, which prompted this study.
The study has limitations and strengths. First, we examined patients during an 8-year period, during which CMS implemented the policy, but findings were similar before and after COPD became a target condition. Second, Kaiser Permanente Northern California serves a small number of fee-for-service Medicare patients but a large number of Medicare Advantage patients. We felt that these results were applicable to the Medicare population being counted in the current HRRP metric, given that mean age was approximately 74 years, and the all-cause non-elective inpatient rehospitalization rate was consistent with other reports examining COPD readmissions (approximately 18%-20%).4,17 Using our data as opposed to administrative data allowed us to include granular, longitudinal data elements, like smoking status, referral to hospice and lab-based severity of illness score to describe the population being studied.
In summary, the counts of patients change significantly if the HRRP quality metric for COPD includes patients who died or were rehospitalized under observation status, despite the outcome rates for these 2 outcomes being low. Further research is needed to understand how hospital-level risk adjusted rates for Medicare patients would change if the metric were to be expanded and whether restricting the measurement to cause-specific rehospitalizations (respiratory-related, for example) would change the behavior of hospitals and providers to focus on more specific, respiratory-related prevention strategies.
Acknowledgments
The authors wish to acknowledge Colleen Plimier, MPH who did the data extraction and Patricia Kipnis, PhD who consulted on the statistical analysis.
Author contributions: LCM designed the study, conducted the analysis, drafted the manuscript and revised the manuscript in response to feedback. CC designed the study and interpreted the results. GE provided institutional review board approval, oversaw data extraction and interpreted the results. VXL designed the study, interpreted the results and critically revised the manuscript. All authors contributed to the intellectual content of the article and gave final approval of the version being published.
Declaration of Interest
The authors do not have any conflicts of interest or disclosures.