Running Head: COPD Triple Therapy in Western Europe/North America
Funding Support: This study was funded by GlaxoSmithKline (GSK) (study number CTT116855; NCT02164513). The funders of the study had a role in the study design, data analysis, data interpretation, and writing of the report. Editorial support (in the form of writing assistance, assembling figures, collating author comments, grammatical editing and referencing) was provided by Chrystelle Rasamison, Fishawack Indicia Ltd, United Kingdom, and was funded by GSK.
Date of Acceptance: August 21, 2020 ǀ Published online: November 5, 2020
Abbreviations: InforMing the Pathway of COPD Treatment, IMPACT; fluticasone furoate, FF; umeclidinium, UMEC; vilanterol, VI; chronic obstructive pulmonary disease, COPD; St George’s Respiratory Questionnaire, SGRQ; Global initiative for chronic Obstructive Lung Disease, GOLD; COPD Assessment Test, CAT; forced expiratory volume in 1 second, FEV1; adverse events, AEs; serious AEs, SAEs; AEs of special interest, AESI; intent-to-treat, ITT; standard deviation, SD; body mass index, BMI; inhaled corticosteroids, ICSs; long-acting beta2-agonists, LABAs; long-acting muscarinic antagonists, LAMAs; Standardized Medical Dictionary for Regulatory Activities Query, SMQ; bone mineral density, BMD; diabetes mellitus, DM; lower respiratory tract infection, LRTI
Citation: Bourdin A, Criner G, Devouassoux G, et al. Informing the pathway of COPD treatment (IMPACT) single-inhaler triple therapy (fluticasone furoate/umeclidinium/vilanterol) versus fluticasone furoate/vilanterol and umeclidinium/vilanterol in patients with COPD: analysis of the Western Europe and North America regions. Chronic Obstr Pulm Dis. 2021; 8(1): 76-90. doi: http://doi.org/10.15326/jcopdf.2020.0158
Online Supplemental Material: Read Online Supplemental Material (300KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is a lung disease characterized by airflow limitation and progressive respiratory symptoms.1 Global public health trends estimate that the COPD burden will continue to rise, with COPD deaths estimated to increase to 4.4% of all deaths in Europe and 6.3% in the World Health Organization-defined region of the Americas by 2060.2 There are differences in the COPD burden in different regions reflecting variations in etiology,3,4 disease severity,5 symptoms,6 medication use,7 and health care systems and utilization.7 These differences may help inform therapeutic strategies to optimize therapeutic approaches to reducing symptoms and exacerbation risk.1
In the global InforMing the PAthway of COPD Treatment (IMPACT) trial, single-inhaler triple therapy fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) reduced moderate/severe exacerbation rates and improved lung function and health-related quality of life versus FF/VI or UMEC/VI dual therapy in patients ≥40 years of age with symptomatic COPD and a history of exacerbations.8 Within trial populations, regional differences such as patient characteristics, treatment patterns, access to care and cultural/socioeconomic factors may dictate treatment choices and influence disease severity and progression in particular geographical locations. For example, a meta-analysis conducted in 2015 comprising 123 studies between 1990 and 2010 found that the overall prevalence of COPD as well as the rate of increase was higher in the Americas (including both North and South America) compared with Europe.9 Furthermore, a cross-sectional study assessing the burden of COPD symptoms in the United States and Europe found variations between patients across countries who had experienced at least 1 symptom of COPD.10 In Europe, patients with more frequent symptoms were more likely to experience worsening of symptoms and unexpected hospitalization. Whereas in the United States, patients with more frequent symptoms were not only more likely to experience worsening of symptoms but also longer lasting symptoms and a longer length of exacerbations.10 A further difference was that treatment adherence was higher in the United States than Europe, however, adherence was consistent across patients in Europe when assessed by modified Global initiative for chronic Obstructive Lung Disease (GOLD) 2014 groups11 but varied in the United States with adherence highest in the GOLD Group C and lowest in Group A.10 Therefore, it is important to evaluate how overall population results pertain to patients treated in particular regions. As IMPACT is one of the largest trials conducted in patients with COPD to date, we have the unique opportunity to analyze study outcomes in patients enrolled in Western Europe and North America, the 2 main regions from an enrollment perspective.
Materials and Methods
Study Design and Patients
IMPACT (GSK Study CTT116855; NCT02164513) was a 52-week, randomized, double-blind, parallel-group, Phase 3 trial conducted in 37 countries.8 The trial design has been previously described.8,12 Briefly, eligible patients with COPD were ≥40 years of age, symptomatic (COPD Assessment Test [CAT] score ≥10), and had a forced expiratory volume in 1 second (FEV1) <50% predicted and ≥1 moderate/severe exacerbation in the preceding year, or FEV1 50%–<80% predicted and ≥2 moderate or ≥1 severe exacerbation(s) in the preceding year. Patients were randomized (2:2:1) to once-daily FF/UMEC/VI 100/62.5/25µg, FF/VI 100/25µg, or UMEC/VI 62.5/25µg administered via the ELLIPTA dry powder inhaler. Patients continued their existing COPD medications during a 2-week run-in period and were provided with as-needed salbutamol (rescue medication). All patients provided written informed consent. The trial was conducted in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki and received approval from local institutional review boards and independent ethics committees.
Endpoints, Assessments, and Data Analysis
The primary endpoint was the annual rate of on-treatment moderate/severe exacerbations with FF/UMEC/VI versus FF/VI and UMEC/VI. Other efficacy endpoints included time-to-first moderate/severe exacerbation, change from baseline in trough FEV1 and St George’s Respiratory Questionnaire (SGRQ) total score at Week 52, and proportion of SGRQ responders (≥4 unit decrease from baseline in SGRQ total score) at Week 52. Treatment by region interaction for SGRQ total score, moderate/severe exacerbation rate and trough FEV1 was assessed. Moderate exacerbations were events requiring treatment with antibiotics and/or oral/systemic corticosteroids. Severe exacerbations were events resulting in hospitalization or death. The incidence of on-treatment adverse events (AEs), serious AEs (SAEs), AEs of special interest (AESI), and mortality was also assessed. In this post hoc analysis, outcomes were evaluated in the subgroups of patients enrolled in North America (United States [including Puerto Rico], Canada) and Western Europe (pre-defined prior to unblinding as the European Economic Area and included Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Netherlands, Norway, Poland, Romania, Spain, Sweden, United Kingdom). Details of sample size calculations for the intent-to-treat (ITT) population have been described previously.8,12 The trial was not powered for subgroup analysis by region. The ITT population included all randomized patients, except those randomized in error. The North America and Western Europe subgroups were derived from the ITT population. Statistical analyses are described in the online supplement.
Results
Patients
Of 10,355 patients in the ITT population, 3164 (31%) were enrolled in Western Europe (1252 to FF/UMEC/VI, 1274 to FF/VI, 638 to UMEC/VI) and 2639 (25%) in North America (1071 to FF/UMEC/VI, 1046 to FF/VI, 522 to UMEC/VI). Baseline characteristics for each region (all treatments combined), are shown in Table 1 and were similar between the 3 treatment groups within each population (Supplementary Table 1 in the online supplement).
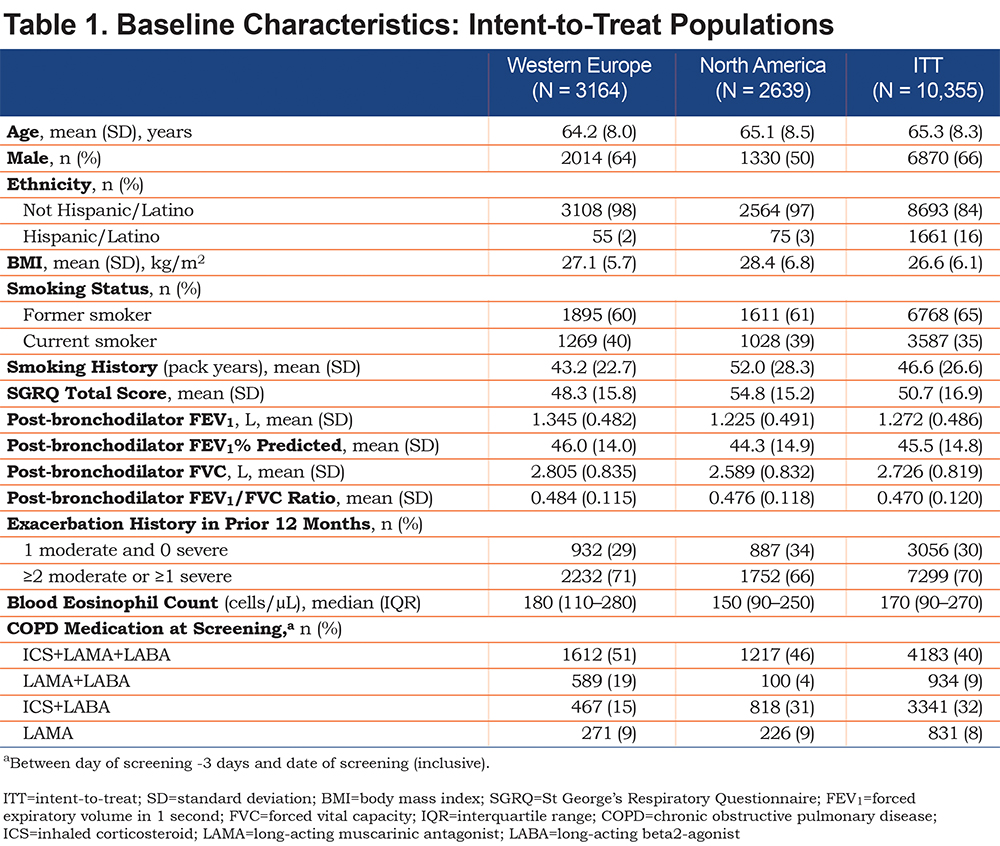
There were some between-region differences in baseline characteristics, notably a lower proportion of males in North America (50%) than in Western Europe (64%) and the ITT population (66%), a higher mean number of smoking pack years in North America (52.0) than in the ITT population (46.6) and Western Europe (43.2), a higher baseline SGRQ total score in North America (54.8) than in Western Europe (48.3) or the ITT population (50.2), and a lower proportion of patients experiencing ≥2 moderate or ≥1 severe exacerbation in the prior year in North America (66%) than in Western Europe (71%) and the ITT population (70%). The proportion of patients on an inhaled corticosteroid (ICS)+long-acting beta2-agonist (LABA)+long-acting muscarinic antagonist (LAMA) triple therapy at screening was higher in Western Europe (51%) than in North America (46%) or the ITT population (40%), as was the proportion on LAMA+LABA at screening (19% versus 4% and 9%, respectively), while fewer patients were on ICS+LABA at screening in Western Europe (15%) than in North America (31%) or the ITT population (32%). Most patients had blood eosinophil levels ≥100 cells/µL in all populations (Figure 1). A lower proportion of patients had blood eosinophil counts <100 cells/µL or <300 cells/µL in Western Europe (18% and 76%, respectively) than North in America (28% and 82%, respectively) or the ITT population (25% and 78%, respectively). Baseline blood eosinophil counts by country are given in Supplementary Table 2 (mean), and Supplementary Figure 1 and Supplementary Figure 2 (distribution) in the online supplement.
On-treatment Moderate/Severe Exacerbations
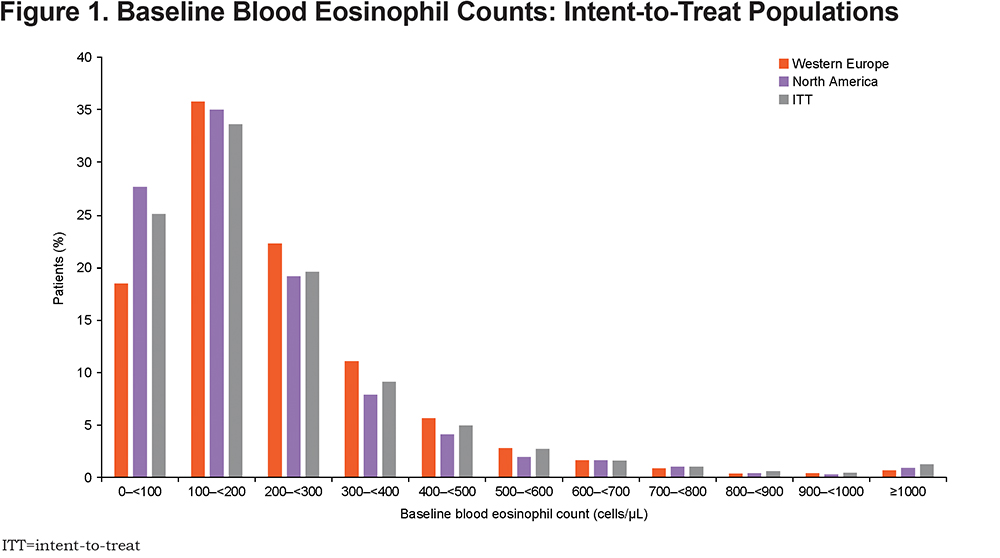
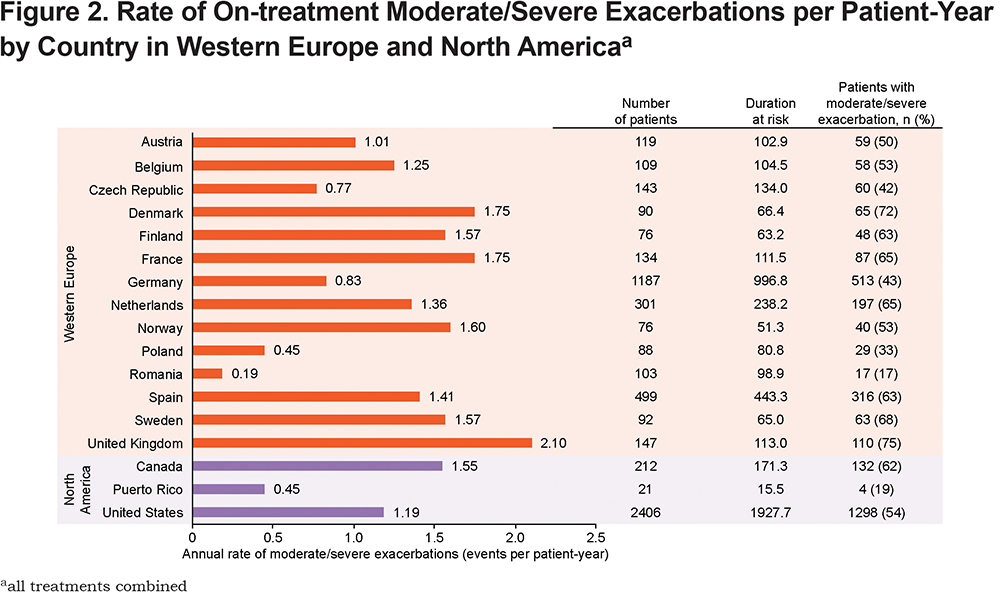
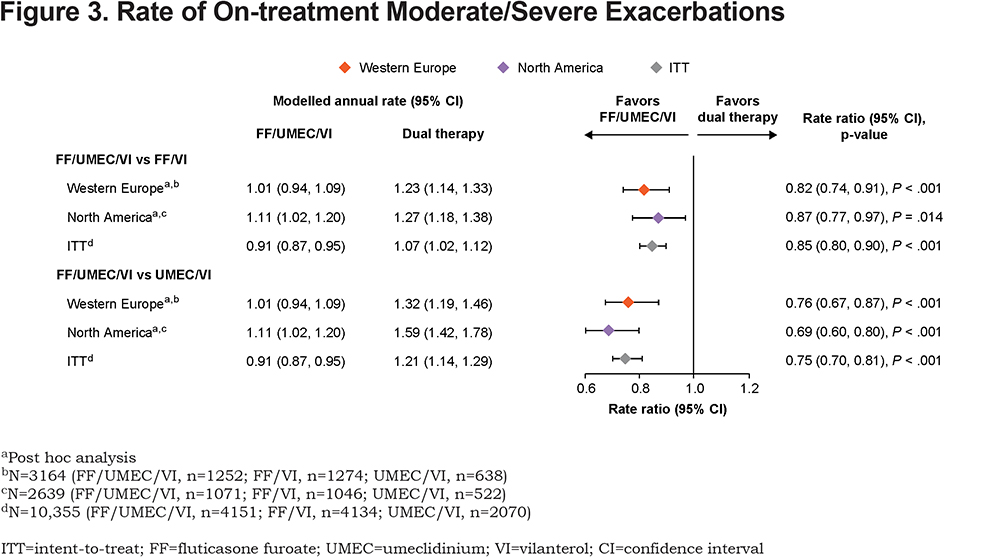
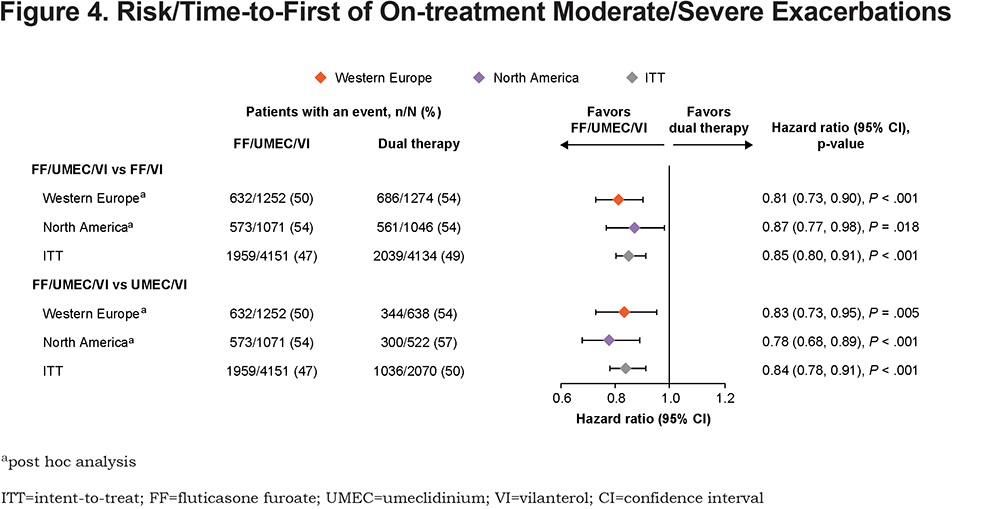
In Western Europe, moderate/severe exacerbation rates were highest in the United Kingdom, followed by France and Denmark, and lowest in Romania and Poland (Figure 2). In North America, rates were higher in Canada (Figure 2). FF/UMEC/VI significantly reduced moderate/severe exacerbation rate and risk (time-to-first) versus either dual therapy in both regions, consistent with ITT results (Figure 3 and Figure 4). Rate and risk reduction with FF/UMEC/VI versus FF/VI were numerically greater in Western Europe than in North America or the ITT population, whereas for FF/UMEC/VI versus UMEC/VI, it was numerically greater in North America than in Western Europe or the ITT population (Figure 3 and 4). There was no significant interaction between treatment and region for this endpoint (Supplementary Table 3 in the online supplement).
Trough Forced Expiratory Volume in 1 Second
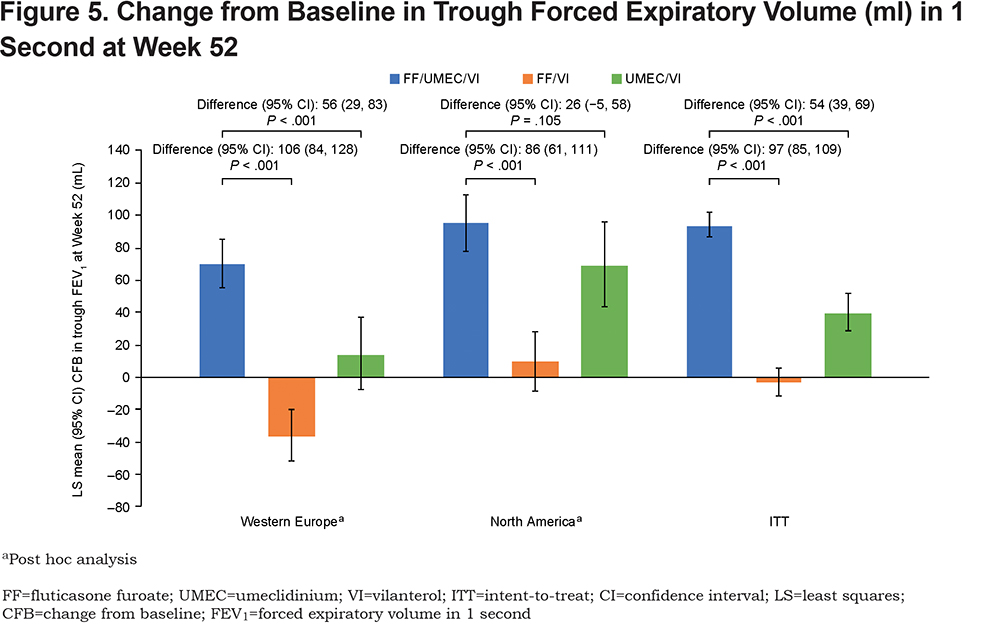
Consistent with ITT results, FF/UMEC/VI significantly increased trough FEV1 at Week 52 versus FF/VI in both regions, with numerically greater between-treatment increases in Western Europe than in North America or the ITT population (Figure 5). FF/UMEC/VI significantly increased trough FEV1 versus UMEC/VI in Western Europe and the ITT population. The point estimate favored FF/UMEC/VI over UMEC/VI in North America but was not statistically significant (Figure 5). Improvement in trough FEV1 with FF/UMEC/VI versus UMEC/VI was numerically greater in Western Europe than in North America, and similar between Western Europe and the ITT population (Figure 5). There was no significant interaction between treatment and region for this endpoint (Supplementary Table 3 in the online supplement).
SGRQ Total Score
All treatments improved SGRQ total score in both regions and in the ITT population, but statistically significant improvements were only demonstrated with FF/UMEC/VI versus FF/VI and UMEC/VI in Western Europe and the ITT population (Figure 6A). The magnitude of improvement from baseline in SGRQ total score with FF/UMEC/VI was greatest the ITT population and smallest in Western Europe. Patients receiving either dual therapy regimen in Western Europe also experienced the smallest improvement in SGRQ total score compared with patients in North America and the ITT population. However, between-treatment differences for FF/UMEC/VI versus both dual therapies were numerically greater in Western Europe than in North America or the ITT population (Figure 6A). There was evidence of an overall treatment difference for SGRQ total score between regions (overall P=.054), which was mainly driven by the comparison between FF/UMEC/VI and FF/VI (P=.018) (Supplementary Table 3 in the online supplement).
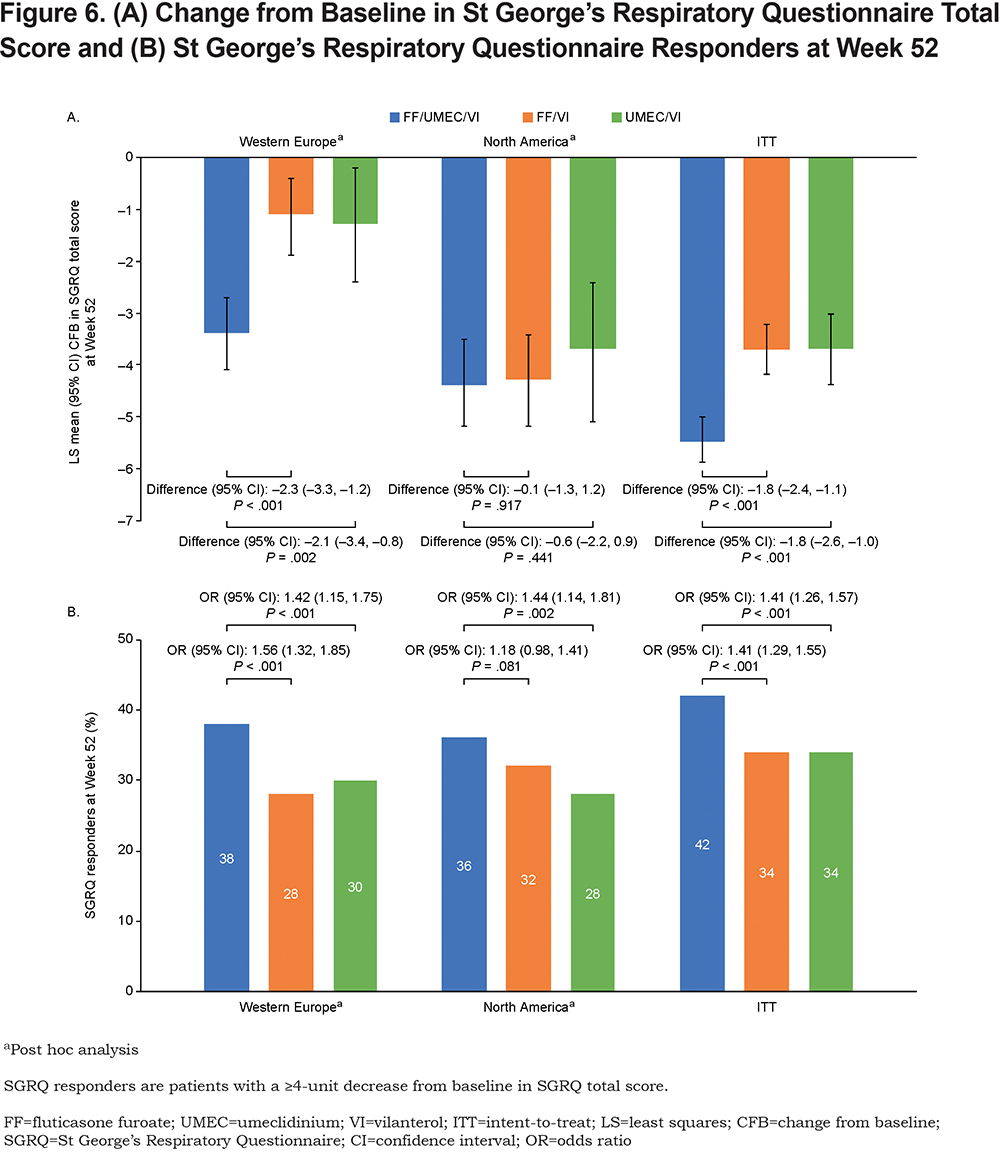
The proportion of SGRQ responders at Week 52 was significantly higher with FF/UMEC/VI than FF/VI or UMEC/VI in Western Europe and the ITT population. In North America, statistically significant differences were seen with FF/UMEC/VI versus UMEC/VI (P=.002) but not FF/VI (P=.081) (Figure 6B).
Safety
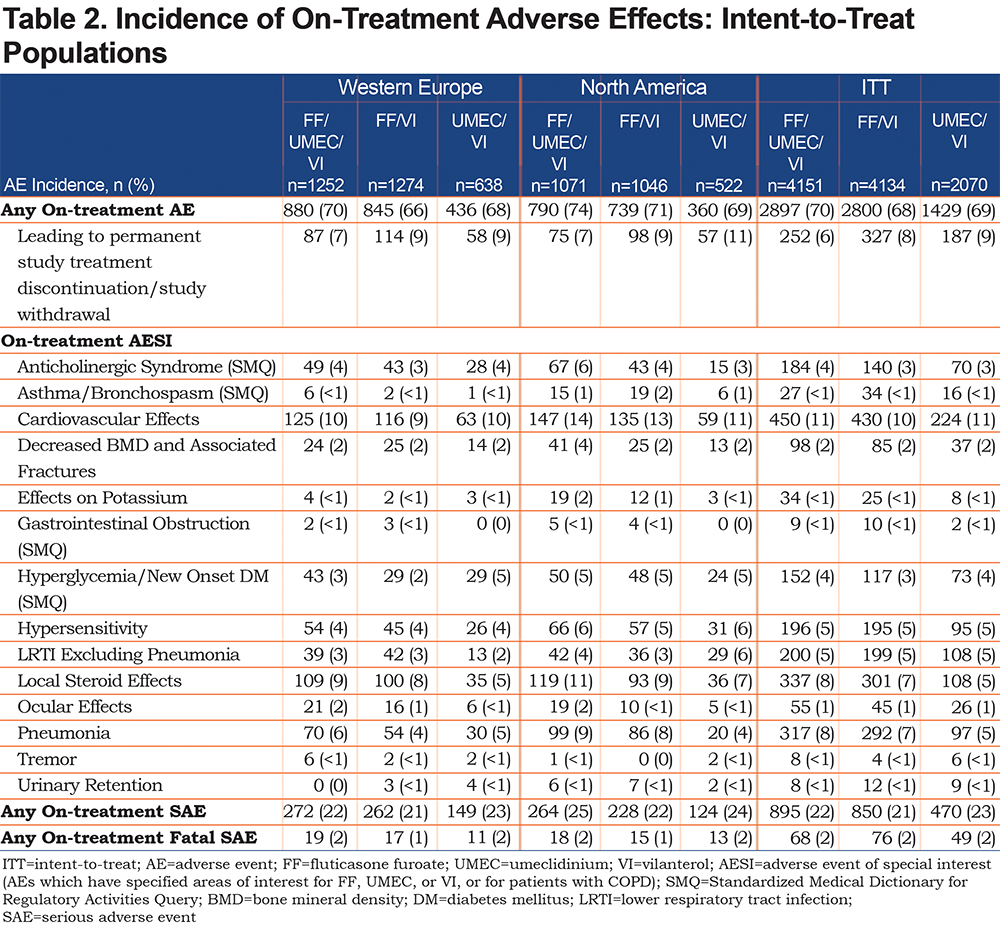
In both regions, the overall AE profile of FF/UMEC/VI was broadly similar to that of FF/VI and UMEC/VI. However, while pneumonia AESI incidence was higher in ICS-containing arms compared with UMEC/VI in North America and the ITT population, this was not seen in Western Europe where incidences were similar across all treatment arms (Table 2). Incidence of SAEs and fatal SAEs of pneumonia was low (≤5% and <1%, respectively), with no difference between treatment groups and across regions.
Discussion
The IMPACT trial demonstrated the superiority of once-daily single-inhaler FF/UMEC/VI triple therapy over FF/VI or UMEC/VI dual therapy in reducing on-treatment moderate/severe exacerbation rates in a global population of patients with symptomatic COPD and a history of exacerbations.8 Results from this geographical analysis in Western Europe and North America were broadly consistent with the benefits shown in the overall ITT population, and reductions in moderate/severe exacerbation rate and risk and improvements in lung function and health status were seen with FF/UMEC/VI compared with FF/VI or UMEC/VI. The safety profile of all treatments in both regions was generally in line with that in the ITT population. As expected, based on the class effect for ICS,13 the incidence of pneumonia was higher in ICS-containing arms compared with UMEC/VI in North America and the ITT population; interestingly this was not seen in Western Europe.
Studies have highlighted the burden of COPD in Western Europe and North America, revealing considerable variation across countries in patient characteristics, patterns of disease severity, symptoms, medication availability, access, and health care utilization.10,14,15 These differences could impact the efficacy of COPD therapies in different populations. In this analysis, improvements in the rate and risk of moderate/severe exacerbations, trough FEV1 and SGRQ responders with FF/UMEC/VI compared with UMEC/VI and FF/VI in both regions were generally of a similar magnitude to those in the ITT population. FF/UMEC/VI significantly increased trough FEV1 at Week 52 versus FF/VI in both regions; while a numerically greater between-treatment increase was seen in Western Europe compared with in North America and the ITT population, this likely reflects the worsening of lung function in the FF/VI group in Western Europe rather than an increase in efficacy with FF/UMEC/VI. Statistically significant improvements in the SGRQ total score were demonstrated with FF/UMEC/VI versus FF/VI and UMEC/VI in Western Europe and the ITT population but not in North America. The interaction term indicated an overall treatment difference between regions for this endpoint, mainly driven by the comparison between FF/UMEC/VI and FF/VI. Reasons for this are unknown but it is worth noting that the baseline SGRQ total score was higher in the North American region compared with the Western Europe and ITT populations, indicating worse health status and greater changes from baseline were seen in all treatment groups in North America compared with Western Europe. The proportion of SGRQ responders at Week 52 was consistent across all regions for each treatment group; however, the difference between FF/UMEC/VI and FF/VI in North America was not statistically significant.
Baseline characteristics were similar across the 2 regions and the ITT population, with a few exceptions. The median blood eosinophil count in North America was lower than in Western Europe and the ITT population, and the mean number of smoking pack years was higher. However, the percentage of patients with blood eosinophil counts <100 cell/µL was lower in Western Europe than in North America. Given the association between cigarette smoking and reduced ICS sensitivity,16 along with improved ICS sensitivity in patients with higher eosinophil levels,17 patients in North America may have been expected to have slightly lower sensitivity to ICS-containing therapies based on their baseline characteristics. However, this was not reflected in the treatment effect of FF/UMEC/VI versus UMEC/VI and FF/VI on moderate/severe exacerbations. Differences in baseline treatment were noted according to region prior to randomization. The proportion of patients on LAMA+LABA therapy at screening was higher in Western Europe (19%) than in North America (4%), while the proportion on ICS+LABA or ICS+LAMA+LABA at screening was higher in North America (77%) than in Western Europe (66%). This may indicate different therapeutic requirements for patients enrolled in North America, which may explain why patients in this region appeared more responsive to FF than patients in Western Europe. Nevertheless, these differences in baseline treatment across regions did not appear to affect the treatment effect of FF/UMEC/VI versus either dual therapy.
There were large between-country variations in moderate/severe exacerbation rates, from 0.19 per patient-year in Romania to 2.10 per patient-year in the United Kingdom. These may be due to differences in patient demographics and clinical characteristics between countries and highlight the potential difficulties in performing cross-trial comparisons unless correction for baseline demographics can be performed. When interpreting these between-country differences, it is worth noting that while the individual patient time at risk was broadly similar between countries, there was a large range of total duration at risk across countries, reflecting the varying sample sizes. As greater duration at risk would give more precision to the point estimates for annual rates of moderate/severe exacerbation, results in countries of small sample size need to be interpreted with caution.
Other studies have evaluated single-inhaler triple therapy versus dual or monotherapies;18-21 however, regional analyses have not been reported. The large sample size and global scope of the IMPACT trial allows for a robust comparison across different regions and the ITT population. Differences in national and international guidelines for COPD exist, including differences in treatment recommendations, potentially leading to regional differences in patient care.1,22 It is important to understand these similarities and differences and how they may potentially affect patient management and inform future guideline development both globally and nationally. While the distribution and prevalence of COPD in different geographic regions has been well-studied,23,24 a large-scale comparative assessment of how treatment efficacy can vary by region has not been previously described. Results in these 2 IMPACT regional subpopulations were consistent with the overall study population and demonstrate the favorable benefit-risk profile of single-inhaler FF/UMEC/VI triple therapy over FF/VI or UMEC/VI dual therapy in patients with symptomatic COPD and a history of exacerbations. However, it should be noted that these analyses are descriptive and were conducted post hoc, and the study was not powered to demonstrate statistical significance for any endpoints by or between regions.
Conclusions
In this regional analysis of the IMPACT trial, FF/UMEC/VI significantly reduced the rate and risk of moderate/severe exacerbations versus FF/VI and UMEC/VI in both the Western Europe and North America regions. Treatment responses were similar with respect to exacerbations and lung function for both regions and the ITT population. However, there were some differences in SGRQ total score between regions, with no differential effect observed between FF/UMEC/VI and dual therapies in North America, unlike in Western Europe and the ITT population. Safety profiles with FF/UMEC/VI, UMEC/VI and FF/VI were similar in both regions and the ITT population although the ICS class effect of increased pneumonia incidence was seen in North America and the ITT population, but not in Western Europe. These efficacy and safety results in patients with symptomatic COPD and a history of exacerbations continue to support a positive benefit-risk profile with FF/UMEC/VI.
Acknowledgements
Author contributions: The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors, take responsibility for the integrity of the work as a whole, contributed to the writing and reviewing of the manuscript, and have given final approval for the version to be published. All authors had full access to the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. A Bourdin, G Criner, G Devouassoux, M Dransfield, DMG Halpin, JM Echave-Sustaeta María-Tomé, NA Hanania, R Kalhan, and TM Siler contributed to the acquisition of data and data analysis and interpretation. MK Han, CE Jones, P Lange, S Lettis, DA Lomas, N Martin, FJ Martinez, H Quasny, L Sail, D Singh, B Thomashow, H Watz and R Wise contributed to data analysis and interpretation. D.A. Lipson contributed to study conception and design, and data analysis and interpretation.
Data availability:
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Declaration of Interests
Editorial support (in the form of writing assistance, assembling figures, collating author comments, grammatical editing and referencing) was provided by Chrystelle Rasamison, Fishawack Indicia Ltd, United Kingdom, and was funded by GSK.
A Bourdin was an investigator in the IMPACT trial and has received personal fees and a grant from Boehringer Ingelheim, personal fees from AstraZeneca, Chiesi, GSK, Novartis and Sanofi-Regeneron, and is an investigator in clinical trials from Nuvaira, Pulmonx, Actelion, MSD, United Therapeutic, Vertex, Acceleron, and Galapagos. G Criner has received personal fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, CSA Medical, Eolo, GSK, HGE Technologies, Novartis, Nuvaira, Olympus, Pulmonx, and Verona. G Devouassoux has received personal fees from AstraZeneca, Novartis Pharma, GSK, Chiesi, and Boehringer Ingelheim, and contracted clinical trial support from AstraZeneca, GSK, Novartis Pharma, ALK, Chiesi, and Boehringer Ingelheim. M Dransfield has received personal fees from Boehringer Ingelheim, PneumRx/BTG, Genentech, Quark Pharmaceuticals, Mereo, AstraZeneca and GSK, a grant from the Department of Defense, and contracted clinical trial support from PneumRx/BTG, Novartis, AstraZeneca, Yungjin, Pulmonx, Boston Scientific, Boehringer Ingelheim, and GSK. DMG Halpin reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, and Pfizer, and non-financial support Boehringer Ingelheim and Novartis. MK Han has received personal fees from AstraZeneca, Boehringer Ingelheim, Merck, Mylan and GSK and research support from Novartis and Sunovion. CE Jones, S Lettis, DA Lipson, N Martin, H Quasny, and L Sail are GSK employees and hold stock/shares in GSK. R Kalhan reports grants from the National Heart, Lung, and Blood Institute during the conduct of the study, grants and personal fees from Boehringer Ingelheim, grants from PneumRx (BTG), grants from Spiration, grants and personal fees from AstraZeneca, personal fees from CVS Caremark, personal fees from Aptus Health, grants and personal fees from GSK, personal fees from Boston Scientific, and personal fees from Boston Consulting Group. P Lange reports personal fees from GSK, AstraZeneca, Chiesi and Boehringer Ingelheim, and grant support from Boehringer Ingelheim.
DA Lomas reports personal fees from GSK and Grifols; he chaired the GSK Respiratory Therapy Area Board in 2012–2015. JM Echave-Sustaeta María-Tomé has received personal fees from GSK and was an investigator in the IMPACT trial. FJ Martinez has received personal fees and non-financial support from, AstraZeneca, Boehringer Ingelheim, Genentech, GSK, Inova Fairfax Health System, Miller Communications, National Society for Continuing Education, Novartis, Pearl Pharmaceuticals, PeerView Communications, Prime Communications, Puerto Rico Respiratory Society, Chiesi, Sunovion, Theravance, Potomac, University of Alabama-Birmingham, Physicians Education Resource, Canadian Respiratory Network, Teva and Dartmouth; and personal fees from Columbia University, MD Magazine, Methodist Hospital Brooklyn, New York University, UpToDate, WebMD/MedScape, Patara/Respivant, the American Thoracic Society, Rockpointe, Rare Disease Healthcare Communications and the France Foundation, and has taken part in advisory boards for AstraZeneca, Boehringer Ingelheim, ProterrixBio, Genentech, Novartis, Pearl Pharmaceuticals, Theravance, Zambon, Gala, Chiesi, GSK, Sunovion, and Teva, steering committees for AstraZeneca, Pearl Pharmaceuticals, Afferent/Merck, Gilead, Nitto, Patara/Respivant, Biogen, Veracyte, Prometic, Bayer, ProMedior and GSK, and been an advisor for Bridge Biotherapeutics. TM Siler has received research grants from AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Compleware, Evidera (PPD), Forest Research Institute (now AstraZeneca), GSK, Novartis, Pearl Therapeutics, Proterix BioPharma, Oncocyte, Sanofi, Seer, Sunovion, Teva, Theravance BioPharma, Vapotherm, Restorbio and Westward, and personal fees from GSK, Sunovion, Theravance Biopharma and Vapotherm. D Singh reports personal fees from GSK, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Genentech, Glenmark, Menarini, Mundipharma, Novartis, Peptinnovate, Pfizer, Pulmatrix, Theravance, and Verona, and grant support from AstraZeneca, Boehringer Ingelheim, Chiesi, Glenmark, Menarini, Mundipharma, Novartis, Pfizer, Pulmatrix, Theravance and Verona. B Thomashow has taken part in advisory boards for AstraZeneca and GSK. H Watz reports personal fees from AstraZeneca, Boehringer Ingelheim, BerlinChemie, Chiesi, GSK, Novartis, Takeda, and Verona Pharma. R Wise reports personal fees from AstraZeneca/Medimmune/Pearl, Boehringer Ingelheim, Contrafect, Pulmonx, Roche, Spiration, Sunovion, Circassia, Pneuma, Verona, Mylan/Theravance, Propeller Health, AbbVie GSK, Merck, Kiniksa and Galderma, and has received research grants from AstraZeneca/MedImmune/Pearl, Boehringer Ingelheim, Pearl Therapeutics, Sanofi-Aventis and GSK. NA Hanania was an investigator on the IMPACT trial and reports receiving personal fees from GSK, AstraZeneca, Boehringer Ingelheim, Sanofi Genzyme, Novartis, Regeneron, Genentech, Sunovion, and Mylan. He also received research support from GSK, Boehringer Ingelheim, Sanofi Genzyme, Novartis and Astra Zeneca. ELLIPTA is owned by or licensed to the GSK Group of Companies.