Running Head: Spirometry Can Mask COPD/Emphysema in Smokers
Funding Support: None
Date of acceptance: October 22, 2020 | Published online: December 11, 2020
Abbreviations: forced expiratory volume in 1 second, FEV1; lower limit of normal, LLN; computed tomography, CT; forced expiratory flow, FEF; maximal expiratory flow, MEF; residual volume, RV; American Thoracic Society, ATS; European Respiratory Society, ERS; SubPopulations and InteRmediate Outcome Measures In COPD, SPIROMICS; COPD Assessment Test, CAT; high resolution computed tomography, HRCT; National Health and Nutrition Examination Survey, NHANES; diffusing capacity corrected for alveolar volume, DLCO/VA(L); maximal mid-expiratory airflow, MMEF; total lung capacity, TLC; specific airway conductance, sGAW; maximal expiratory flow-volume, MEFV
Citation: Gelb AF, Yamamoto A, Verbeken EK, et al. Normal routine spirometry can mask COPD and emphysema in symptomatic smokers. Chronic Obstr Pulm Dis. 2021; 8(1): 124-134. doi: http://doi.org/10.15326/jcopdf.2020.0176
Online Supplemental Material: Read Online Supplemental Material (96KB)
Introduction
Normal and preserved routine spirometry can mask chronic obstructive pulmonary disease (COPD) and emphysema in symptomatic smokers who have abnormal expiratory airflow at forced expiratory flow(FEF) at 75% of the forced vital capacity (FEF75). Guidelines for the initial detection of COPD, developed by the American College of Physicians, American College of Chest Physicians, American Thoracic Society(ATS), and the European Respiratory Society (ERS), all recommend the presence of respiratory symptoms.1 Furthermore, there has been increasing interest in earlier detection of COPD in symptomatic smokers.2-6 Woodruff et al2 reported results from the SubPopulations and InteRmediate Outcome Measures In COPD (SPIROMICS) study that approximately 50% of former or current symptomatic smokers with COPD, and an abnormal COPD Assessment Test7 (CAT) >10 had “preserved pulmonary function.” This was based on post-bronchodilator forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio ≥70% and FVC (L)>80% predicted, and both also above the lower limit of normal (>LLN). Therapeutic intervention during the 3 months before enrollment in the study noted 42% having used inhaled bronchodilators and 23% inhaled glucocorticoids. It was reported that 33% of the cohort had chronic bronchitis based on CAT,7 43% had been previously diagnosed with COPD, and 27% with asthma.2 The prospective rate of exacerbation was also higher among these individuals compared to both healthy nonsmokers, and current and former smokers with preserved spirometry and lower CAT scores.7 Thin section lung high resolution computed tomography (HRCT) was consistent with minimal if any emphysema, but mild thickening of airway walls were noted by Martinez et al.2,4 In a similar COPD Genetic Epidemiology study, Regan et al5 reported a higher incidence of emphysema in lung HRCT in symptomatic smokers, also with similar “preserved pulmonary function” studies as noted above.2,4 Bowler et al6 also emphasized increased COPD exacerbations in an outcome study from a similar cohort. The designation of normal or “preserved” spirometry including FEV1 (L), FVC (L) and FEV1/FVC% ignores the potential presence of isolated abnormal expiratory airflow limitation at lower lung volumes. This is consistent with intrinsic obstruction in small airways without cartilage, upstream from 10-12 divisions, with normal diameter ≤2.0mm, and /or emphysema. These physiologic observations have been recently emphasized by Hoesterey et al.8 The goal of the current study was to confirm our previous observations that detection of small airways obstruction and/or emphysema, in symptomatic smokers despite normal routine spirometry, requires analysis of expiratory airflow at low lung volumes, including FEF75.
Materials and Methods
Study Population
We initially selected 16 symptomatic patients over 18 months, and 5 were subsequently seen over 2-5 years. All 16 patients, post 270µg inhaled aerosolized albuterol sulfate, had initial spirometry values greater than predicted LLN and FEV1(L)≥80% predicted in 15 cases, and FVC(L)≥80% predicted and FEV1/FVC≥70% in all 16 Cases. They were originally referred for further evaluation and management of suspected COPD, including either small airways obstruction and/or emphysema. They had variable complaints of shortness of breath, cough, wheezing, chest tightness, and CAT7 scores >10. Seven of the 16 patients also had a non-calcified lung nodule <12mm diameter detected on lung HRCT. There was no evidence for cardiac decompensation or other comorbidities and smoking history was from 30-75 pack years. Six of 16 patients were current smokers when initially seen and subsequently quit within 4 months. These 16 symptomatic patients were started on varying combinations of inhaled corticosteroids, short- and long-acting beta2-agonists, and/or muscarinic receptor antagonists for relief of symptoms. Pulmonary function studies were performed consistent with ATS/ERS recommendations.9 Predicted normal values included: spirometry before and after 270µg inhaled aerosolized albuterol sulfate administered using a space chamber from Morris et al,10 maximal inspiratory and expiratory flow-volume curves for FEF at 50% and 75% of FVC from Knudson et al,11 National Health and Nutrition Examination Survey (NHANES) III routine spirometry data from Hankinson et al,12 single breath diffusing capacity from Burrows et al,13 and static lung volumes measured by volume plethysmography from Goldman et al.14 Normal data for specific airway conductance was obtained from Zarins et al.15 Whole-lung thoracic HRCT without IV contrast was obtained at peak inspiration in all 16 cases, and technically included high-resolution, thin-section (1mm) lung slices, 0.8mm slice spacing, Toshiba standard kernel FC 18, sharp kernel 52, and pitch 0.8-1.0, and Siemens sharp kernel B35.
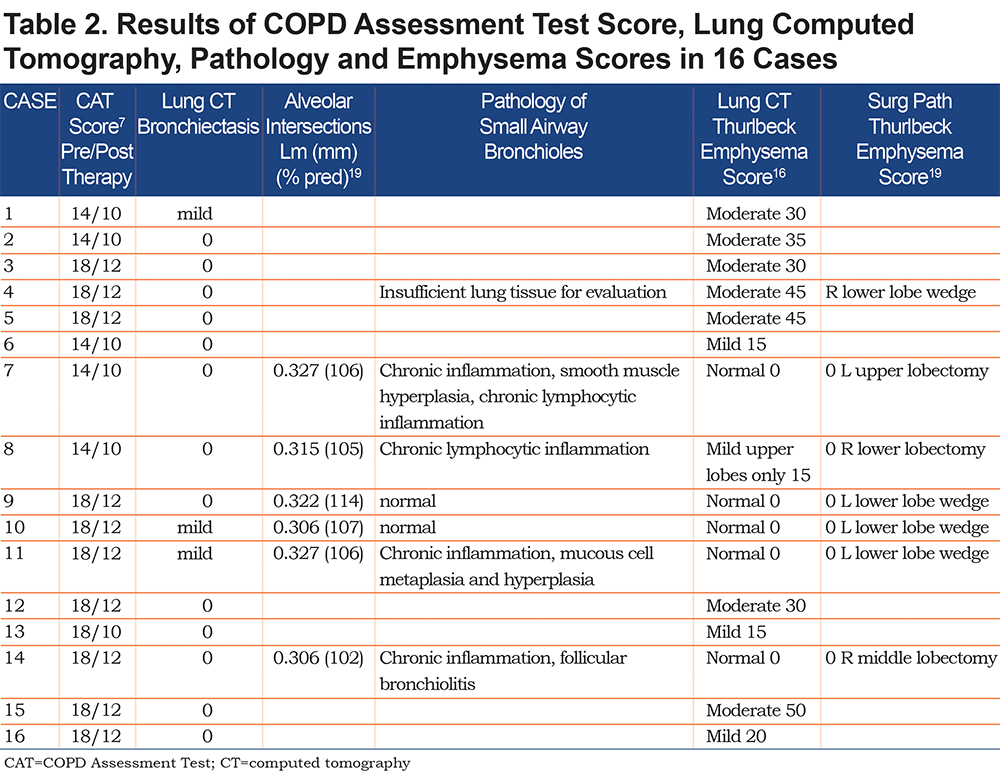
All 7 patients with suspicious non-calcified lung nodules underwent surgical resection for suspected lung cancer. One patient (case 4) underwent wedge resection right lower lobe, 3 patients (cases 9-11) underwent wedge resection of the left lower lobe, 1 patient (case 7) underwent lobar resection of the left upper lung, 1 patient (case 8) underwent a right lower lobectomy and 1 patient (case 14) underwent a right middle lobectomy. This provided formalin non-inflated lung specimens for potential limited pathologic identification of extent of emphysema and small airways disease and for correlation with whole-lung CT in 6 of 7 cases. Inadequate lung tissue prevented emphysema scoring in Case 4.
In determining the extent of whole-lung emphysema on inspiratory lung HRCT, we divided both the right and left lungs into 3 zones each (total 6 zones). The upper zone is above the carina, the mid zone extends from the carina to the inferior pulmonary veins and the lower zone extends further below. We have relied on the classic Thurlbeck et al air inflated lung standards for scoring extent and severity of emphysema as adopted for lung CT,16 and based on our correlative structure-function studies.17,18 If the zonal extent of emphysema was 1%-25%, it was considered minimal to mild, 26%-50% moderate, 51%-75% severe, and >75% very severe. The HRCT whole-lung emphysema score was determined by author MJS based on our previous studies.17,18 The microscopic distance between 100 to 107 alveolar walls (Lm) was measured in 6 surgical cases by author EKV. Predicted mean value for Lm=0.0011 X age (year)+0.2174 and normal value to 120% predicted exclude emphysema.19 Current data in this study has not been previously reported and informed consent was obtained from every patient included in the present study. The study was approved by the Western Institutional Review Board, Olympia, Washington, (Protocol 20172049; NCT Registration Number 00576069).
Results
Study Population
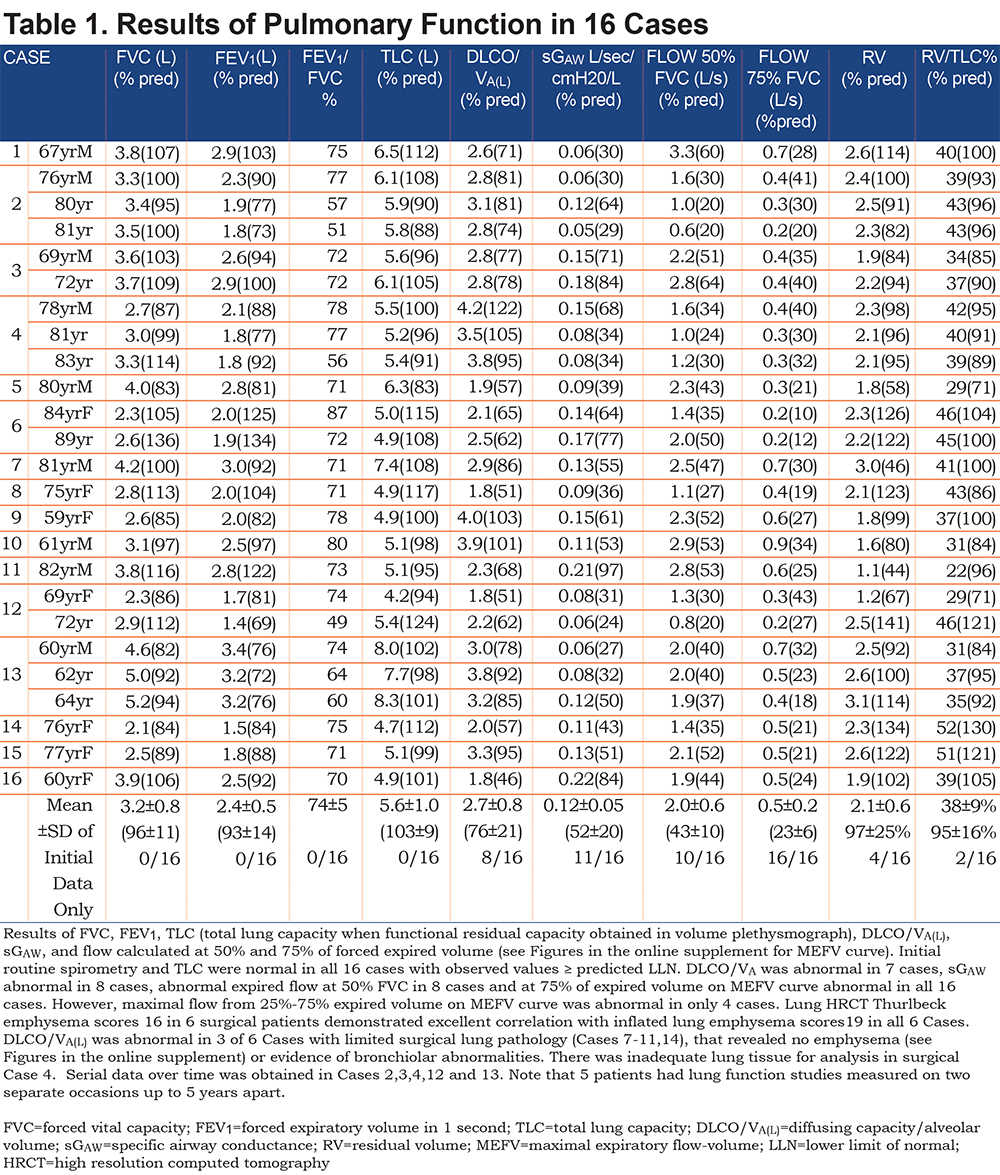
In the Tables 1-2 and in Figures 1-16 (in the online supplement), are results of pulmonary function studies post inhaled 270µg albuterol and lung HRCT in 16 cases, and lung pathology in 6 cases. The FVC was >80% predicted in every case and the ratio ofFEV1/FVC≥0.70%in all 16 cases. FEV1 was >80% predicted in 15 cases but > than LLN in all. Functional residual capacity and calculated total lung capacity (TLC) were all normal. In 8 of 16 cases, the diffusing capacity corrected for alveolar volume (DLCO/VA(L)) was abnormal, implying there was loss of integrity of the alveolar capillary surface area as in emphysema. Specific airway conductance was abnormal <60% predicted in 11 of 16 cases.15 Maximal expiratory airflow (MEF) at 50% expired FVC was abnormal in 10 of 16 cases, whereas at 75% expired FVC, FEF75 was abnormal in all 16 cases, according to Knudsen et al,11 NHANES III, Hankinson et al,12 and Quanjer et al20 predicted mean minus 1.64SD (see Figures 1-16 in the online supplement which correspond to Cases 1-16 in Table 1 and Table 2). Maximal mid-expiratory airflow (MMEF25-75) was abnormal in only 4 of 16 cases,11,12 increased residual volume (RV) in 4 cases, and RV/TLC% in only 2 cases. Whole- lung CT emphysema scoring as noted in the tables and the figures (in the online supplement) in 9 non-surgical resection cases ranged from mild in 4 cases, to moderate in 5 cases, with predominance in mid to upper lung zones. Whole lung HRCT in 7 surgical resection patients (cases 4, 7-11, and 14) suggested no emphysema in Cases 7, 9, 10, 11, and 14, and mild emphysema only in the upper zone in Case 8, and moderate emphysema in Case 4 based on our previous correlative studies.17,18 Moreover, in Case 8, lung resection RLL pathology confirmed the absence of emphysema, despite lung CT-scored mild emphysema, which was limited to upper lung zones. Inadequate lung tissue prevented pathologic emphysema scoring in Case 4. Corresponding lung pathology not shown was consistent with Stage 1 lung malignancy in all 7 surgically resected cases. There was nosignificant breakdown of alveolar attachments in lung tissue surrounding terminal bronchioles in the 6 patients studied (figures in the online supplement and Cases 7-11, and 14). Furthermore, the distance between 100-107 alveolar walls (Lm) was normal in every surgical case. In Case 7 Lm=0.327mm 106% predicted, in Case 8 Lm=0.315mm 105% predicted, in Case 9 Lm=0.322mm 114% predicted, in Case 10 Lm=0.306mm 107% predicted, in Case 11 Lm=0.327mm 106% predicted and in Case 14 Lm=0.306 102% predicted. Emphysema was excluded microscopically in all 6 surgical cases evaluated. Bronchioles were morphologically within normal limits, and luminal diameters remained intact and comparable to luminal diameters of accompanying pulmonary arteries.
Discussion
The 16 cases of symptomatic smokers presented here provides further examples of patients with apparently “preserved spirometry” but symptoms consistent with COPD, as well as HRCT lung evidence of emphysema in 11 of the 16 cases. All had normal FEV1, FEV1/FVC%, and FVC. However, none of the patients had truly “preserved lung function,” as indicated by the presence of variable abnormally low values of FEF rates at low lung volumes in all 16 cases. This also included a significant reduction in specific airway conductance (sGAW) in 11 of the 16 cases, and a significantly reduced DLCO/VA(L) in 8 of the cases. Interestingly, despite significant reductions in FEF75 in all 16 cases, MMEF25-75 was abnormal in only a quarter of the cases. Eleven of the 16 cases had mild to moderate predominantly upper zone emphysema by systematic visual assessment.16-18 The absence of HRCT evidence of an emphysema score was confirmed by pathologic examination of inflated resected lung specimens in all 6 patients who underwent lobectomy or wedge resection and provided adequate material for pathology. The 5 patients without HRCT lung evidence of emphysema most likely had small airways disease without parenchymal involvement, although the HRCT lung images from these patients could not be systematically evaluated for evidence of small airways disease. Furthermore, lung HRCT images were obtained at TLC and not at RV.
Overview: Historical and Current Perspective
In an accompanying editorial referring to Woodruff et al,2 Fabbri21 reiterated that patients with normal FVC, FEV1 and FEV1/FVC% do not fulfill the definition of COPD, by current COPD guidelines (GOLD).22 Moreover, Fabbri21 emphasized that COPD may be a disease of the “lungs quiet zone,” as originally defined by Woolcock et al.23 This view was later expanded upon in an editorial by Mead24 almost 50 years ago: “a place where there can be pathologic changes that are not detected by changes in the FEV1”.The editorial by Mead24 was in affirmation of 2 original articles identifying the pathophysiology of small airway disease.25,26 Sentinel pathophysiologic studies by Macklem and Mead27 demonstrated that the resistance offered by the peripheral, small airways without cartilage, upstream from 10-12 divisions, and ≤2.0mm id, normally contributed <20% of total airway resistance. Moreover, Mead24 and Hogg et al28 reported that in the presence of COPD, with or without emphysema, the distal quiet small bronchiolar zone was the major site of increased airway resistance. These studies were performed in post-mortem lung specimens, when the larynx was excluded.27,28 When all upper airways resistance is included, the contribution of small airways resistance to total airways resistance is probably ≤̇ 15%. This paradigm could be further obscured by normal expiratory spirometry measurements consistent with “preserved pulmonary function” as previously reported.2-6
In the early 1970`s, mentored by Jay A. Nadel, we published several sentinel pathophysiologic translational observations to help facilitate earlier detection of both subclinical emphysema29,30 and intrinsic small airway disease, including asthma in non-smokers.29-31 Despite the presence of normal routine spirometry, the physiologic diagnosis of suspected moderate-to-severe emphysema, was proven by pathology of resected lobe(s) for malignant lung nodules after air or formalin inflation.29,30 Furthermore, additional physiologic abnormalities obtained prior to lung surgery in suspected emphysema included the combination of abnormal diffusing capacity, loss of static lung elastic recoil, and abnormal expiratory airflow limited to the distal part of the maximal expiratory flow-volume (MEFV) curve at 80% expired FVC. However, mid-expiratory airflow rates, airway resistance and TLC remained normal.29-31
The MEFV curve was developed by Fry and Hyatt32 and was obtained simultaneously during volume-time spirometry. We emphasized the reproducibility of analyzing expiratory flow at 75% and 80% of expired FVC.29-31 Furthermore, intrinsic small airway abnormalities in non-smoking asthmatics with bronchiolar obstruction were similarly identified using MEFV curves, despite normal static lung elastic recoil, normal diffusing capacity, and normal routine spirometry.28-31 Abnormal expiratory airflow in the distal part of the MEFV curve, namely FEF75was as sensitive as abnormal closing volume, extent of heliox isoflow,33 frequency dependence of dynamic lung compliance, and increased arterial-alveolar oxygen gradient.29-33 It should be further emphasized that expiratory airflow at any effort independent lung volume is directly related to the static lung elastic recoil pressure and inversely to the intrinsic airway resistance.34,35 When static lung elastic recoil pressure and expiratory flow are measured at identical effort independent lung volumes, it is relatively easy to determine the individual contribution of loss of lung elastic recoil versus peripheral intrinsic airway obstruction in limiting expiratory airflow.29-31,34,35 The structure-function studies by Cosio et al36 subsequently confirmed our initial physiologic observations in smokers with normal or borderline spirometry and diffusing capacity, and minimal or no emphysema. They could be separated from normal individuals by exhibiting an abnormal MEFV curve at mid lung volume, abnormal closing capacity, volume of isoflow, and phase 3 of the single-breath nitrogen washout.36 However, in the Cosio et al36 study, MEFV curves were not analyzed at low lung volumes. In a subsequent pathophysiology study, Hogg et al37 reported that smokers with GOLD Stage 0 with normal spirometry and GOLD Stage 1 with mild expiratory airflow limitation FEV1/FVC ≤70% and FEV1≥80% predicted, had mild thickening of the walls of small airways and accumulation of inflammatory mucous exudates in their lumen. In a more recent pathological study, McDonough et al38 reported that narrowing and disappearance of small conducting airways and destruction of terminal bronchioles occurred before the onset of overt emphysematous destruction and was probably responsible for the increased peripheral airway resistance in COPD. In a sentinel pathophysiologic study, Hogg et al39 reported that 10 of 54 (18.5%) chronic smokers with localized lung cancer, despite FEV1 100%-110% predicted, and normal static lung volumes, had diffusing capacity 81%±18% predicted (mean±SD), and maximal mid-expiratory flow MMEF25-75 80%±33% predicted, and Thurlbeck emphysema score 23±13 (mean ± SD) in resected lobes or lungs.15,19 When the FEV1 was 90%-99% predicted, the prevalence of emphysema using Thurlbeck score 22 ± 9 was 33% predicted, and diffusing capacity was 102%±34% predicted, and MMEF25-75 66%± 24% predicted.39 When the FEV1 was between 80%-89% predicted, the prevalence of emphysema score 25±14 was 33% and diffusing capacity was 83%±26% predicted and MMEF25-75 59%±23% predicted.39 Tan et al40 have recently reported that 11% of life-time never-smokers demonstrated emphysema on CT scans, and the prevalence increased to 30% among smokers despite normal lung function. Furthermore, Hogg, Pare, and Hackett have provided the interested reader with an in-depth review of the contribution of small airway obstruction to the pathogenesis of COPD and emphysema.41
Obstruction of small, peripheral airways <2mm id is the major site of pathophysiology in COPD.23-28,41 Because peripheral resistance is normally small, there may be considerable intrinsic obstruction in peripheral airways and/or emphysema that could affect ventilation distribution and gas exchange but would have little effect on lung function tests designed to detect expiratory airflow obstruction. When total airway resistance is elevated to a clinically recognized level in the small airways, obstruction is much more severe than is generally recognized.24,31,41 The NHANES III data reported by Stanojevic et al42 noted that median value for FEV1/FVC% in healthy, non-smoking males, 40 years of age was 80% and lower limit of normal was 70%, corresponding to 50th percentile predicted. The median value for FEV1/FVC% in healthy males 60 years of age was 75% and LLN was 65%, corresponding to 50th percentile predicted.42 The median value for FEV1/FVC% in healthy males 80 years of age was 72% and lower limit of normal was 57%, also corresponding to 50th percentile predicted.42 Values for FEV1/FVC% in healthy females compared to males at the same age, were slightly greater by 2%.42 Moreover, these data emphasize the limitations of using FEV1/FVC <70% to identify potential expiratory airway obstruction in smokers 60 years of age and older.42 We also have emphasized that when the FEV1/FVC is equal or greater than 75%, the MMEF25-75 is always normal.43 However, if MMEF25-75 is reduced, the FEV1/FVC% is always less than 75%, although not always outside predicted limits.43 Detection of mild COPD in presence of a normal FEV1/FVC, using novel isolated spirometry reductions including FEV3/FVC, FEV3/FEV6 and FEV1/FEV6 have been proposed.44,45 However, in our 16 cases, only 3 cases could be identified as abnormal using these novel spirometry measurements.44,45 A normal diffusing capacity may be misleading, and not always detect mild to moderate emphysema observed on thoracic HRCT, as noted in 3 cases in the present study. We have previously noted normal diffusing capacity in never smoked, chronic asthmatics with persistent expiratory airflow limitation and measured loss of lung elastic recoil with a thoracic HRCT scan consistent with normal or mild emphysema.46 Subsequent autopsy and/or explanted whole lung when formalin is inflated confirmed mild to moderate emphysema.46
The current challenge is to more easily detect COPD, emphysema, and/or intrinsic small airway obstruction masked by otherwise “preservedpulmonary function,” i.e., normal routine spirometry. This challenge is important in symptomatic smokers,2-6,29,30 and in symptomatic non-smoking asthmatics,31,46 and can be achieved by analyzing expiratory flow at low lung volumes on the MEFV curve29-31 using applicable reference values.10-12,42 However, it is important for the reader to acknowledge the 2017 ATS Technical Statement with recommendations for standardized pulmonary function studies.47 The original Global Lung Function Initiative (GLI) pre-albuterol “normal spirometry” data reported by Quanjer et al48 was obtained in 15,661 presumably non-smoking individuals aged 3-94 years. They were referred for pulmonary function testing in Poland and the United States for clinical evaluation of asthma, cystic fibrosis, cough, dyspnea, and other miscellaneous clinical problems. Only 2.75% had an abnormal FEF25-75, and 1.29% an abnormal FEF75. Alternatively, despite normal routine spirometry, FEF25-75 remained abnormal in 2.9% of cases and FEF75 abnormal in 12.3% of cases. Based on these data, Quanjer et al,48 and Lukic et al49 and subsequently the ATS47 concluded that measuring expiratory flow at 25%-75% and at 75% expired FVC had not demonstrated added value for identifying expiratory airflow obstruction in adults and children.49 Therefore, these tests were not recommended for routine use. This was based on epidemiologic and not clinical studies in symptomatic non-smokers. When using GLI normal data for spirometry including FEF75 (lps) from Quanjer et al,48 our results were similar to using reference normal data from Morris et al10 and Hankinson et al (NHAMES III).12 The findings presented here confirm our previous29-31and other studies50-53 underscoring the value of further pursuing earlier detection of COPD in symptomatic patients, with normal “preserved” routine spirometry. Future investigations are needed to provide additional clinical data, including physiologic, whole-lung thoracic HRCT, and when available, correlative pathologic evidence of underlying obstructive lung disease to challenge current recommendations regarding not analyzing expiratory flow at low lung volumes.47-49 We believe that clinicians need to reject the conclusion suggested by Sator and colleagues (BOLD study)54 that normal routine spirometry is consistent with a false positive clinical diagnosis of COPD in symptomatic patients with respiratory symptoms including in smokers. They never measured FEF75. The accompanying editorial by Vanfleteren et al55 highlighted the complexity of making the correct diagnosis of COPD in symptomatic patients with normal routine spirometry. However, they55 did not acknowledge the importance of measuring expiratory airflow at low lung volumes, i.e., FEF for detection of unsuspected small airway obstruction and/or emphysema, especially in symptomatic smokers.29-31 Recent updates56,57 on earlier detection of COPD in symptomatic smokers with normal “preserved” routine spirometry have not further addressed the issue of analyzing expiratory airflow at lower lung volumes including FEF75. Moreover, the recent editorial by Barnes et al58 emphasized the pressing need to redefine symptomatic “GOLD 0 COPD”.
Acknowledgements
Author contributions: AFG, JCH, JAN, AY, and EKV provided conception and design. Analysis and interpretation were provided by AFG, JCH, JAN, AY, MS, EKV, EFG, MD, and DPT. Intellectual contributions and drafting of the article were provided by AFG, JCH, JAN, AY, MS, EKV, EFG, MD, and DPT. DNTT, RMM, and CF were responsible for the lung function studies. AY, JCH, EKV, and EFG were responsible for the pathology studies and MS was responsible for the radiology studies.
The authors thank pathologists Be Huynh, MD, from Long Beach Memorial Medical Center, Long Beach, California for Case 8 (Figure 8c,d) and Yuki Takasumi, MD, from Providence Saint John's Health Center, Santa Monica, California for Case 9 (Figure 9c,d), and thoracic surgeons Navrose Grewal MD, Robert J. McKenna, Jr., MD, and George Panagiotides, MD. The authors also thank graphic artist Janet Ward, Bob Ward, BS, MS, electrical engineering, and Richard F. Rideout, and Amanda Post for the lung function studies and Noe Zamel, MD, Pulmonary Division, Department of Medicine, University of Toronto, Ontario, Canada for the pulmonary physiologic discussion.
Declaration of Interest
The authors have nothing to declare.