Running Head: COPD Management Adherence Pre and Post Intervention
Funding Support: The work described herein was part of a continuing medical education and quality improvement program funded by an independent educational grant from AstraZeneca LP to PRIME Education, LLC. The grantor had no role in the study design, execution, analysis, or reporting.
Date of acceptance: February 17, 2021 | Published online: February 19, 2021
Abbreviations: chronic obstructive pulmonary disease, COPD; quality improvement, QI; Global initiative for chronic Obstructive Lung Disease, GOLD; modified Medical Research Council dyspnea scale, mMRC; COPD Assessment Test, CAT; standard deviation, SD; COPD Questionnaire, COPD-Q
Citation: Martinez FJ, Thomashow B, Sapir T, Simone L, Carter J, Han M. Does evaluation and management of COPD follow therapeutic strategy recommendations? Chronic Obstr Pulm Dis. 2021; 8(2): 230-242. doi: http://doi.org/10.15326/jcopdf.2020.0175
Online Supplemental Material: Read Online Supplemental Material (574KB)
Introduction
The approach to COPD diagnosis and evaluation has evolved over time and transformed therapeutic strategy recommendations. This has traditionally highlighted the importance of spirometry to ensure a correct diagnosis and characterize physiological disease severity.1 Over the past decade, the multidimensional nature of COPD has led to explicit recommendations regarding the approach to defining disease impact and personalizing approaches to therapy.2 The Global initiative for chronic Obstructive Lung Disease (GOLD) scientific strategy in 2011 pointedly recommended the routine assessment of patient symptom burden with available instruments (modified Medical Research Council, [mMRC], or the COPD Assessment Test, [CAT]), and a systematic assessment of exacerbation risk.3 Numerous groups have confirmed limited adherence to published therapeutic strategies.4-8 Rigorous, prospective data to determine the level of penetration of multidimensional assessment at an individual patient level have not been crisply defined. Here, we examine the impact of a comprehensive quality improvement (QI) program introduced at a series of clinical sites in the Southeastern United States.
Methods
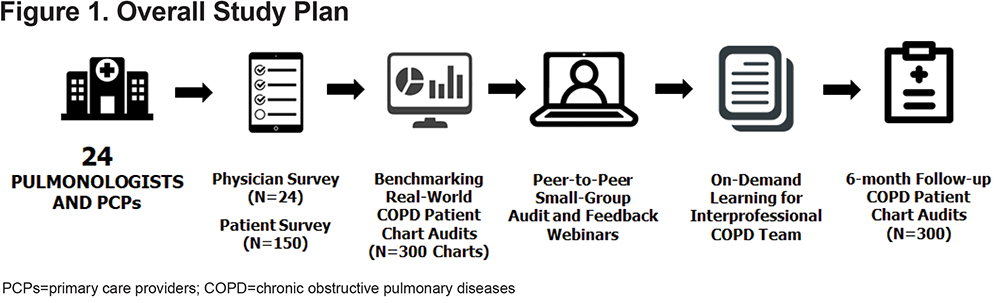
The parent study was conducted as a QI project from 2017 to 2018 at a national health care system in the Southeastern United States. The overall schema is illustrated in Figure 1. An independent institutional review board approved the study methods (IRB ID 5429; Sterling IRB, Atlanta, Georgia). All physicians gave informed consent to participate in the educational program and study. Patients gave informed consent for their participation in the survey.
Physician Recruitment and Patient Inclusion Criteria
The recruitment strategy was focused on reaching specialty and primary care providers among a large national health care system. Among 52 physicians invited to participate, a total of 24 physicians enrolled and participated in the project, including 9 pulmonologists and 15 primary care physicians. Due to pragmatic considerations, including the limited time commitment that the practices could devote to administering patient surveys and identifying patient charts, as well as funding restrictions, the study was designed to review 150 patient surveys and 300 baseline patient charts. Since 24 physicians participated in the study, each physician was asked to identify 6 to 7 patients who met the following criteria: age>18 years, COPD diagnosis, using maintenance COPD medications, and at least 2 office visits in the past year. These patients are referred to herein as the “COPD patient survey group.” A separate group of COPD patients (n=300; 135 managed by pulmonary and 165 by primary care clinicians) from the same practices were evaluated to further refine our knowledge of physicians and their practice management patterns.
Physician and Patient Surveys
Data regarding evaluation and management of patients with a diagnosis of COPD were collected from physicians using a systematic survey (Supplemental Table 1 in the online supplement). COPD patients, who were identified by their physicians, were queried about their evaluation and management using a complementary, systematic survey (Supplemental Table 2 in the online supplement). The survey items were developed to align with the QI program’s objectives, which were to close gaps in: (1) alignment of clinical practices with evidence-based recommendations for the assessment of COPD symptoms and exacerbation risk, (2) referrals and care coordination, and (3) supporting patients in self-efficacy and adherence. The physician survey included items for assessing use of spirometry and validated instruments for assessing COPD symptoms, estimation of exacerbation burden and hospitalizations, referrals provided for patients with COPD, perceptions of their patients’ main barriers to COPD medication, and potential areas for quality improvement. The patient survey was designed at a fifth grade reading level and included items for assessing perceptions of their disease, how often their COPD provider performed spirometry and asked about symptoms, referrals received from their COPD provider, and main barriers to COPD medication adherence.
Educational Interventions
After the physician and patient survey data had been collected, the physicians underwent focused, educational, peer-to-peer small group sessions based on the learning principles of audit and feedback, which were led by co-authors (FJM, BT, MH). The physicians invited their interprofessional team members to join them in participating in the sessions, which were administered through web conference software. Each session was organized by the presentation of slides with graphs showing the physicians’ and patients’ responses to survey items related to spirometry utilization, symptom assessment, patient-reported and physician-estimated exacerbations and hospitalizations, barriers to medication adherence, and referral patterns. For each topic, the COPD expert led the study participants in discussions about the evidence-based rationale for recommended clinical practices. The discussions focused on identifying systems-based barriers to alignment with evidence-based recommendations, as well as effective methods for performing and documenting the clinical practices. At the end of each session, the presenter guided the participants in developing an individualized action plan for improving performance and documentation.
To reinforce the education, the authors developed a 1.25 hour accredited, continuing medical education video. In the video, co-authors (FJM, BT, MH) presented evidence-based recommendations and leading practices for COPD diagnosis, clinical assessment, therapy selection, patient education, and referrals for COPD management services, including pulmonary rehabilitation. Both the small-group sessions and video activity focused on the 2017 GOLDguidelines,1 and also referred participants to the COPD Foundation Pocket Consultant Guide.9
Pre- and Post-intervention Retrospective Chart Abstraction and Analysis
Chart audits were assessed at 2 time periods to quantify improvements in documented performance measures as a result of the educational intervention (Figure 1). The baseline audits reviewed 12 months of patient chart data from an index date prior to the cohort's participation in the audit-feedback webinar. The post-education chart data collection was initiated at an index date 6-months after participants completed the webinar and reviewed 6-months of patient data.
Charts were retrospectively abstracted by 1 of 3 trained medical record reviewers who were blinded to the assessment period of the charts that they were reviewing. To assess inter-rater reliability, each reviewer compared samples of their colleague’s charts through an internal quality assurance process. The assessment was based on numbers of chart variables for which the reviewers agreed in their abstraction. Among metrics reported in this article there was >95% agreement among auditors on abstracted variables. The data presented in this manuscript reflect: (1) the data from physicians and their COPD patients, and (2) changes in care after physician education.
Statistical Analysis
Pearson chi square test and Fisher’s exact were performed on categorical variables to examine differences in the relationship between patient and provider survey responses and pre-/post-intervention chart audits. Significance level was set at p ≤ 0.05. Data were analyzed using IBM SPSS statistical software version 22.
Results
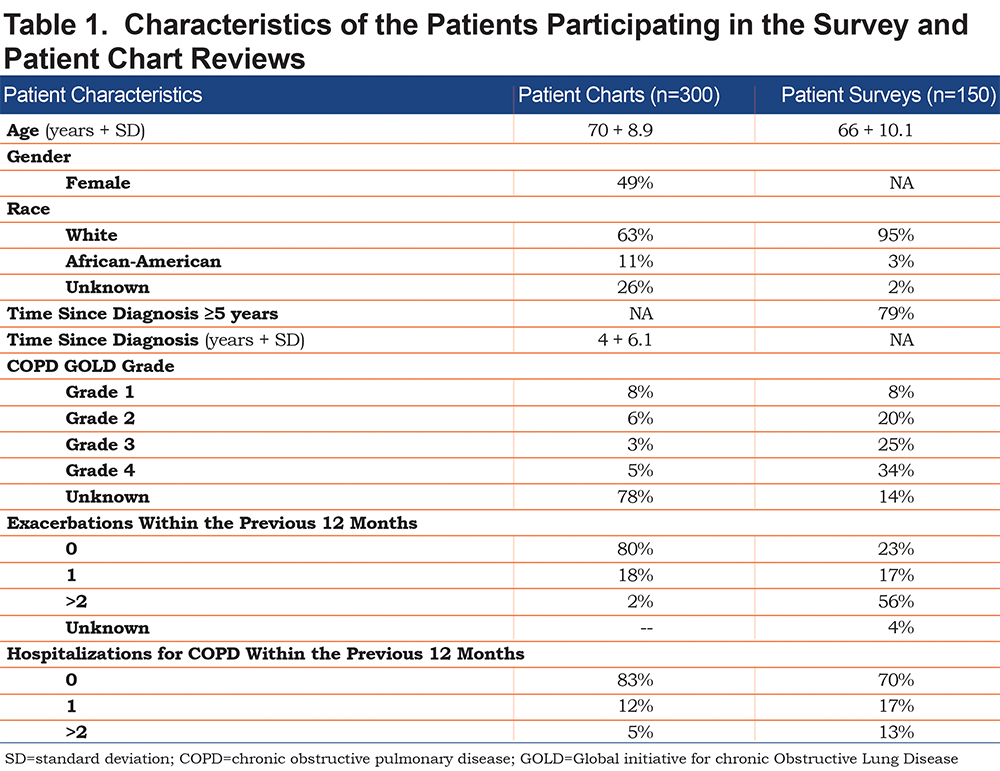
The participating physicians had an average of 20 years in practice. The majority of physicians (67%) saw ≥ 20 COPD patients/week and worked in clinical settings with an average of 4 physicians per practice. The characteristics of the participating patients are enumerated in Table 1.The patients who were surveyed exhibited a wide range of spirometric severity and most (79%) had been diagnosed with COPD for ≥ 5 years. The patients whose charts were reviewed were of a similar age and gender. The majority had not undergone spirometry or experienced an exacerbation or hospitalization in the year before chart review.
Perceptions of Providers and Their Patients About COPD Assessment and Care
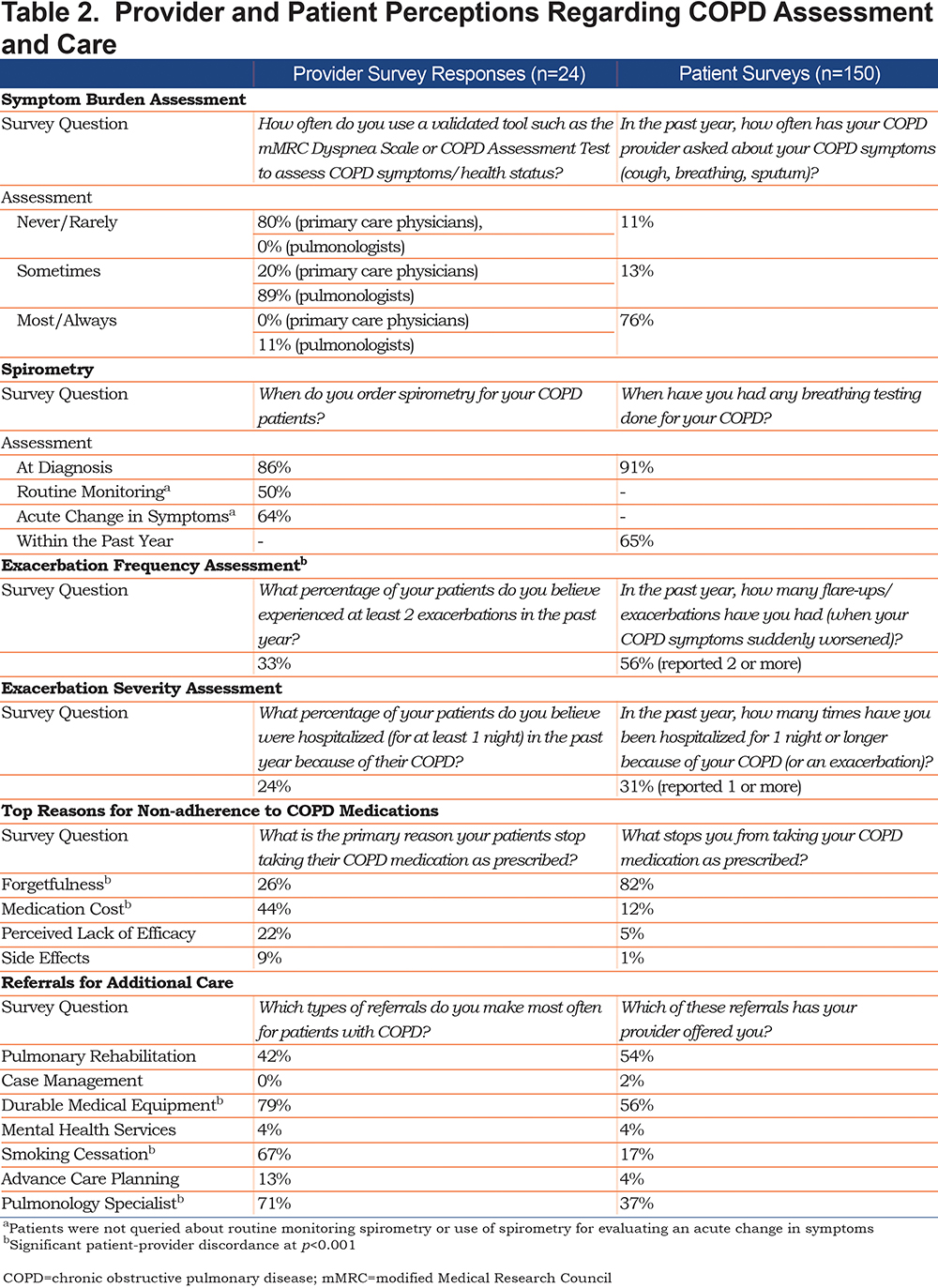
Results from the survey study provided insights into how clinicians and their patients perceived certain aspects of disease assessment and care (Table 2). The majority (80%) of the primary care physicians reported that they rarely used formal instruments to assess respiratory symptom burden, whereas the majority of the pulmonologists (89%) reported that they sometimes used such instruments. In response to a separate survey item, the majority of patients (76%) noted that their clinician inquired about respiratory symptoms at most or every clinical encounter. There was concordance between providers (86%) and patients (91%) in the reporting of spirometric testing at diagnosis. Clinicians estimated that one-third (33%) of their patients had experienced at least 2 exacerbations in the past year. In contrast, more than half (56%) of their patients reported at least 2 exacerbation events in the past year. There was better concordance for hospitalizations reported by the clinician versus patient. There was significant discrepancy between the clinician’s interpretation of the reason for suboptimal adherence to COPD medication (medication cost – 44%) versus the patient’s reason for not taking medication as prescribed (forgetfulness – 82%). There were similar discrepancies in the use of various referrals; this was particularly evident in referrals for smoking cessation.
Clinician Insights Before and After Focused Training
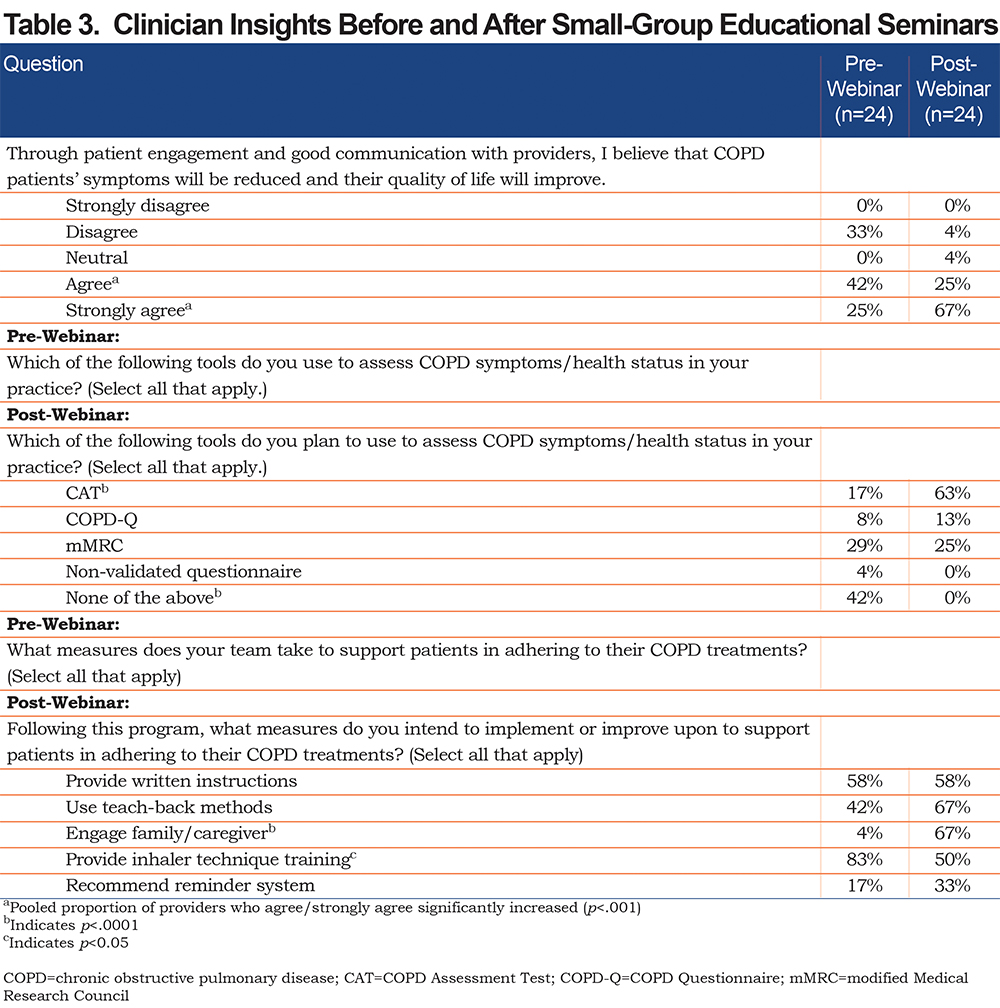
Self-reported changes were noted by the clinicians regarding their approach to care before and after educational webinars (Table 3). There appeared to be an increase in acceptance of the importance of good communication with patients to improve outcomes. Similarly, there was an increase in understanding of the role of the CAT for symptom burden assessment. There was a numerical increase in the proportion of clinicians who anticipated using teach back methods, family/caregiver engagement, and reminder systems to improve adherence. Interestingly, a lesser proportion noted anticipated use of inhaler technique training.
Change in Patient Care and Outcomes Before and After Education in Broader Patient Population
There were notable improvements in numerous measures of patient management in the year following the QI intervention as compared to the year before the intervention (Table 4). Chart review documented improvement in patient engagement and shared decision-making, although we noted a trend towards more events in a few individuals as opposed to incremental gains across all patients. There were improvements in general COPD assessment, including COPD staging. A notable exception was documentation of quality-of-life assessment, which was recorded in approximately three-fourths of patients. Documentation of referral for various COPD management services was noted in a distinct minority of participants, although overall documentation of referral was low in the medical records. There was a slight increase in provider documentation of adherence; where commented upon, the majority of patients were noted to be adherent. No difference in exacerbations was seen although we were not powered to examine this outcome specifically.
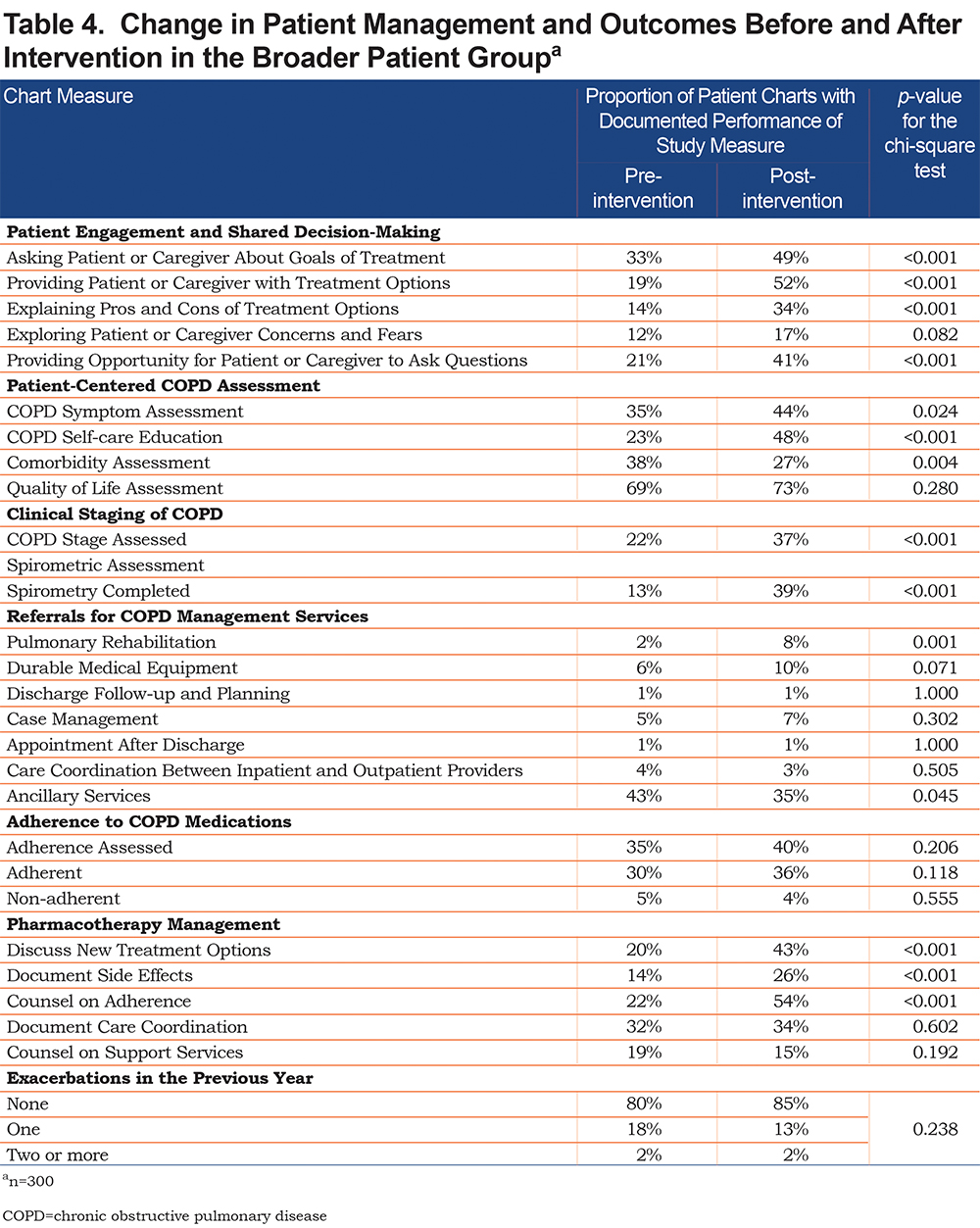
There were improvements in discussion of new treatment options, documentation of side effects, adherence counseling, and documentation of care coordination. Nevertheless, all but adherence counseling was noted in a minority of the patients. The majority of patients did not experience an exacerbation in the years monitored with a numerical improvement pre- and post-QI intervention.
Discussion
The approach to COPD diagnosis and evaluation has evolved to the current recommendation of a multidimensional assessment and resulting personalized therapeutic approaches. Despite these logical recommendations, documentation of widespread implementation has been lacking. Similarly, comprehensive quality improvement programs have not been carefully evaluated. In this prospective study we examine the components of care as enumerated by a mixture of primary care and pulmonary specialty physicians and as recalled by their patients. Similarly, a multipronged QI program was introduced allowing an examination of changes in patient care. We documented: (1) clinicians’ perceptions of the components of COPD care that they provided and those recalled by their individual patients, (2) improvements in processes of care after an educational intervention employing audit-feedback methods, and (3) suggestions of a change in patient care and outcomes after the education intervention.
The majority of therapeutic guidelines have recognized the multidimensional nature of COPD and have explicitly led to evaluation that includes lung function, symptom burden and exacerbation risk. The GOLD guidelines in 2011 pointedly recommended the routine assessment of patient symptom burden or exercise limitation with available instruments (mMRC, CAT).3 Similarly, this group recommended a systematic assessment of exacerbation risk.3 To our knowledge, no specific data have been presented contrasting the impressions of clinicians regarding their adherence to COPD evaluation, the reported compliance of their patients, or confirmed assessment with dedicated chart review of patients cared for by these physicians. Our data suggest that the majority of clinicians do not routinely use formal mechanisms for symptom burden assessment, although their patients note that the majority query about COPD symptoms in some fashion. The majority of clinicians and patients recollect undergoing spirometric assessment, while objective confirmation is documented in a minority of patients. The difference in clinicians’ estimates of COPD exacerbations and patients’ self-reported exacerbations could be due in part to clinical differences in the subset of patients who were queried as compared with the providers’ entire population of patients with COPD. Additionally, clinicians and patients may define exacerbations differently. However, it is notable that the providers’ estimate of patients who experienced at least 2 exacerbations in the past year (33%) also does not align with the chart review, which showed that 2% of patients had documentation of at least 2 exacerbations in the past year. Together, the discordance in exacerbations as estimated by clinicians, reported by patients, and documented in patient charts, underscores the importance of a systematic approach to assessing COPD exacerbation risk, as recommended by the GOLD guidelines.
Numerous groups have confirmed limited adherence to published therapeutic strategies,4-17 although these generally have assessed compliance with therapeutic recommendations. Limited data have been collected assessing compliance with recommendations for symptomatic and exacerbation risk assessment. A retrospective chart review of COPD patients evaluated in 2 outpatient primary care provider offices in the United States confirmed that no patients had been evaluated with either the CAT or mMRC, 21% had documented spirometric confirmation of airflow obstruction, and 31.5% of patients were incorrectly diagnosed and mislabeled as suffering from COPD.18A survey of 500 U.S. primary care physicians confirmed that only 23.6% adhered to guideline spirometric recommendations.10 A subsequent cross-sectional chart review study conducted in 11 U.S. primary care sites documented spirometric confirmation in only 27% of patients; no description of dyspnea or exacerbation assessment was described.19 A survey of clinicians in 2 general practices in New York City confirmed low use of spirometry but no query was provided regarding assessment of symptom burden or exacerbations.20 The Spanish Community Assessment of COPD Health Care (COACH) Study was an observational, retrospective audit study of consecutive clinical cases of COPD.21 Evaluation of dyspnea was seen in only 11.1% of cases while 81.4% had exacerbations in the previous year recorded. A retrospective, chart audit22 across 14 Swiss primary care practices conducted in 2012 confirmed spirometric confirmation of obstruction in 83% of patients and queries regarding respiratory symptoms in the same proportion; exacerbation management was noted in 24% of patients and documented use of the mMRC in 25%. A multinational survey of primary care and pulmonary clinicians performed in 2013 suggested broad use of spirometry (82%-100%) and validated patient-reported outcome measures (67%-81%).12 A clinical record audit at 9 hospital outpatient respiratory clinics in Spain completed between 2013-2014 noted frequent use of spirometry and objective assessment of respiratory symptoms (mMRC use > CAT use) and exacerbation assessment.23 A 2016 UK population-based cross-sectional study using the Clinical Practice Research Datalink for primary care of spirometrically diagnosed COPD patients, noted that 84% had documented evidence of mMRC (74%) or CAT (10%) assessment.7 Our U.S.-based data confirm global differences in COPD assessment while providing unique insights regarding clinician impressions of disease assessment and their patient’s recollections.
We confirm that an educational intervention employing audit-feedback methodology alters clinician impressions of the approach to evaluation, as has been employed in other settings.24,25 This was principally evident in consideration of using validated instruments for symptom burden assessment and increasing overall communications with patients. Similarly, there appeared to be improvements in multiple measures of processes of care and patient outcomes in reviewing charts from the included practices before and after the intervention. There have been several previous attempts to improve guideline use and COPD management in the outpatient setting. A QI intervention in an internal medicine outpatient clinic suggested modest improvements in spirometry utilization and pneumococcal vaccination; symptom and exacerbation assessment was not addressed.26 A cluster randomized trial in the Netherlands27 suggested improved quality of life among patients with spirometrically-confirmed COPD who were managed using the Assessment of Burden of COPD tool28 which is largely based on the Clinical COPD Questionnaire.29 An Italian study focused on a Sicilian general practice setting confirmed modest improvements in selected components of COPD care (e.g., spirometry utilized) after a limited educational program.30
Our findings should be interpreted in the pragmatic context of the QI educational program in which the surveys and chart reviews were conducted. Our study is limited by the relatively small number of clinicians who were enrolled in several practice settings in the Southeastern United States. Nevertheless, these clinicians were active participants in this COPD QI program with their individual patients and clinicians within their practices providing additional insights.
The patient and clinician queries assessed similar broad concepts although, the specific questions varied based on their knowledge and expertise. It is also possible that the perceptions reported by patients and clinicians could have been influenced by recall bias. These factors limit the conclusions that can be drawn in comparing the provider and patient perceptions. However, a key goal of studies designed to assess and compare patient and provider perceptions is to guide efforts to promote appropriate individualized care. Consider again our finding that 56% of patients reported at least 2 COPD exacerbations in the past year, whereas providers estimated that 33% of their patients had experienced at least 2 exacerbations in the past year, and just 2% of patients had documentation of at least 2 exacerbations in their charts. These findings accurately reflect the perceptions of the study participants and highlight the need to establish shared definitions among providers and patients regarding signs and symptoms that constitute COPD exacerbations, as well as the need for improvements in systematically assessing and documenting exacerbations.
The pre- and post-intervention changes provide limited evidence assigning causality to the intervention as it was not clear the patients were those cared for by the active participants. The pragmatic nature of the study in the context of a QI education program did not afford inclusion of a control group of providers who did not participate in the educational activities and whose charts were reviewed over the same time periods. On the other hand, the participating clinicians served as champions for quality-of-care improvement in the respective practices where the patients whose charts were reviewed, using a systematic approach, were treated, which affords some control over clinic-related extraneous variables. The changes in physicians’ practice patterns as assessed through the surveys and chart reviews may also reflect self-selection biases as providers who are motivated to implement practice improvements may be more likely to enroll in a QI program.
The timing of the study did not address the most recent GOLD recommendations regarding treatable traits and circulating eosinophils.31 On the other hand, data collection and the intervention took place many years after the previous, major revision of the GOLD recommendations.
Our study provides important insights regarding clinician insights in COPD processes of care and a similar view from their patients. Given the importance of multidimensional assessment, as advocated by recent COPD therapeutic strategies,1 these data suggest areas where future studies should focus their efforts. Despite a relatively straightforward intervention engaging local physician champions, there were suggestions of improved processes of care and potential patient endpoints.
Acknowledgements
Author contributions:
FJM was responsible for conceptualization of project, training of participants, data analysis, writing of first manuscript and revision of manuscript. BT was responsible for training of participants, data analysis, revision of manuscript. TS was responsible for conceptualization of project, securing funding, data collection, data analysis, revision of manuscript.
LS was responsible for conceptualization of project, securing funding, developing educational content, data collection, data analysis, writing of first manuscript and revision of manuscript. JC was responsible for recruitment of participants, data collection, data analysis, revision of manuscript and MH was responsible for conceptualization of project, training of participants, data analysis, writing of first manuscript and revision of manuscript.
Declaration of Interest
F.J. Martinez reports grants and personal fees from AstraZeneca during the conduct of the study; personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Boehringer Ingelheim, non-financial support from ProterrixBio, personal fees and non-financial support from GlaxoSmithKline, personal fees from MD Magazine, personal fees from Methodist Hospital Brooklyn, personal fees and non-financial support from Miller Communications, personal fees and non-financial support from the National Society for Continuing Education, personal fees from New York University, personal fees and non-financial support from PeerView Communications, personal fees and non-financial support from Chiesi, personal fees and non-financial support from Sunovion, personal fees from UpToDate, personal fees from WebMD/MedScape, other from Afferent/Merck, non-financial support from Gilead, non-financial support from Nitto, personal fees from Patara/Respivant, other from Biogen, other from Veracyte, non-financial support from Zambon, personal fees from the American Thoracic Society, grants from the National Institutes of Health, personal fees and non-financial support from Physicians Education Resource, personal fees from Rockpointe, other from Prometic, grants from Rare Disease Healthcare Communications, personal fees and other from Bayer, other from Bridge Biotherapeutics, personal fees and non-financial support from Canadian Respiratory Network, grants from ProMedior/Roche, personal fees and non-financial support from Teva, personal fees from CME Outfitters, personal fees and non-financial support from Csl Behring, personal fees from Dartmouth University, personal fees from DevPro, from Gala, personal fees from Integritas, personal fees from IQVIA, personal fees from Projects in Knowledge, personal fees and non-financial support from Sanofi/Regeneron, from twoXAR, personal fees from Vindico, other from AbbVie, personal fees from the Academy for Continuing Healthcare Learning, personal fees from United Therapeutics, from Novartis, outside the submitted work.
B. Thomashow reports advisory board and consultancy work for GlaxoSmithKline and is the Chief Medical Officer of the COPD Foundation.
M. Han reports personal fees from PrimeInc during the conduct of the study; personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Cipla, Chiesi, Teva, Verona, Merck, Mylan, and Sanofi and other support from Novartis and Sunovion outside the submitted work.
The other authors have nothing to declare.