Running Head: Symptom Burden in Patients With NTM Lung Disease
Funding statement: BronchandNTM360social was funded by a grant from Insmed Incorporated.
Date of Acceptance: February 17, 2021 ǀ Published online: February 19, 2021
Abbreviations: nontuberculous mycobacteria, NTM; NTM lung disease, NTMLD; mycobacterium avium complex, MAC; cystic fibrosis, CF; chronic obstructive pulmonary disease, COPD; analysis of variance, ANOVA; odds ratio, OR; adjusted odds ratio, AOR; computed tomography, CT; confidence interval, CI
Citation: Pravosud V, Mannino DM, Prieto D, et al. Symptom burden and medication use among patients with nontuberculous mycobacterial lung disease. Chronic Obstr Pulm Dis. 2021; 8(2): 243-254. doi: http://doi.org/10.15326/jcopdf.2020.0184
Online Supplemental Material: Read Online Supplemental Material (374KB)
Introduction
The growing recognition of nontuberculous mycobacteria (NTM) as contributors to chronic lung infection and the increasing prevalence of NTM lung disease (NTMLD) has led to greater concern about their impact on patients, especially with other underlying pulmonary diseases. Among various clinical manifestations of NTM, pulmonary disease is the most frequent.1
Global increase in incidence and prevalence of NTMLD, as well as increased mortality rates due to NTMLD, have been widely discussed in the literature.1-6 The following types of mycobacteria most commonly cause progressive NTMLD: the slow-growing species, Mycobacterium aviumcomplex (MAC) and Mycobacterium kasassii, as well as a rapid-growing group known as Mycobacterium abscessus.1,7 Any household or hospital water supply, mainly tap water or indoor showerhead, is considered a potential source of NTM.1,7 Of the 2 main species of MAC, Mycobacterium avium has been most closely associated with water exposures, whereas, Mycobacterium intracellulare is most associated with soil exposures.
Recent studies have demonstrated that immunocomprised individuals, including but not limited to people with HIV/AIDS,8 as well as thin postmenopausal women and the elderly9-11 can be at particular risk for NTMLD. The prevalence of NTM is also notable among patients with pre-existing respiratory illnesses,9,12,13 including persons with bronchiectasis, cystic fibrosis (CF), or chronic obstructive pulmonary disease (COPD).1,13-18
Previous studies conducted in the United States have found that place of residence also matters. People residing in the central, and especially in the southern and southeastern parts of the United States., may have a higher risk of exposure to NTM.1,2,7,19 More recently, particularly high rates of NTMLD have been identified in Hawaii.20 However, the existing data on prevalence and incidence rates of NTMLD in the United States are incomplete due to NTMLD not being reportable at a national level as well as at the state level in most states.5 The lack of active surveillance makes it difficult to determine the prevalence and incidence of NTMLD accurately.1,21 Ongoing studies are currently exploring more deeply the relationships between environmental exposures and the development of NTMLD.
Nontuberculous Mycobacterial Lung Disease Symptomatology and Treatment
NTM can lead to asymptomatic pulmonary infection and symptomatic illnesses.1 Evidence from previous studies has shown that NTMLD symptomatology varies.10,22,23 In general, symptoms are nonspecific and similar to symptoms attributed to often co-existing underlying pulmonary disorders. Signs and symptoms of the clinical presentation of NTMLD include but are not limited to cough, sputum production, dyspnea, hemoptysis, fever, night sweats, depression, fatigue, malaise, and weight loss.1,10,22,23
In patients with NTMLD, the decision of establishing treatment depends on the type of species, co-existing illnesses, and disease/symptom burden.1,13 Patients with MAC or Mycobacterium kansasii lung disease can benefit from multiple antibiotic and/or other mycobacterial therapies, whereas, those with Mycobacterium abscessus lung disease may also require surgery to remove localized areas of disease to increase the likelihood of complete cure.1 However, not every patient with an NTMLD-established diagnosis will require medication if, for instance, their disease progression is slow; and not every patient will benefit from NTM medication due to drug intolerance.1,13 In fact, because of the potential risks of serious adverse events, NTMLD treatment may not even be initiated or may need to be discontinued prematurely.1,13 An alternative treatment such as another medication or surgical procedure should be considered instead.1
Symptomatic improvements are one of the primary endpoints for monitoring of patients on NTM medication.1,13 Evidence shows that the duration to observe successful treatment outcomes with symptomatic improvements of NTMLD varies and may last from several months to several years.13 Although improvements in symptom severity while on medication are generally expected,1,13 some patients, especially those with other pulmonary comorbidities, may experience complications.1
The Present Study
Currently, there is little information about differences in NTMLD-related symptom burden among people who are or are not undergoing treatment for their NTMLD. The present study aimed to explore what symptoms are associated with NTMLD with or without the concurrent use of mycobacterial therapy.
For this investigation, we used responses to a “Burden of NTM Survey” developed by the COPD Foundation. The Foundation is a not-for-profit organization whose mission is to prevent and eliminate the progression of COPD.24 Created in 2004, the COPD foundation is developing and undertaking initiatives to educate and encourage the COPD community, and engage health care professionals, elective officials, and academic and industry leaders with the goal of COPD prevention and improvement of the quality of life of patients with COPD and related disorders.24 The 3 main areas of focus are (1) education and awareness, (2) public policy and advocacy, and (3) research.24
The “Burden of NTM Survey” was designed to better understand how much, and in what way, a patient may be affected by NTM lung infection or disease. A cohort of patients to survey was identified on the Bronchiectasis and NTM Initiative's 360social website. BronchandNTM360social is a free, interactive, online social community and network for patients with bronchiectasis and NTM, their family members, caregivers, and health care providers.25 It was also developed by and maintained by the COPD Foundation. The “Burden of NTM Survey” was posted on the BronchandNTM360social website. We aimed to determine the proportion of respondents of this survey with NTMLD, with or without NTM treatment, who self-reported symptoms. Results from this study will potentially assist with the future development of instruments for symptom surveillance of patients with NTMLD with and without the concurrent use of mycobacterial therapy.
Methods
Study Design and Data Source
The “Burden of NTM Survey” was made available online on the BronchandNTM360social website from September 12, 2016 through January 11, 2017. A total of 287 individuals completed the questionnaire. The collection of the informed consent was not required as the survey was optional and anonymous. This study qualified for exemption by the institutional review board at the University of Kentucky (#17-0085-X6B).
We identified study participants with a self-reported NTMLD diagnosis through this cross-sectional survey. Participants were excluded if they had never been told by a doctor or a health care professional that they had an NTM lung infection or disease. The surveyed participants were asked to report whether they experienced 12 symptoms during the past 2 weeks (yes/no). Namely, those symptoms included: (1) cough, (2) a productive cough (i.e., coughing up blood, phlegm, or mucus), (3) fatigue or lack of energy, (4) sleep problems, (5) feelings of sadness or depression related to illness, (6) difficulty in walking 500 meters without stopping, (7) difficulty in interacting with others, (8) difficulty with sensitivity to cold or heat, (9) difficulty with fever, chills, or night sweats, (10) loss of appetite, (11)shortness of breath, wheezing or other breathing difficulties, and (12) body pain. The survey asked about the duration of living with an established diagnosis of NTMLD (less than 1 year, 1 to less than 2 years, 2 to 5 years, more than 5 years), whether participants considered themselves as having an ongoing NTM lung infection or disease (yes/no), current use of any medication(s) to treat their NTMLD (yes/no), duration of undergoing any medication(s) for NTMLD (less than 3 months, 4-6 months, 7-12 months, more than 12 months), and current experience of other lung illnesses such as asthma, bronchiectasis, COPD or emphysema, and CF. Participants could also list other pulmonary or non-pulmonary comorbid diseases in the “other, please specify” option. Demographic characteristics such as age category (less than 35 years, 35-49 years, 50-64 years, 65-79 years, and more than 80 years), gender, and place of residence (state and/or country as the survey was not limited only to patients residing in the United States) were obtained from the respondents.
Statistical Analysis
All statistical analyses were carried out using SAS, version 9.4 (SAS Institute Inc., Cary, North Carolina). Pearson χ2 (or Fisher’s Exact Test, if the expected cell was ≤5) was used to assess differences in categorical characteristics of the study sample by the 2 comparison groups: those with versus without NTM medications as well as to examine age- and gender-related differences among participants of the 4 U.S. regions (the Midwest, the Northeast, the South, and the West). We used Student’s t-tests to assess significant differences in the mean number of self-reported symptoms within the 3 two-level, categorical variables describing age, gender, and the duration of NTMLD medication (for participants with any NTM therapies). The analyses of variance (ANOVA) followed by the Tukey’s Studentized Range (HSD) post-hoc tests were used to determine differences in the mean number of self-reported symptoms between all pairs of the 3-level age variable as well as among the 4 residence groups for patients residing in the United States. Initially, we examined differences among the 3 age groups: (1) those who were less than 50 years old, (2) those who were 50 to less than 65 years old, and (3) those who were 65 years and older. However, evidence shows that NTMLD is more prevalent among people aged 50 years and older.11 Therefore, the survey participants were divided into 2 age categories for further comparison and adjustment in the regression modeling: (1) those who were younger than 50 years old, and (2) those aged 50 years and older.
Pearson χ2 tests were conducted to identify differences in the percentages of individuals in the 2 groups (those who were versus those who were not currently on any medication to treat their NTMLD) having reported cough, fatigue or lack of energy, sleep problems, feelings of sadness or depression related to illness, difficulty in walking 500 meters without stopping, and difficulty in interacting with others. Single- and multivariable penalized logistic regression models with 95% confidence intervals based on profile likelihood26 (as suggested,27 a superior method in small samples and sparse data28) were carried out to evaluate, respectively, unadjusted and adjusted, associations between self-reported symptoms (as separate outcomes of interest) and the use of any medication to treat NTMLD as the main exposure of interest. In multivariable modeling, the associations were adjusted for demographics (age and gender), duration of NTMLD, and having 1 or more of the respiratory illnesses such as asthma, bronchiectasis, CF, and COPD/emphysema. The survey participants could skip questions. We used complete case analysis with listwise deletion of missing data in the procedure steps for all types of comparison analyses. Two-sided p-values <0.05 were considered statistically significant.
Results
Description of the Study Sample
A total of 266 patients said that they had been told by a health care provider that they had an NTM lung infection or disease. Excluding missing data from the denominator for each variable of interest showed that most survey participants were aged 50 years or older (250, 95.1%), of female gender (244, 93.1%), and were living with NTMLD for more than 5 years (142, 55.7%) (see Table 1). Half of the respondents reported being currently on medication for any NTMLD. As compared to participants without NTMLD therapies, a significantly larger proportion of those currently on any NTM medication reported living with NTMLD for less than a year or less than 2 years (respectively, 8.6% versus 1.6% and 17.2% versus 11.9%, p=0.0231). The survey did not ask to specify the type of medication(s) nor mycobacteria or infection causing the disease; only 35 patients (13.2%) could recall having been told by a health care provider that they had MAC/Mycobacterium avium-intracellulare (MAI) using “other, please specify” option of the question about comorbid pulmonary conditions (results not shown in the table). Many of the participants who were currently on any NTM medication were undergoing their therapies for more than a year (90, 70.9%); some were undergoing treatment for 7-12 months (17, 13.4%), and few were undergoing treatment for 4-6 months (8, 6.3%) or less than 3 months (12, 9.5%). Most of the survey participants who were without any NTM therapies skipped this question; only 1 person mentioned undergoing NTM treatment for more than 12 months. Many respondents (247, 92.9%) reported having other lung illnesses such as bronchiectasis (80.5%), COPD or emphysema (29.7%), asthma (24.6%), or CF (2.6%). A significantly larger proportion of participants who were versus who were not currently on any NTMLD medication reported having asthma (30.2% versus 19.4%, p=0.0436) and COPD or emphysema (36.4% versus 24.8%, p=0.0428); however, more participants without any NTMLD medication reported having bronchiectasis (76.7% versus 89.2%, p=0.0081).
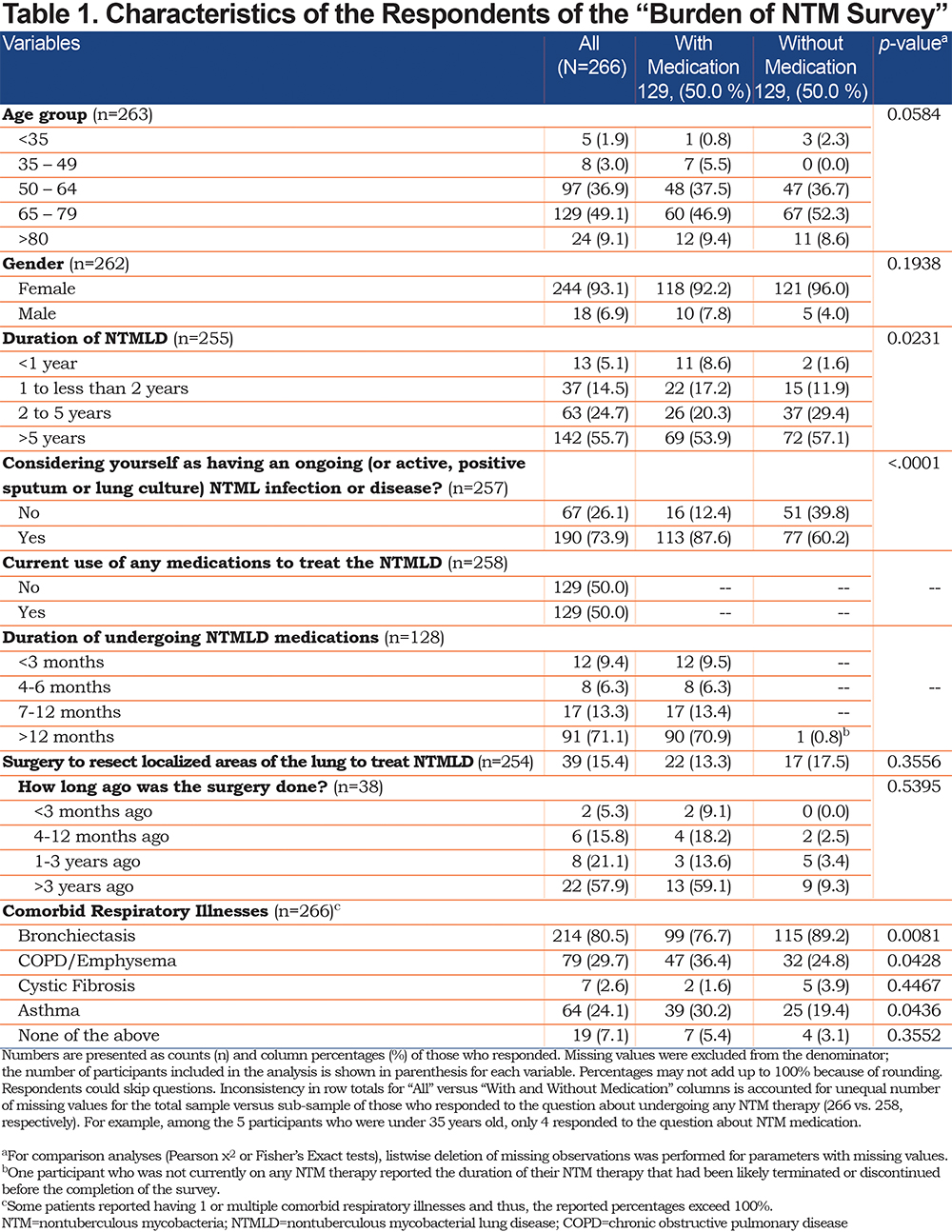
Two-thirds of respondents experienced half or more of the 12 listed symptoms (167, 66.3%). As seen in Table 2, the 3 most troubling symptoms were cough (61.0%), fatigue or lack of energy (60.6%), and shortness of breath, wheezing, or other breathing difficulties (51.2%). For those who were on any NTMLD medication, the average number of self-reported symptoms did not vary significantly by the duration of undergoing any NTMLD treatment (F=0.96, p=0.4161 – results not shown in the table). Table 1 in the online supplement shows the percentages of those experiencing each self-reported symptom during the past 2 weeks by age and gender group. There were no significant age- or gender-related differences between the means of the number of self-reported symptoms (those less than 50 years versus those 50 years and older: μ=8.3 versus μ=6.5, t=1.86, p=0.0638; women versus men: μ=6.5 versus μ=7.6, t=-1.36, p=0.1754, see Figure 1 and Figure 2 in the online supplement, respectively). Of note, when treating the age variable as a 3-level variable (those less than 50 years versus those from 50 to less than 65 years versus those 65 years and older), we did not observe significant differences regarding the mean number of self-reported symptoms either (μ= 8.3 versus μ=6.7 versus μ=6.4, F= 1.93, p=0.1469 – results not shown in the table). Table 2 in the online supplement shows the percentages of self-reported symptoms by the region of residence for the subsample of 235 patients residing in the United States. Of note, participants of the 4 U.S. regions did not differ with respect to age or gender (χ2=1.22, p=0.7490; χ2=2.68, p=0.4444, respectively – results not shown in the table). The results of ANOVA suggested significant differences in the average number of self-reported symptoms among the 4 residence groups (F=2.77, p=0.0423). As seen in Figure 3 in the online supplement, patients from the Midwest reported a higher mean number of symptoms (μ=7.1), followed by patients from the South region (μ=6.8), the West (μ=6.5), and finally, from the Northeast region (μ=5.5). However, the post-hoc analysis did not reveal any statistically significant differences in pairwise comparisons among these 4 regions.
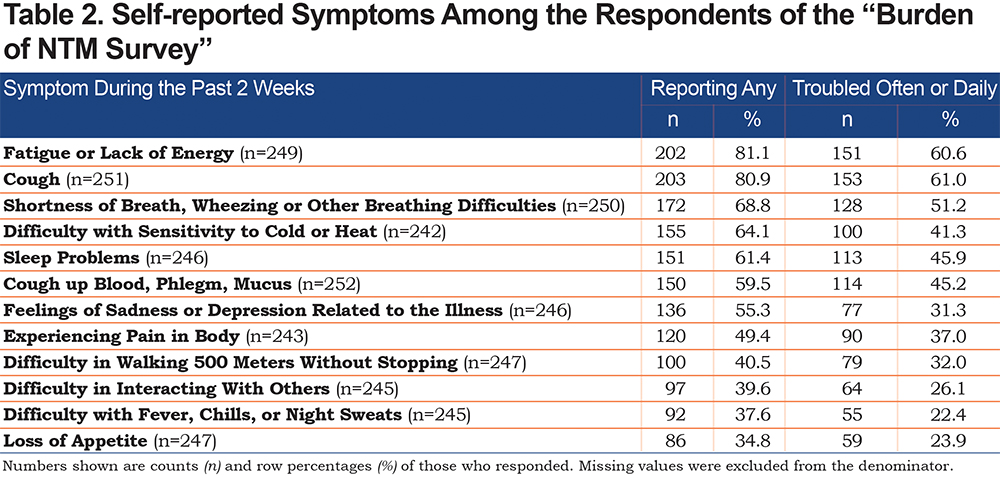
Bivariate Results
We observed significantly larger percentages of those who reported shortness of breath, wheezing or other breathing difficulties (80.0% versus 57.6%, p=0.0002), fatigue or lack of energy (87.9% versus 74.4%, p=0.0091), feelings of sadness or depression related to illness (65.6% versus 45.2%, p=0.0014), difficulty in walking 500 meters without stopping (48.8% versus 32.3%, p=0.0096), and difficulty in interacting with others (47.9% versus 31.5%, p=0.0092) among those who were currently taking any medication to treat their NTMLD as compared to those who were not on medication (see Table 3). The findings from the unadjusted logistic regression models were consistent with these results and revealed a significant association between being on medication and reporting of the 5 symptoms listed above (see Table 4).
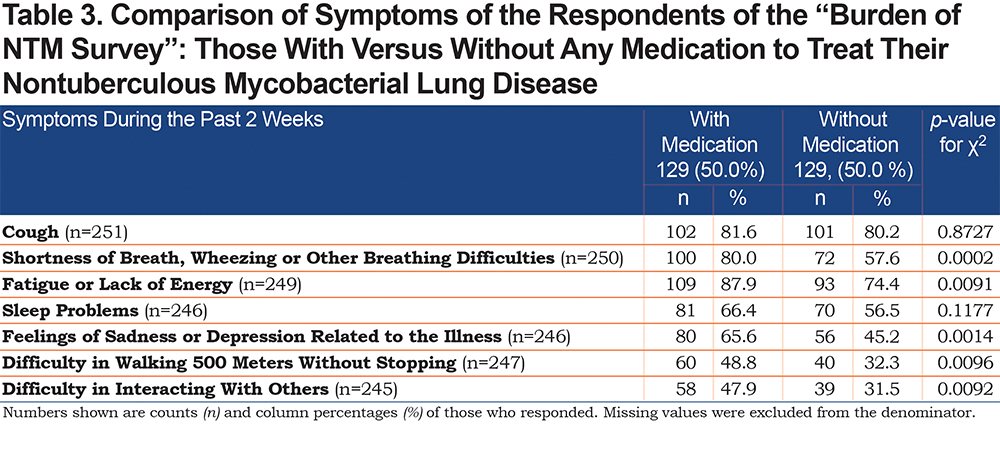

Multivariable Results
After adjusting for demographics, duration of living with NTM disease, and other respiratory illnesses, patients currently on medication, as compared to those who were not on medication, had significantly greater odds of reporting shortness of breath, wheezing or other difficulties (adjusted OR, (AOR)=2.8, 95% CI: 1.6–5.1), fatigue or lack of energy (AOR=2.0, 95% CI: 1.1–4.1), feelings of sadness or depression related to illness (AOR=2.1, 95% CI: 1.2–3.6), difficulty in walking 500 meters without stopping (AOR=1.9, 95% CI: 1.1–3.2), and difficulty in interacting with others (AOR=2.0, 95% CI: 1.2–3.6). The odds of reporting cough and problems with sleep were similar in the 2 groups (see Table 4).
Discussion
In the United States and throughout many areas of the world, increasing prevalence rates for NTM respiratory infections have become a concern for patients and health care providers.7,11,17,19 NTM may cause both asymptomatic lung infection and symptomatic disease.1 Resolution of or even improvements in symptoms is an important endpoint for therapeutic drug monitoring of patients with NTM therapies.13 This cross-sectional study describes a population of individuals with a self-reported NTMLD with or without NTM treatment and associated symptoms. Many respondents reported having at least 1 comorbid pulmonary illness in addition to their NTMLD confirming at-risk populations. Understanding the burden of NTMLD may ultimately assist caregivers in focusing on symptom management in addition to impact of therapeutic intervention.
As one of the priorities of the patient-centered research for NTM lung infection and disease,29 development of an NTMLD severity assessment tool has become a topic of emerging discussions.29,30 Due to the lack of active surveillance, NTMLD is under-investigated, and the outcomes of NTM treatment are not well understood. Undergoing NTM therapies, which can last on average from 18-24 months,1 may not guarantee successful treatment results.1 Previous studies have shown evidence of NTM reinfection and recurrence among patients on NTM medication.31-33 Given a long-term duration of living with NTMLD (which some may posit as a chronic condition),9 development of a validated composite score of NTMLD-related symptom burden and a measure of disease severity is very important. This knowledge can improve the monitoring of existing and assessment of new NTM therapies.29,30,34 Similar to assessment tools for patients with CF35 and bronchiectasis,36,37 Quittner et al created a health-related quality of life module and a draft measure specifically for NTM.30 Based on the concerns reported by patients and pulmonologists, the module included NTM and digestive symptoms, body image and eating problems.30 Quittner et al also proposed an algorithm for administering the NTM module to accompany assessment tools for patients with CF and bronchiectasis.38 More data collection and research is needed for further development of the module’s themes and their validation.30,38
Principal Findings
To our knowledge, this is the first study in the United States that examined associations of symptoms of patients with self-reported NTMLD with and without the use of NTM medication. More than half of the surveyed participants reported living with an NTMLD for more than 5 years, highlighting the chronic nature of NTMLDs.17,39-41 In contrast to earlier studies noting a predominantly male distribution in those with NTM infection, our study is in alignment with recent research characterizing NTMLD predominately in women and older (those aged 50 years or older) patients.42
The findings revealed that experiencing symptoms was common among NTMLD patients with and without treatment. The 3 most troubling symptoms were cough, fatigue or lack of energy, and shortness of breath, wheezing or other breathing difficulties. After adjusting for demographics, duration of living with an NTMLD, and other respiratory disorders, we observed a greater burden of symptoms among those receiving NTM medication. Participants with NTM therapies were more likely to report shortness of breath, wheezing or other breathing difficulties, fatigue or lack of energy, feelings of sadness or depression related to illness, difficulty in walking 500 meters without stopping, and difficulty in interacting with others. The present study, however, may be subject to confounding by indication. Since NTMLD severity is one of the factors influencing the initiation of NTM treatment,1 it is likely that NTM medications were prescribed to patients with a greater disease severity and symptom burden.
Our study could not determine why eligible participants who supposedly had NTMLD were without any NTM therapies at the time of the survey. It is possible that participants who were not on NTM medication had asymptomatic NTM infection confirmed by skin and/or antibody tests,1 or that they had been successfully treated in the past, and thus, no longer required NTM treatment. Contrasting answers to the questions about the duration of living with NTMLD and duration of NTM medication (which was missing for those currently not on medication) could have provided more explanations. Other reasons for not undergoing NTM treatment could be related to either drug non-adherence or drug intolerance.13 Many participants without NTM medication (60.2%) reported considering themselves as having an ongoing NTMLD, which was likely an indication of non-negligible symptom burden among them. A significant reason, such as drug intolerance, could have influenced the decision of not establishing or halting NTM medication for such individuals. Indeed, compared to those with any NTM therapy, more (although not statistically significantly) respondents without NTM therapies reported undergoing surgery to treat their NTMLD, which is important given that surgery could be one of the alternative treatments for NTMLD patients with severe NTM drug intolerance.1 Future research should include follow-up questions for respondents without NTM therapies to determine reasons for not initiating or stopping NTM treatment and its impact on symptom burden.
Limitations
In the present study, the information about specific mycobacteria or infection causing the disease was not available, and data on what type of treatment was used to treat patients’ NTMLD were also limited. Future similar studies should include NTM species identification to the questionnaires as this can explain the choice for NTM medication.1 To establish successful treatment outcomes, it is critically important to fully evaluate, correctly diagnose, and treat NTMLD and other co-existing lung diseases which are common and are often difficult to manage.28 Although NTM therapies are presumed to help with symptoms, they can have substantial side effects (including but not limited to gastrointestinal, hearing, and visual effects).13 Recently published results from a 10-year retrospective cohort study from Italy showed that approximately 40% of NTMLD patients had experienced adverse effects from NTM medication, among whom almost one-third of patients could not continue their therapies.43 The effect of unmeasured confounding in our study, due to unknowing what kind of treatment was used and for which NTM species, could be significant. Not accounting for other co-existing lung disease medication and its impact on patient’s symptoms can raise similar issues.
Furthermore, this was a cross-sectional survey, and no longitudinal data were collected. The present study could not assess the effect of treatment on symptoms over time, nor did the survey ask the sequence number of the NTM diagnosis or treatment, which is a substantial limitation given the possibility of reinfection and recurrence of NTMLD.31-33 The study used a convenience sample of people associated with the BronchandNTM360social, and thus, there is a possibility of selection bias that might have led to over-representation of participants with some demographic characteristics or with co-existing pulmonary disorders. No causal inferences should be applied to the general population.
Finally, the identification of NTMLD is not always straightforward. Establishing a diagnosis of NTMLD for patients with pulmonary symptoms requires 3 criteria: (1) a compatible chest radiograph or chest high-resolution computed tomography (CT) scan, (2) a minimum of 2 positive sputum cultures for nontuberculous acid-fast bacilli or 1 positive bronchial wash/lavage or 1 positive biopsy culture, and finally, (3) the exclusion of other diseases to account for symptoms and radiographic abnormalities.1 Our analysis was based on self-reported data; the diagnosis of NTM was not confirmed. Our study did not use any standard measures of disease severity such as CT scan results or a pulmonary function test to account for differences in symptomatology observed in NTMLD patients with versus without NTMLD medication. Previous research has shown significantly greater declines in forced expiratory volume in 1 second and forced vital capacity among unsuccessfully treated patients with NTMLD as compared to those without NTMLD treatment or those with successful treatment outcomes.44
Despite its limitations, the present study is a good example of a research initiative that can be undertaken in the absence of any ongoing surveillance. The investigators can no longer explain global growth in prevalence and incidence rates of NTMLD by advanced diagnostic procedures only.45 We aimed to better understand the burden of NTMLD-related symptoms. Our findings showed how common symptoms were and how often they had troubled patients. Further investigations are needed to explore whether increased symptoms are related to differences in disease severity and/or medication effects.
Conclusion
Patients currently on any medication to treat their NTMLD reported more symptoms associated with their NTMLD. Although more research is still needed, the present study provides insights that may help with the development of a practical instrument (e.g., a composite symptom score) for symptoms surveillance and assessment of the burden of NTMLD.
Acknowledgements
Author contributions: VP conducted data analysis and interpretation, drafted and revised the manuscript. DM, VP, DP, EM, QZ, TA, RC provided substantial contributions to the concept and design. RC, DM, QZ, TA, DP, EM critically revised the manuscript for important intellectual content. DM provided supervision, administrative, technical, and material support. DP and EM were involved in obtaining the funding.
The NTM survey was developed by the COPD Foundation with input from Amy Leitman and Susan Wisliceny from NTM Info & Research and from Insmed Incorporated. The findings achieved herein are solely the responsibility of the authors.
Declaration of Interest
The authors report no conflicts of interest in this work. VP served as a Research Graduate Assistant at the University of Kentucky and assisted the COPD Foundation analysis team during this project. DM, DP and EM are staff members of the COPD Foundation. QZ is a former employee of Insmed Incorporated. RC is a Research Assistant Professor at the University of Kentucky. TA serves as the Chair of the COPD Foundation’s Bronchiectasis and NTM Research Registry.