Running Head: COPD and Emphysema and Outcomes in COVID-19
Funding Support: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Date of acceptance: March25, 2021 │ Published online: March 26, 2021
Abbreviations: coronavirus-disease-2019, COVID-19; chronic obstructive pulmonary disease, COPD; body-mass index, BMI; high-flow nasal therapy, HFNT; noninvasive positive pressure ventilation, NIPPV; interquartile range, IQR; intensive care unit, ICU; acute respiratory distress syndrome, ARDS; computed tomography, CT; confidence interval, CI; reverse transcription polymerase chain reaction, RT-PCR; C-reactive protein, CRP; lactate dehydrogenase, LDH; interleukin-6, IL-6; forced expiratory volume in 1 second, FEV1; coronary artery disease, CAD; congestive heart failure, CHF; chronic kidney disease, CKD; end-stage renal disease, ESRD; hemodialysis, HD; mechanical ventilation, MV; absolute lymphocyte count, ALC; hazard ratio, HZ; Thoracic Medicine and Surgery department, TMS
Citation: Marron RM, Zheng M, Romero GF, et al.Impact of chronic obstructive pulmonary disease and emphysema on outcomes of hospitalized patients with coronavirus 2019 pneumonia. Chronic Obstr Pulm Dis. 2021; 8(2): 255-268. doi: http://doi.org/10.15326/jcopdf.2020.0200
Background
Coronavirus disease 2019 (COVID-19) presents with a wide range of severity and has caused a worldwide pandemic.1,2 Initial reports from China described a disease where 81% of individuals who test positive are asymptomatic, have mild symptoms, or a mild pneumonia, while 19% have disease severe enough to warrant hospitalization, and 5% overall progressing to critical illness and requiring intensive care.3 Predictors for disease severity, intensive care unit admission, use of mechanical ventilation, or death reported initially out of China included age over 65 years and presence of comorbid disease.4 Comorbid disease included cardiovascular disease, diabetes, hypertension, chronic obstructive pulmonary disease (COPD), and immunosuppression.
Severity of outcomes in patients with COPD hospitalized with COVID-19 in reported data varies. An early report out of China of 1099 patients with COVID-19 had 137 current smokers, 12 former smokers, and only 12 patients (1.1%) with COPD.4 A study of 191 hospitalized patients with COVID-19 from China only reported 6 patients with COPD, with 4 dying (7% of total deaths) and 2 surviving to hospital discharge and only 11 patients of the 191 were smokers.5 A report of 90 patients infected reported only 1 with COPD, with more common comorbidities including coronary artery disease, diabetes, and hypertension.6 A series of 1591 patients admitted to the intensive care unit (ICU) in Italy with COVID-19 reported 4% of patients with COPD, while other comorbidities such as hypertension were more prevalent.7 In the United States, a case series describing 21 patients admitted to ICUs in the Seattle area with COVID-19 included 5 smokers and only 1 person with COPD.8 Two cohorts from New York City had the prevalence of COPD between 5.1%–5.4%.9,10
Chronic lung disease was only present in 2.5% of patients in a cohort study of patients assessing risk of progression to acute respiratory distress syndrome (ARDS) or death in China11 and emphysema was present in <2 % in multiple studies assessing computed tomography (CT) imaging features of COVID-19 disease.12,13 A meta-analysis of 7 studies with a total of 1592 patients with COVID-19 found only 1 study where COPD was a significant predictor of severe COVID-19 disease. When pooling the data of all 7 studies, however, COPD was associated with severe COVID-19 disease with an odds ratio of 5.69 (confidence interval, [CI] 2.49 to13.00).14 Another analysis of 1590 patients with COVID-19 found that a diagnosis of COPD carried an odds ratio of 2.68 (CI 1.42 to5.04) for ICU admission, mechanical ventilation, or death.15 COPD was predominantly reported as a comorbidity in these studies either by patient report on hospital admission or via extraction of an existing diagnosis in the medical record.5,16,17
Herein, in this single-center retrospective study we seek to assess the impact of a diagnosis of COPD and/or the presence of emphysema on CT on the severity of presentation and short-term outcomes in patients with COVID-19.
Methods
Patient Population
We performed a retrospective chart review of adult patients 18 years or older admitted to Temple University Hospital in Philadelphia, Pennsylvania with COVID-19 from March 18, 2020 to May 04, 2020. Data was collected through manual data extraction via the electronic medical record with the use of quality evaluations by the investigators.
Diagnosis of Coronavirus 2019 Infection
The diagnosis of patients with COVID-19 pneumonia was based on symptoms, presence of infiltrates on chest X-ray or high-resolution CT scan with a positive reverse transcription polymerase chain reaction (RT-PCR) nasopharyngeal swab for COVID-19. High resolution CT scans were performed for clinical utility and graded and interpreted by a board-certified radiologist.
Patients with negative RT-PCR for COVID-19 test were excluded. The study was approved by the Temple University Institutional Review Board (Protocol number 26854).
Definition of COPD/Emphysema
Patients were divided into 2 cohorts for analysis - patients with a history of COPD and/or emphysema on the CT of the chest and those with neither. All patients included in the COPD/emphysema cohort had either available spirometry showing irreversible airflow obstruction or a history of cigarette smoking with outpatient use of an inhaled bronchodilator, and/or findings of emphysema on CT as interpreted by a board-certified radiologist.
Clinical Variables
Clinical variables collected included age, sex, race, body mass index (BMI), comorbidities, and smoking status. Laboratory markers suggestive of severity of COVID-19 collected included absolute lymphocyte count (ALC), such as C-reactive protein (CRP), lactate dehydrogenase (LDH), interleukin-6 (IL-6), D-dimer, and ferritin. Also analyzed were troponin and brain natriuretic peptide. Outcomes analyzed included maximal ventilatory support required (oxygen supplementation via nasal cannula, high-flow nasal cannula oxygen therapy [HFNT], noninvasive positive-pressure ventilation [NIPPV], or invasive mechanical ventilation), need for intensive care, total hospital length-of-stay, and mortality.
Propensity Scores Matching
To identify comparable groups with and without COPD/emphysema, we used propensity scores matching to derive the 2 matched groups for data analysis. A nearest neighbor 1:1 variable ratio, parallel, balanced propensity scores matching method was used after generating the propensity scores from the variables age, BMI, d-dimer, CRP, ferritin, fibrinogen, absolute lymphocyte count, percentage of lymphocytes, and LDH. Given the relatively small sample size and to confirm that bias was not introduced through propensity matching, univariate and multivariate regression analyses were also performed for the outcomes of mortality, invasive mechanical ventilation, intensive care unit admission, and hospital length-of-stay.
Primary Objective
The primary objective of this study was to determine if patients admitted with a clinical history of COPD and/or radiographic diagnosis of emphysema have worse outcomes associated with COVID-19 pneumonia as compared to patients without COPD/emphysema.
Statistical Analysis
Data were expressed as mean ± standard deviation or median (interquartile range [IQR]) where applicable for continuous variables and frequencies with percentages for categorical variables. Continuous variables were compared between the 2 groups using 2-sample t-test and categorical variables with the use of the Pearson chi-square test. The non-normally distributed laboratory variables were compared between the 2 groups using a 2-sample Wilcoxon rank-sum test. Survival data was assessed by Kaplan-Meier curve and compared by log-rank test. A p-value <0.05 was considered statistically significant. Univariate and multivariate regression analysis was performed for 4 primary outcomes–mortality, invasive mechanical ventilation, intensive care unit admission, and hospital length of stay–in order to confirm the findings of the propensity-matching analysis. For each outcome, COPD/emphysema was analyzed as well as several variables that were deemed clinically relevant and had significant differences on initial analysis with 2-sample t-test, Pearson chi-square test, or 2-sample Wilcoxon rank-sum test. All the data were analyzed using SAS 9.4 (SAS Institute, Cary, North Carolina) and Stata 14.0 (StataCorp. LP, College Station, Texas).
Results
A total of 577 patients with nasopharyngeal RT-PCR positive for COVID-19, admitted to the hospital between March 18-May 4, 2020, were included in the analysis. Of these patients, 103 had either a historical diagnosis of COPD and/or radiographic evidence of significant emphysema.
Patients with COPD/Emphysema
Fifty-seven of the 103 patients in the COPD/emphysema cohort had a clinical diagnosis of COPD with 46 of the 103 patients having significant radiographic evidence of emphysema. Sixteen patients had pulmonary function testing available via our electronic medical records, with a median forced-expiratory volume in 1 second (FEV1) of 1.5 liters (69% of predicted). Eleven patients had evidence of chronic hypercapnia on arterial blood gas and 10 patients were on supplemental oxygen at home.
Fifty-three patients (51.5%) were using either a long-acting muscarinic agent, long-acting beta2-agonist, or inhaled corticosteroid as an outpatient. Forty-nine of these patients (47.6%) were on combination therapy. Sixty-one patients (59.2%) were initiated on a long-acting muscarinic, long-acting beta2- agonist, or inhaled corticosteroid delivered via nebulized or hand-held metered dose inhaler while hospitalized. Ninety-seven patients (94.1%) received systemic corticosteroids while hospitalized for COVID-19. The 7 patients who did not receive steroids had either no pulmonary symptoms, a relative contraindication such as sepsis from bacterial infection or active diverticulitis or left the hospital against medical advice before treatment was initiated.
All patients received CT scans for clinical care purposes and all imaging was interpreted and reviewed by a board-certified radiologist. CTs showed evidence of emphysema in 46 patients. The degree of emphysema was quantified via CT densitometry with a cutoff criterion of –950 Hounsfield units. Of these patients, the average percentage of parenchymal emphysema was 5.6% (+5.11%) in the left lung and 5.6% (+5.3%) in the right lung.
Patient Demographics
The COPD/emphysema cohort had a median age of 67 years (62–75), which was significantly older than the non-COPD cohort with a median age of 58 years (46–67) (p=<0.0001). Gender was statistically similar between the 2 cohorts. The COPD/emphysema cohort had a lower median BMI (28.3) compared to the non-COPD cohort (31.1) (p=<0.0001). Smoking history determined through intake history showed the COPD/emphysema cohort had higher median pack years (35, IQR 10.5–45)) compared to the non-COPD cohort (20, 11.8–36), but this was not a statistically significant finding. More patients in the COPD/emphysema cohort (28.2%) were still actively smoking. Complete demographics are shown in Table 1.
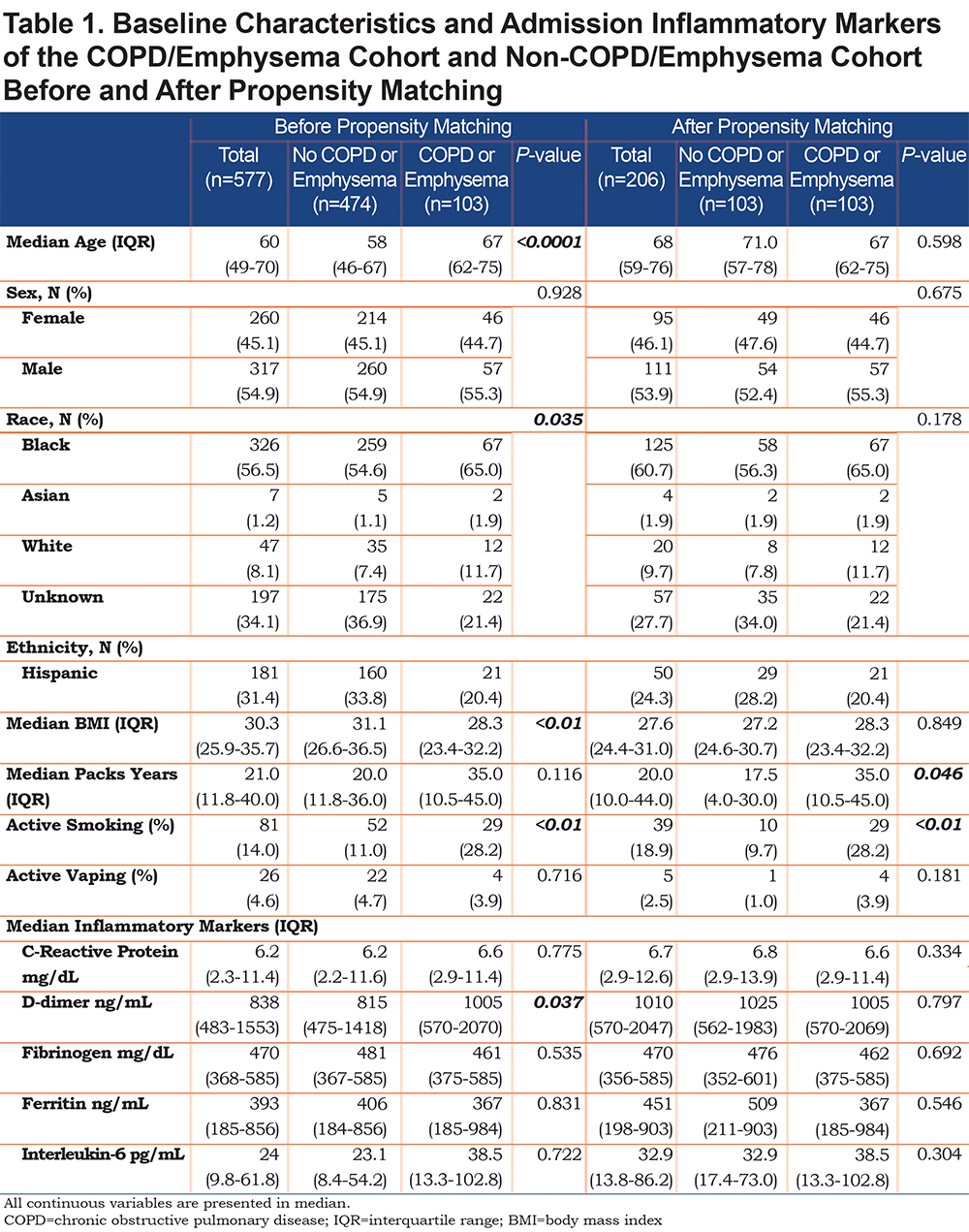
Laboratory Findings
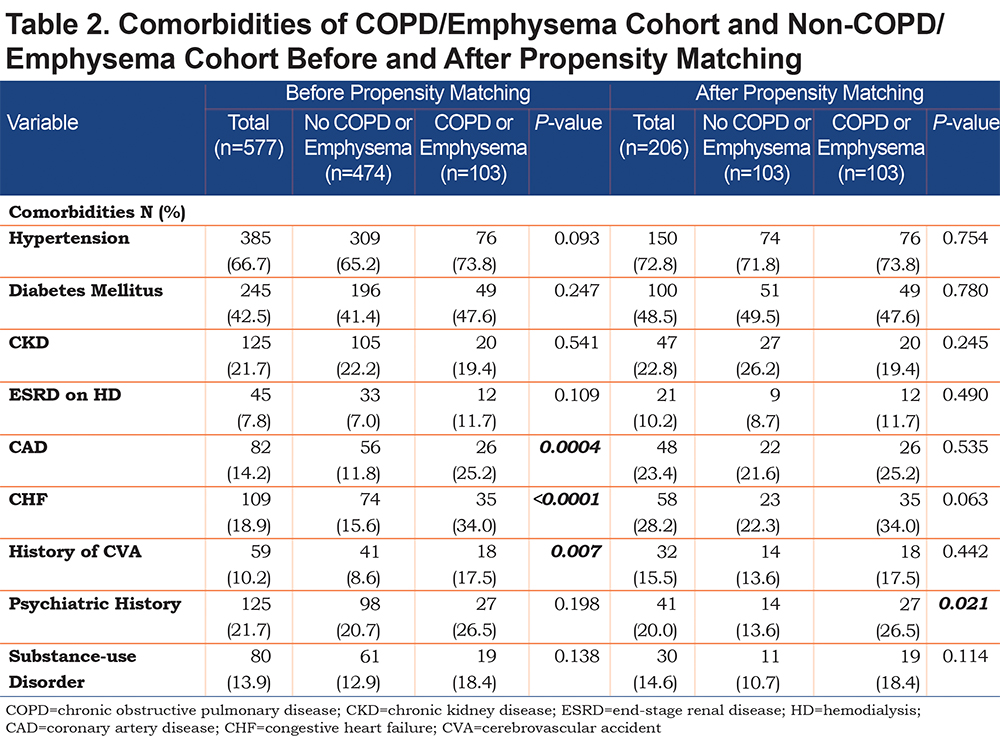
On admission, we used inflammatory markers as a surrogate to represent the severity of a COVID-19 associated cytokine storm. The COPD cohort had higher IL-6 levels compared to the non-COPD cohort, but the difference was not statistically significant. However, the COPD cohort had a significantly higher median D-Dimer (1005 ng/mL) compared to the non-COPD cohort. As expected, the COPD cohort had more comorbidities than the non-COPD cohort (represented in Table 2), with the difference in coronary artery disease (25.2% versus 11.8%), congestive heart failure (34% versus 15.6%) and history of stroke (17.5% versus 8.6%) being statistically significant.
Clinical Outcomes
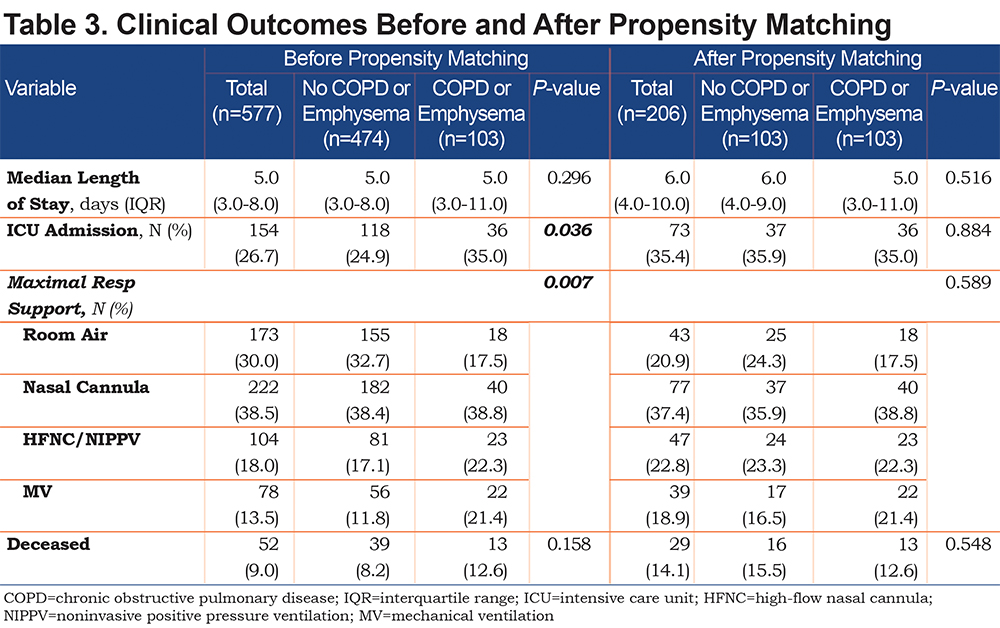
Outcomes between the 2 cohorts are shown in Table 3. The COPD/emphysema cohort had significantly more ICU admissions (35% versus 24.9%, p=0.036) and needed more advanced respiratory support, including oxygen via HFNT, NIPPV (22.3% versus 17.1%) and mechanical ventilation (21.4% versus 11.8%) compared to the non-COPD cohort. Mortality was not significantly different between the 2 cohorts.
Propensity Matching
We applied propensity matching for potential confounders of age, BMI, and severity of COVID-19 on presentation. After propensity matching, the 103 patients of the COPD cohort were compared to 103 patients in the non-COPD cohort. Full demographic, comorbidities, and laboratory data are shown in Table 1.
After propensity matching, there was no statistically significant difference observed between the COPD and the non-COPD cohort in age, BMI, or any of the selected inflammatory markers. Median pack years and active smoking status were statistically higher in the COPD cohort. Of note, the statistical difference seen prior in coronary artery disease, congestive heart failure, and stroke history was not observed in the cohorts after propensity matching (Table 2).
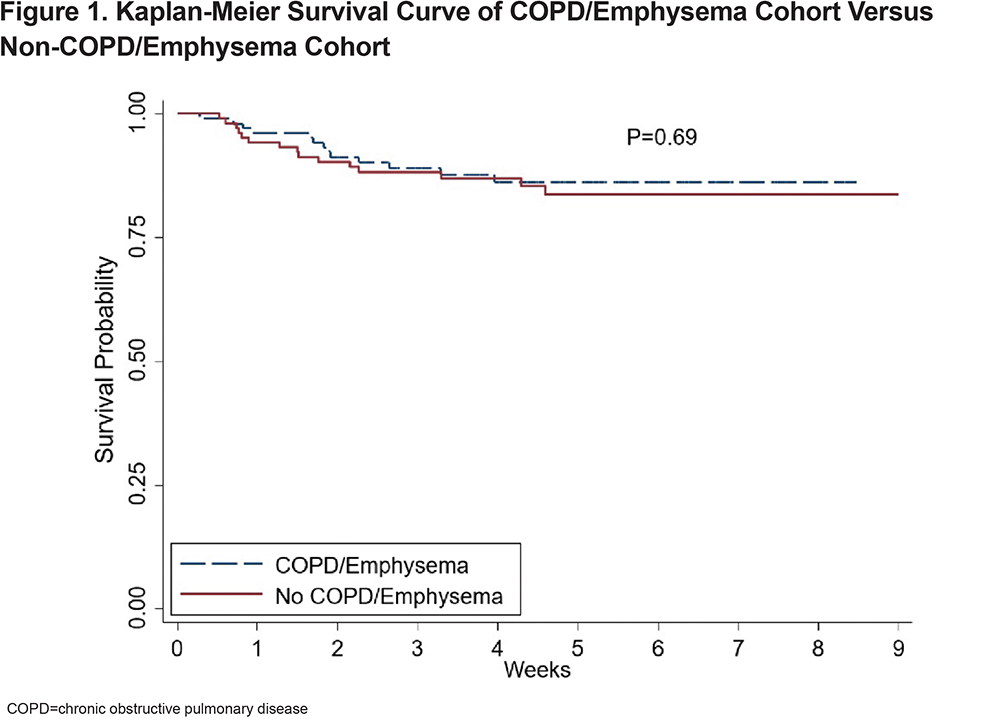
Outcomes of the matched cohorts are shown in Table 3. There was no statistically significant difference between ICU admissions and level of respiratory failure after propensity matching between the COPD and non-COPD cohort. Survival probability was assessed via Kaplan-Meier and there was no difference in survival between the COPD and non-COPD cohort over the first 80 days after initial admission for COVID-19. (Figure 1)
Univariate and Multivariate Regression Analysis
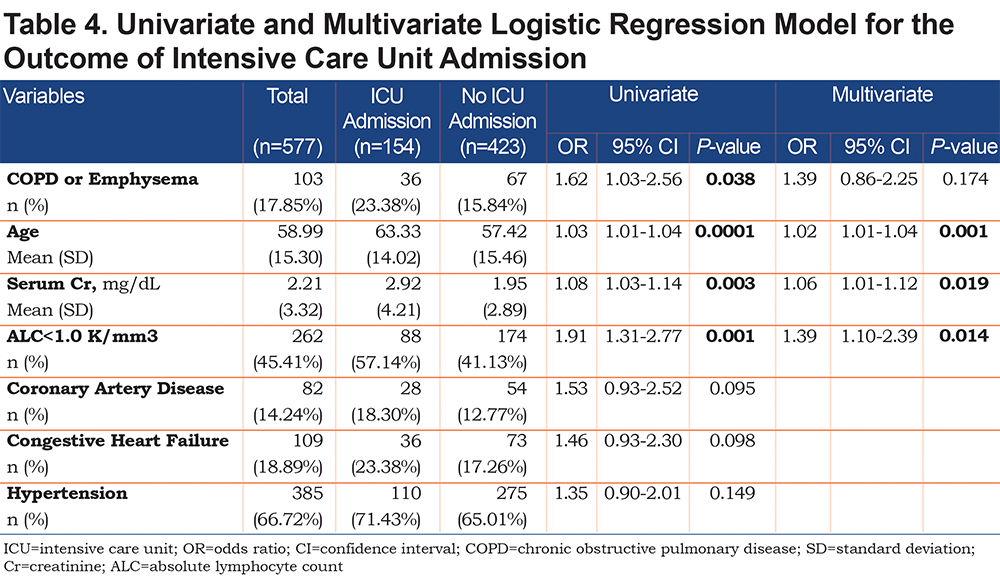
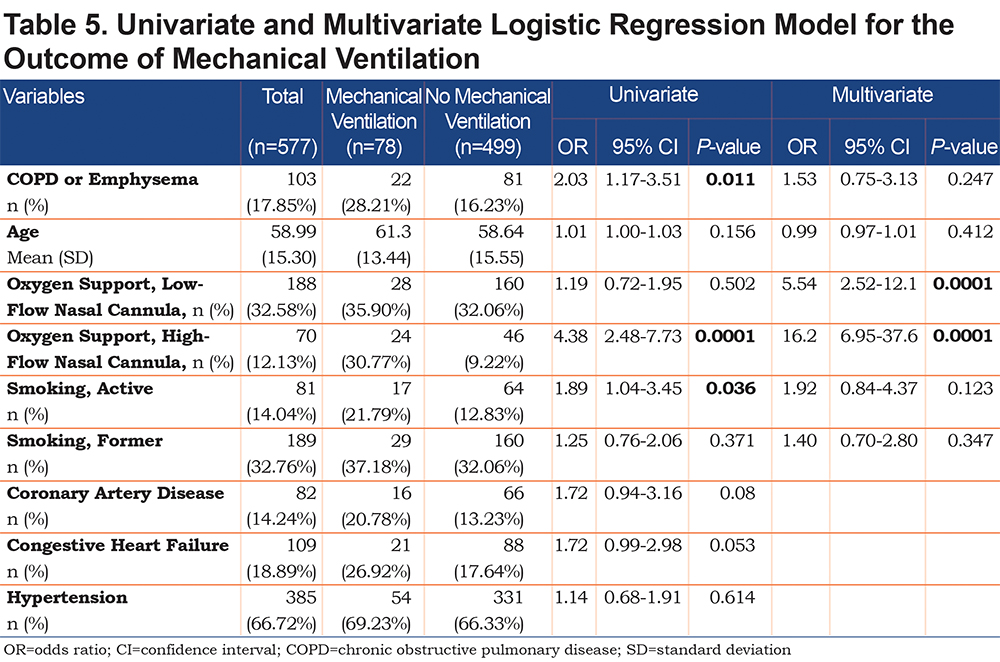
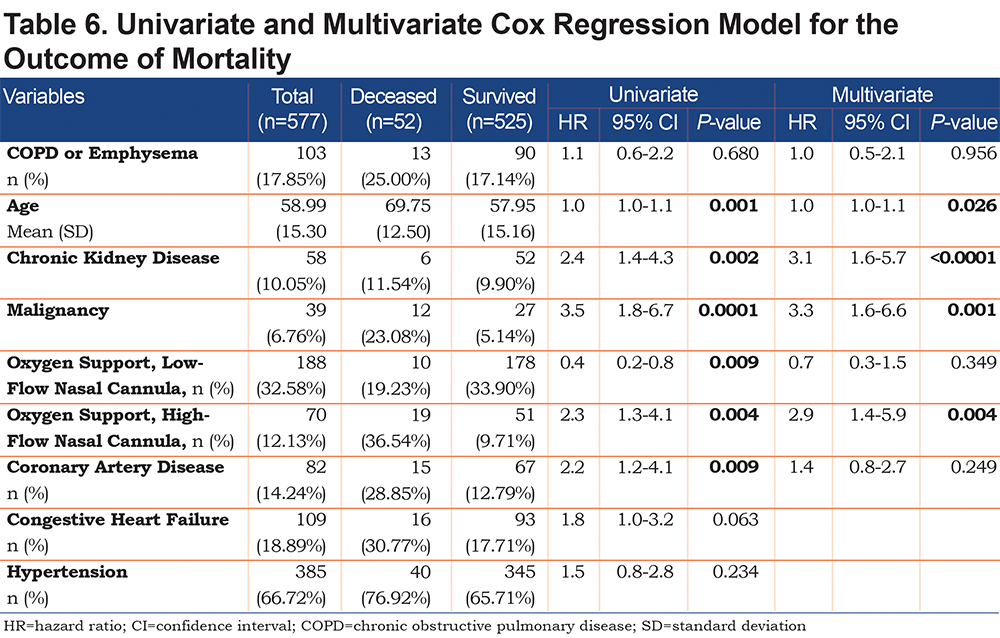
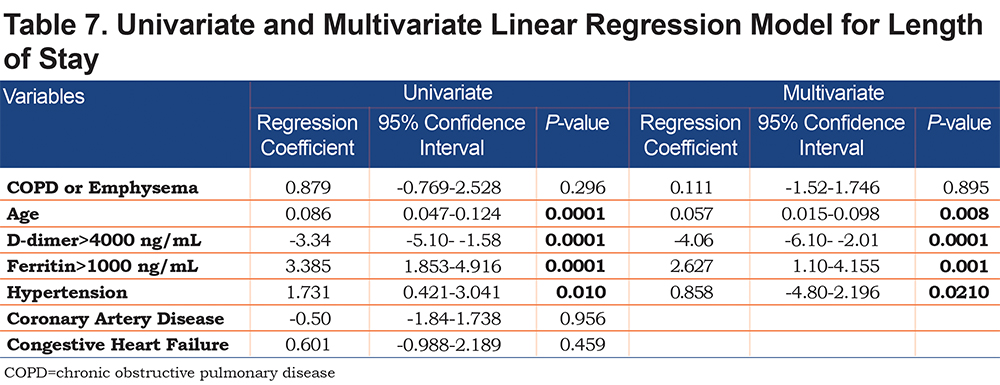
On univariate analysis a diagnosis of COPD emphysema was associated with a higher rate of ICU admission with an odds ratio of 1.62 (1.03–2.56, p=0.038) and with a higher risk of invasive mechanical ventilation with an odds ratio of 2.03 (1.17–3.51, p=0.011). There was no significant difference for mortality with an odds ratio of 1.01 (0.6–2.2, p=0.68) or hospital length-of-stay (p=0.296). When multivariate regression analysis with variables significant on univariate analysis was performed, a diagnosis of COPD/emphysema was not associated with significant differences in any of the primary outcomes. This analysis is shown in Tables 4, 5, 6, 7.
Discussion
This retrospective observational study of patients hospitalized with COVID-19 found no major differences in the outcomes between patients with and without COPD and/or emphysema after propensity matching for age, BMI, and markers associated with COVID-19 disease (ALC, ferritin, D-Dimer, LDH, fibrinogen, and CRP). In the unmatched cohorts we do not report a higher rate of mortality or hospital length of stay in patients with COPD and/or emphysema but did find that they more frequently were admitted to the ICU and required higher levels of maximal respiratory support. Univariate and multivariate regression analyses yielded similar results.
Patients with COPD and/or emphysema were significantly older, had a lower BMI, had higher D-Dimer values, and higher rates of cardiovascular comorbidity (coronary artery disease, congestive heart failure, and stroke). Of note, the higher D-dimer level may have been due to the older age in the COPD/emphysema group. Univariate and multivariate regression analysis did confirm that age, but not COPD/emphysema, was correlated with an elevated D-dimer level.
The cohort of patients had a relatively high prevalence of COPD/emphysema, likely due to high rates of cigarette smoking in the area around the study hospital and the fact that the hospital is a referral center for patients with COPD. While active smokers had a higher rate of mechanical ventilation on univariate analysis, neither active nor former smokers did after multivariate analysis (Table 5). A significant number of patients were on controller-inhaler therapy as an outpatient, some on inhaled corticosteroids. Debate over potential impairment of antiviral responses in those on inhaled corticosteroids with COVID-19 exists, however, a recent meta-analysis and a systematic review have not shown a difference in mortality or rates of severe disease.18,19 We chose to propensity-match for age because older patients with COVID-19 have been shown to have worse outcomes and higher mortality.8,15,16,20 BMI was also matched both because obesity has been associated with worse COVID-19 outcomes21,22 and because both obesity and low BMI are associated with worse outcomes in patients with COPD.23,24 COVID-19 has been associated with lymphopenia as well as elevated inflammatory markers. These markers of elevation can correlate with COVID-19 disease severity so we chose to include it in our propensity matching.5,10,25-27 We did not propensity match for a history of coronary artery disease, congestive heart failure, or history of prior stroke because cardiovascular disease is a known, associated comorbidity in patients with COPD,28,29 but it is worth noting that when controlling for age, our propensity-matched groups did not have significant differences with regards to these comorbidities, nor with others such as hypertension, diabetes mellitus, or chronic kidney disease. Unsurprisingly, active smoking was higher in the COPD/Emphysema cohort both before and after propensity matching.
Comorbid disease has been associated with worse outcomes in patients with COVID-19. Specifically, comorbid hypertension, diabetes, heart disease, and kidney disease have been correlated with increased risk of hospitalization and death.7,9,15,30,31 In a meta-analysis, although only 1 of 7 studies had shown a significant association, pooled data showed COPD to be associated with severe COVID-19 disease. The value of our propensity matching methodology is to control for age, BMI, and known laboratory markers associated with COVID-19 disease in comparing patients with and without COPD/emphysema.
The similar outcomes for patients with and without COPD/emphysema at our large academic center suggest that patients with COPD may not necessarily be at higher risk for worse outcomes when hospitalized for COVID-19. Similar findings have recently been reported on multivariate analysis for the outcomes of ICU admission and death.32 We report data from the earlier months of the pandemic in the United States when practice varied on utilization of invasive mechanical ventilation rather than using NIPPV or HFNT out of a concern for aerosolization of virus particles and increased risk of disease transmission to health care workers.33-36 Invasive mechanical ventilation has been associated with higher mortality in COVID-19 patients,7,37,38 and evidence is accumulating that aerosolization from the respiratory tract on HFNT and NIPPV is less concerning.39,40 Data reported by our institution in a separate study reported relatively low use of invasive mechanical ventilation and favorable outcomes associated with the use of HFNT.41 In addition, many of our patients received glucocorticoids and bronchodilators either in the form of inhalers or nebulized therapy regardless when indicated. The avoidance of invasive mechanical ventilation, use of glucocorticoids, and use of bronchodilators all are factors that may explain why our propensity-matched group of patients with COPD and/or emphysema did not have worse outcomes.
Our study has several limitations, including the following examples. It is a single-center, retrospective study. COPD is a heterogenous disease with various phenotypes and this study is limited in not evaluating these differences such as disease severity and exacerbation history. Our control group had a high rate of active smoking, although the COPD/emphysema group had significantly higher rates before and after matching. We chose to group patients with a diagnosis of COPD along with patients with emphysema on CT imaging as these are related conditions and it allowed a greater number of patients to be compared. We attempted to objectively characterize COPD/emphysema based on a history of smoking, prior prescription of a long-acting bronchodilator and by having all CT scans evaluated by a radiologist to determine presence of emphysema. It is established that COPD is underdiagnosed in the population in general and that may lead to missing some patients affected with early, asymptomatic, or undiagnosed disease which we attempt to mitigate with our radiologic inclusion criteria.42-44
In conclusion, our findings suggest that although patients with COPD and/or emphysema hospitalized with COVID-19 were found to have higher maximal respiratory requirements and more frequent ICU admission prior to propensity matching, they do not necessarily have worse outcomes including mortality. Further investigation into outcomes of patients with these common diseases in the setting of the ongoing COVID-19 pandemic is warranted.
Acknowledgements
Author contributions: RMM, MZ, GFR, JS, and GJC formulated the overall study design. RMM, MZ, GFR, RP, IL, AT, TS, MK, and NP assisted in data collection and consolidation. HZ performed statistical analysis. RMM, MZ, GFR drafted the manuscript. GJC revised and reviewed the manuscript.
Temple University COVID-19 Research Group: Aaron Mishkin, Infectious Disease; Abbas Abbas, Thoracic Medicine and Surgery (TMS); Abhijit S. Pathak, Surgery; Abhinav Rastogi, Admin; Adam Diamond, Pharmacy; Aditi Satti, TMS; Adria Simon, Emergency Medicine; Ahmed Soliman, TMS; Alan Braveman, TMS; Albert J. Mamary, TMS; Aloknath Pandya, TMS; Amy Goldberg, Surgery; Amy Kambo, TMS; Andrew Gangemi, TMS; Anjali Vaidya, Cardiology; Ann Davison, TMS; Anuj Basil, Cardiology; Charles T. Bakhos, TMS; Bill Cornwell, TMS; Brianna Sanguily, TMS; Brittany Corso, Internal Medicine; Carla Grabianowski, TMS; Carly Sedlock, Infectious Disease; Catherine Myers, TMS; ; Chenna Kesava Reddy Mandapati, TMS; Cherie Erkmen, TMS; Chethan Gangireddy, Cardiology; Chih-ru Lin, TMS; Christopher T. Burks, Lab Administration; Claire Raab, Internal Medicine; Deborah Crabbe, Cardiology; Crystal Chen, Internal Medicine; Daniel Edmundowicz, Cardiology; Daniel Sacher, TMS; Daniel Salerno, TMS; Daniele Simon, Emergency Medicine; David Ambrose, TMS; David Ciccolella, TMS; Debra Gillman, TMS; Dolores Fehrle, TMS; Dominic Morano, TMS; Donnalynn Bassler, TMS; Edmund Cronin, Cardiology; Eduardo Dominguez, TMS; Ekamjeet Randhawa, TMS; Eman Hamad, Cardiology; Eneida Male, TMS; Erin Narewski, TMS; Francis Cordova, TMS; Frederic Jaffe, TMS; Frederich Kueppers, TMS; Fusun Dikengil, TMS; Jonathan Galli, TMS; Andrew Gangemi, TMS; Jamie Garfield, TMS; Gayle Jones, TMS; Gennaro Calendo, TMS; Gerard Criner, TMS; Gilbert D'Alonzo, TMS; Ginny Marmolejos, TMS; Matthew Gordon, TMS; Gregory Millio, Internal Medicine; Rohit Gupta, TMS; Gustavo Fernandez, TMS; Hannah Simborio, TMS; John Harwood Scott, TMS; Heidi Shore-Brown, TMS; Hernan Alvarado, Respiratory Care; Ho-Man Yeung, Internal Medicine; Ibraheem Yousef, TMS; Ifeoma Oriaku, TMS; Iris Jung-won Lee, Nephrology; Isaac Whitman, Cardiology; James Brown, TMS; Jamie L. Garfield, TMS; Janpreet Mokha, TMS; Jason Gallagher, School of Pharmacy; Jeffrey Stewart, TMS; Jenna Murray, TMS; Jessica Tang, TMS; Jeyssa Gonzalez, TMS; Jichuan Wu, TMS; Jiji Thomas, TMS; Jim Murrett, Ultrasound Fellow; Joanna Beros, TMS; John M. Travaline, TMS; Jolly Varghese, TMS; Jordan Senchak, Internal Medicine; Joseph Lambert, TMS; Joseph Ramzy, TMS; Joshua Cooper, Cardiology; Jun Song, Medical Student; Junad Chowdhury, TMS; Kaitlin Kennedy, TMS; Karim B. Ahmed, TMS; Karim Loukmane, TMS; Karthik Shenoy, TMS; Kathleen Brennan, TMS; Keith Johnson, TMS; Kevin Carney, TMS; Kraftin Schreyer, Emergency Medicine; Kristin Criner, Endo; Kumaran, Maruti, Radiology; Lauren Miller, TMS; Laurie Jameson, TMS; Laurie Johnson, TMS; Laurie Kilpatrick, TMS; Lii-Yoong Criner, TMS; Lily Zhang, TMS; Lindsay K. Mcgann, Hospitalist; Llera A. Samuels, TMS; Marc Diamond, TMS; Margaret Kerper, TMS; Maria Vega Sanchez, TMS; Mariola Marcinkienwicz, TMS; Maritza Pedlar, TMS; Mark Aksoy, TMS; Mark Weir, TMS; Marla R. Wolfson, TMS; Marla Wolfson, TMS; Martin Keane, Cardiology; Massa Zantah, TMS; Mathew Zheng, TMS; Matthew Delfiner, Internal Medicine; Matthew Gordon, TMS; Maulin Patel, TMS; Megan Healy, Emergency Medicine; Melinda Darnell, TMS; Melinda Darnell, TMS; Melissa Navaro, TMS; Meredith A. Brisco-Bacik, Cardiology; Michael Bromberg, Hematology; Michael Gannon, Cardiology; Michael Jacobs, TMS; Mira Mandal, TMS; Nanzhou Gou, TMS; Erin Narewski, TMS; Nathaniel Marchetti, TMS; Nathaniel Xander, TMS; Navjot Kaur, TMS; Neil Nadpara, Internal Medicine; Nicole Desai, Internal Medicine; Nicole Mills, TMS; Norihisa Shigemura, Surgery; Ohoud Rehbini, TMS; Oisin O'Corragain, TMS; Omar Sheriff, TMS; Oneida Arosarena, Otolaryngology; Osheen Abramian, TMS; Paige Stanley, TMS; Parag Desai, TMS; Parth Rali, TMS; Patrick Mulhall, Pulm; Pravin Patil, Cardiology; Priju Varghese, Internal Medicine; Puja Dubal, TMS; Puja Patel, TMS; Rachael Blair, TMS; Rajagopalan Rengan, TMS; Rami Alashram, TMS; Randol Hooper, TMS; Rebecca A. Armbruster, Chief Medical Officer; Regina Sheriden, TMS; Robert Marron, TMS; Roberto Caricchio, Rheumatology; Rogers Thomas, TMS; Rohit Gupta, TMS; Rohit Soans, Surgery; Roman Petrov, TMS; Roman Prosniak, TMS; Romulo Fajardo, Surgery; Ruchi Bhutani, TMS; Ryan Townsend, TMS; Sabrina Islam, Cardiology; Samantha Pettigrew, Internal Medicine; Samantha Wallace, TMS; Sameep Sehgal, TMS; Samuel Krachman, TMS; Santosh Dhungana, TMS; Sarah Hoang, TMS; Sean Duffy, TMS; Seema Rani, TMS; Shapiro William, TMS; Sheila Weaver, TMS; Shelu Benny, TMS; Sheril George, TMS; Shuang Sun, TMS; Shubhra Srivastava-Malhotra, TMS; Stephanie Brictson, TMS; Stephanie Spivack, Infectious Disease; Stephanie Tittaferrante, Internal Medicine; Stephanie Yerkes, TMS; Stephen Priest, Internal Medicine; Steve Codella, TMS; Steven G. Kelsen, TMS; Steven Houser, Research; Steven Verga, TMS; Sudhir Bolla, TMS; Sudhir Kotnala, TMS; Sunil Karhadkar, Surgery; Sylvia Johnson, TMS; Tahseen Shariff, TMS; Tammy Jacobs, TMS; Thomas Hooper, TMS; Tom Rogers, TMS; Tony S. Reed, Chief Medical Officer; Tse-Shuen Ku, TMS; Uma Sajjan, TMS; Victor Kim, TMS; Whitney Cabey, Emergency Medicine; Wissam Chatila, TMS; Wuyan Li, TMS; Zach Dorey-Stein, TMS; Zachariah Dorey-Stein, TMS; Zachary D. Repanshek, Emergency Medicine
Declaration of Interest
None of the authors report any financial compensation, financial relationships, intellectual properties, or any other relationships that are relevant to this manuscript or that could have affected its composition.