Running Head: Home Exercise Application for Individuals with COPD
Funding Support: Development of Respercise and GlaxoSmithKline (GSK) study 200182 were funded by GSK.
Date of acceptance: March 25, 2021 │Published online: March 26, 2021
Abbreviations: pulmonary rehabilitation, PR; chronic obstructive pulmonary disease, COPD; digital application, app; coronavirus disease 2019, COVID-19; Self-management Programme of Activity Coping and Education, SPACE; modified Medical Research Council dyspnea scale, mMRC; forced expiratory volume in 1 second, FEV1; 5-repitition sit-to-stand, 5STS; short physical performance battery, SPPB; standard errors, SE; standard deviation, SD
Citation: Yonchuk JG, Mohan D, LeBrasseur NK, George AR, Singh S, Tal-Singer R. Development of Respercise® a digital application for standardizing home exercise in COPD clinical trials. Chronic Obstr Pulm Dis. 2021; 8(2): 269-276. doi: http://doi.org/10.15326/jcopdf.2020.0194
Introduction
Physical inactivity and muscle weakness are strong predictors of hospitalizations and mortality in individuals with chronic obstructive pulmonary disease (COPD).1 Pulmonary rehabilitation (PR) is effective in COPD, significantly improving exercise capacity, dyspnea, fatigue and sense of control,2 however, PR uptake remains poor for various reasons, including program availability, transportation barriers, cost and poor access. In the United States there is only 1 accredited PR center per 43,000 COPD patients.3
Additional intervention strategies which improve physical activity and exercise capacity in individuals with COPD are, therefore, much needed, and are especially important at a time of restricted general activity, for example during a global pandemic such as the coronavirus disease 2019 (COVID-19) pandemic.4 Digital technologies and home-based interventions5-7 present an opportunity to reach patients lacking access to PR and offer the added advantage of delivering a standardized program for implementation across global multi-center clinical trials,8 especially since PR content and duration varies significantly across countries and individual physiotherapist supervision can similarly vary from center to center.9 Although a digital application offers limited supervision, which may limit its effectiveness in comparison to personalized in-person rehabilitation, an app offers equal encouragement and a standardized set of instructions and exercises to all trial participants. Pulmonary rehabilitation, however, offers the advantage of social interaction as well as including a strong educational component for disease management, which may make the app more suitable for clinical trials or for maintenance programs with those who have had an initial course of traditional PR.
The Self-management Programme of Activity Coping and Education (SPACE) for COPD program at the University Hospitals of Leicester National Health Service Trust was developed to deliver home rehabilitation via a printed manual, and later the internet,5 with demonstrably comparable results to PR.10 Using content from this program, our objectives were to iteratively develop a digital application (app), Respercise,® for use in clinical trials and conduct usability testing of Respercise before assessing feasibility and success of deployment in a clinical trial.
Methods
App Development
Respercise was designed in 2 parallel paths; content inclusion and design concepts. Content was licensed from the Self-management Programme of Activity Coping and Education (SPACE) for COPD® program, then edited and reviewed by clinical researchers and physicians to include information relevant to physical activity, exercise, goal setting, and safety sections suitable for delivery via a digital application in a clinical trial. A consumer device, Garmin Vivofit®, was used for daily step counts for goal setting, and strengthening exercises were conducted thrice weekly using TheraBands.™
Clinical researchers and information technology professionals developed the concept design of the app. The application code/structure was developed using Android Studio 3.1, HTML5, JavaScript, SQLite, JSON and XML data files as data containers. It is used as minimum Android API 18 (Android 4.3 and SQLite 3.7) up to API 27 (Android 8.1 and SQLite 3.19).
The app was designed with 5 main sections: (1) Administration, (2) How to Respercise, (4) a Walking dashboard, a Strength dashboard, and (5) a Help section. The administration section was restricted to use by the site staff only and allowed for setup of the individual program for each participant. The How to Respercise section includes information and educational content about COPD adapted from the SPACE for COPD program, information about the Respercise exercise program, and lifestyle guidance. The Walking dashboard allows participants to enter their daily steps, see the trend of their step count over 7 days, and to receive encouragement messages/awards towards their daily goals. The Strength dashboard includes subsections for each of the 4 strength exercises: bicep curls, step-ups, upright rows, and a sit-to-stand maneuver. Each subsection contains an infographic displaying progress, a “view results” button, a “learn more” button, an “add result” button, and an “awards” section. The learn more section includes written instructions and a video demonstration for each exercise. (Figure 1)
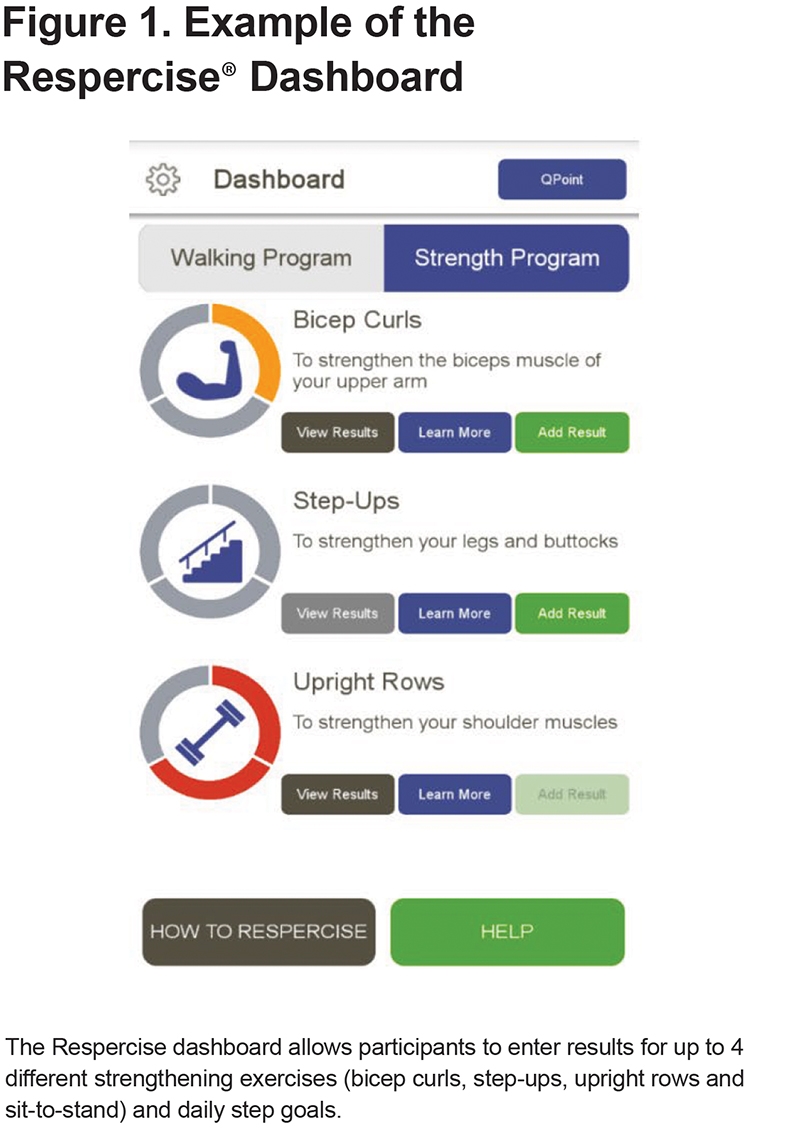
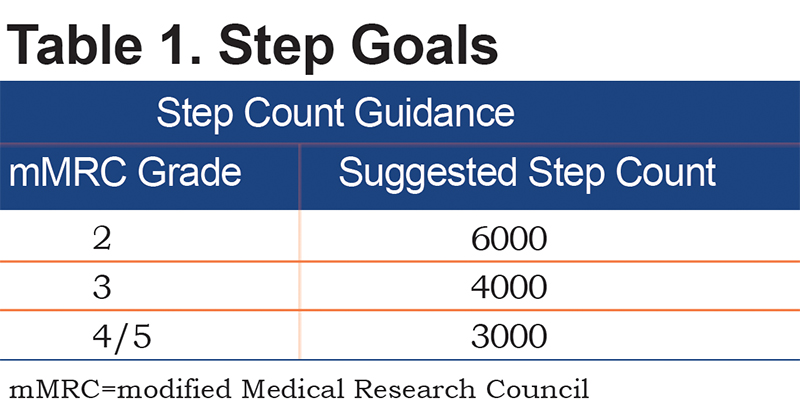
Daily step goals and strength goals for the trial were set in consultation with the principal investigator and based on the participant’s history and modified Medical Research Council dyspnea scale (mMRC) grade (Table 1). At the discretion of the principal investigator, if a participant met or exceeded their daily step goal regularly, it could be increased by 10%. For the strength program, participants were assigned a Theraband for the program and asked to complete 3 distinct sessions consisting of 24 total repetitions (2 sets of 12 or 3 sets of 8) of each exercise in a rolling 7-day period. If successful, they were assigned the next strongest band.
Static buttons for the How to Respercise and Help sections are always available on screen. The Help button, when pressed, displays a dynamic hover screen that provides an overview of the current section, explaining the current section the user is in, and providing buttons to a “Frequently Asked Questions” section, the "End User License Agreement," and contact information for the participant’s site.
Usability Testing in Individuals with COPD and Redesign
A usability test, comprising face-to-face interviews with 12 individuals with COPD aged 50-80 years, was undertaken for the initial app version. Participants were asked about technology attitudes, their COPD condition, and their views on exercise in a semi-structured, qualitative manner, and asked to perform tasks in the app. Responses to app use were categorized into “Like,” “Hmm…,” “Snag,” and “Blocker” categories. The app was redesigned to address major concerns.
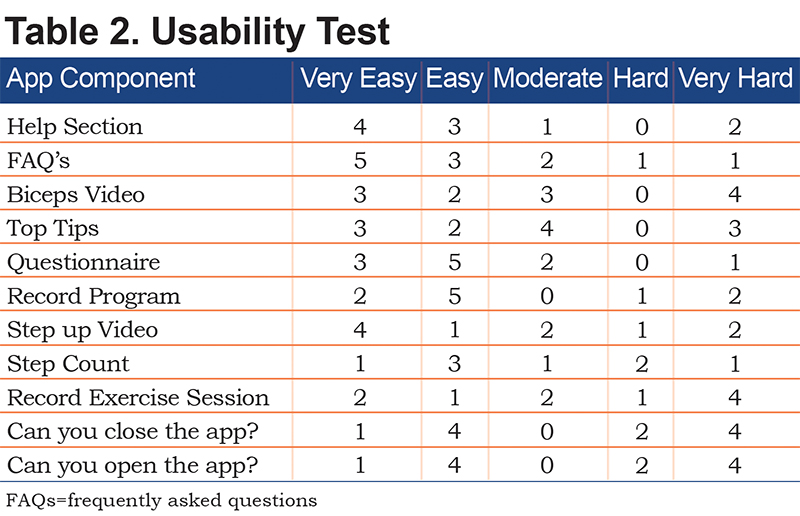
A second round of usability testing was performed in a rehabilitation clinic setting at the University of Leicester; patient feedback was assessed quantitatively via a questionnaire and informally with health care professionals present in the room. Six male and 6 female participants 61-83 years of age were initially asked a series of questions on frequency of various technology utilization. They were then provided devices with Respercise and a physical activity monitor similar to the model used in the clinical trial, were given tasks to complete in the application, and were asked to rate the task on a 5-point scale from Very Easy to Very Hard. Results from the second test were used to make minor alterations (Table 2).
Application in a Clinical Trial
The final production app was deployed in a clinical trial.11 This study was a randomized, placebo-controlled, double-blind, parallel group, phase 2A trial evaluating the safety and efficacy of the selective androgen receptor modulatorGSK2881078 in men and postmenopausal women with COPD. Patients were recruited between February 2018 and June 2019 from 13 clinical sites across the United States, United Kingdom, and Germany. Eligible patients were aged 50–75 years with a confirmed diagnosis of COPD (post-bronchodilator forced expiratory volume in 1 second [FEV1]/forced vital capacity ratio of less than 0.70) and had a post-bronchodilator FEV1 between 30% and 65% of the predicted value. A score of 1–3 on the timed 5-repetition sit-to-stand (5STS) component of the short physical performance battery (SPPB) was used to confirm impaired physical function (a maximum score of 4 for the 5STS denotes no impairment, whereas a patient unable to perform the 5STS would score 0). Patients were either current or former smokers, with a minimum 10 pack-year smoking history, and a body mass index of between 18kg/m2 and 32kg/m2 inclusive.
Key exclusion criteria included use of oral steroids concurrently or within 4 weeks before the screening visit, COPD exacerbation requiring treatment with oral steroids or hospitalization 4 weeks before screening, a score of 0 on any SPPB component, or other conditions or medications that could influence muscle mass or function.
The study protocol was approved by all relevant local institutional review boards or independent ethics committees. The study was conducted in accordance with Good Clinical Practice under the provisions of the Declaration of Helsinki. All patients provided written informed consent.
Patients who met the eligibility criteria were randomly assigned (1:1) to receive either GSK2881078 or placebo once daily orally for 13 weeks. Separate randomizations were generated for each gender. Additionally, all patients undertook the concurrent standardized home-exercise program, Respercise, delivered via a smartphone app for 13 weeks from baseline.
Procedures
Enrolled participants received a Respercise demonstration by site staff, a smartphone with Respercise, TheraBands™ and a Garmin Vivofit® monitor. At the demonstration, Respercise was programmed for an individualized number of strengthening exercises, and baseline step goal based on the participant’s mMRC dyspnea score; subsequent increases in resistance for strengthening exercises and step count goals were automated according to the app’s algorithm and dependent on participant data entry of completed exercises and daily physical activity.
The first 32 study participants to complete the study underwent a semi-structured, in-depth 1-hour phone exit interview using standard exit interview methodology, probing their experiences qualitatively, the results of which were analyzed via a blinded-interim analysis. Due to the qualitative, semi-structured nature of the interviews, the same question may not have been asked to all trial participants. The remaining study participants who completed the study underwent a briefer, 15-minute interview using a check list to confirm findings elicited from full interviews and identify any missing concepts. All interviews were conducted by a third-party, specialist vendor.
The timed 5STS test12 was performed as a measure of lower extremity strength at screening, baseline, day 56 and day 90 for all study participants.
Statistical Analyses
For descriptive results from qualitative interviews, unadjusted means (n or percentage) are presented. For all endpoints with multiple post-baseline assessment such as the 5STS, the analysis was based on a mixed model repeated measures adjusted for treatment, day, treatment x day, and baseline, with day as the repeated factor. Analyses were conducted separately by sex. Results for the per protocol population are included (participants who did not have any acute exacerbations of COPD during the study and who met all trial inclusion/exclusion criteria throughout the study). For change from baseline, adjusted means and corresponding standard error of means (SEs) are presented.
Results
Initial App Usability Testing
Qualitative testing of the initial app version indicated that users liked the activity monitor, videos, texts, and exercises along with the feedback and notifications. Issues of minor concern were the lack of syncing for the Vivofit, the purpose and length of the initial survey, poor screen contrast, and amount of information and clarity on goal setting. “Blocker” items included specific survey questions, small controls, and the resetting of step counts.
Based on input from the initial usability testing, revisions included: a new color scheme, improved navigation and controls, a shorter survey, additional help screens, an introductory video, and background audio information. The main app dashboards were redesigned for uniformity and simpler navigation, ease of use, and data uploading.
Twelve participants with COPD were asked to rate the ease of use of various tasks in the revised app. Tasks such as the survey, looking at tips, watching videos, and finding help were rated as easy by 8 or more participants (Table 1). Recording steps and exercise rated as moderately easy whereas opening and closing the app scored poorly. Minor alterations were made before producing the final app.
Clinical Trial Feasibility
A total of 96 participants were randomized and dosed into the study. Of these, 32 of the participants who first completed the study underwent a 1-hour, in-depth exit interview, with the 35 other trial participants who completed the study given a briefer, 15-minute exit interview.
Overall, across the trial, the home exercise program was reported as easy to fit into daily life (n=45/53; 84.9%).
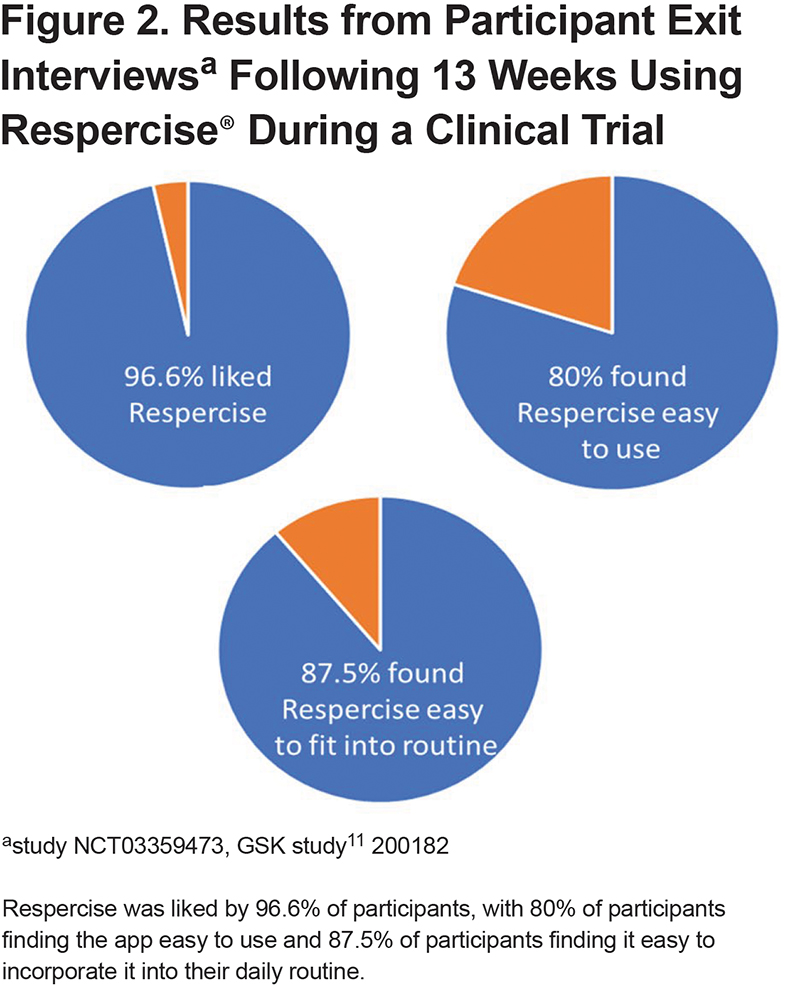
From the first 32 clinical trial participants, feedback was positive; 97% of respondents liked the app, with 1 participant finding the exercises boring. A total of 78% of participants commented on the ease of completing the program, with 80% reporting the program was easy, 16% reporting difficulty with higher resistance TheraBands™ and 1 participant reporting struggling to do upright activities. (Figure 2)
From the briefer interviews of 35 participants, 89% reported that the program was easy to complete with high self-reported adherence (n=17/27; 70.0%). Some participants (n=19/24; 79.2%) reported having to push themselves to complete. Other participants reported feeling stronger (n=6/31; 19.4%) as a result of the home exercises. Most participants (n=12/16; 75.0%) had no issues recording their home exercises.
Some participants (n=3) reported that the device did not work correctly, lost internet connectivity or did not record correctly (n=1 each), suggesting improvements needed prior to use in future trials. Particpants’ suggested improvements to the home exercise program (n=5/19; 26.3%) included: “step exercises could be completed by walking up/downstairs,” “more walking exercises outside the home,” “exercises could accommodate pre-existing physical restrictions,” “increase exercises as participants progress” and “more variety of exercises.”
The percentage of daily step count entries into the app from trial participants was greater than 90% (90% in both treatment arms [females: 96.4%; males: 98.3%] and placebo [females: 95.1 %; males: 92.0%]).
For the per protocol population, there were 14 female and 11 male participants in the placebo arm (Respercise alone, without GSK2881078). Lower extremity function, as measured via 5STS, demonstrated improvement from baseline to day 90 for both males and females with Respercise alone. For females, baseline STS was 16.5 (standard deviation [SD] 7.84) seconds and for males, baseline STS was13.3 (SD 1.52) seconds. Change from baseline at day 90 for adjusted means was, in females -2.22 (SE 0.73) seconds and for males a change of -2.27 (SE 0.58) seconds.
Discussion
Respercise is acceptable to individuals with COPD and can be successfully deployed in a clinical trial as evidenced by participant feedback and clinical improvement in physical function. The improvement in physical function with Respercise, as measured by a greater than 2-second improvement for 5STS in the placebo arm, in a clinical trial setting represents an improvement above the proposed minimally clinically important difference of -1.7 seconds for this measure in COPD.12
Sustained engagement with digital behavior interventions has been shown to be low.13-15 Micro-incentives such as inter-participant gamification, payments linked to usage, or rewards other than congratulatory messages may be more successful in promoting adherence, as has been demonstrated with other digital platforms. Equally, a hybrid approach may be required with tele-coaching alongside the app to retain engagement16 beyond the period studied here. Respercise is currently designed for standardization across clinical trials, and as such, features little in the way of gamification.
For sustained app usage to promote behavioral change, Respercise should incorporate adaptive, personalized features (e.g., different exercises or increasing number of sets of exercises) that change over time in response to users’ behavior and interactions.17 Furthermore, the social interaction encountered within a PR program is often a driver for patients for continued engagement; apps that incorporate social interaction, for example with other patients, may see more sustained use and this could be a goal for future incorporation into the app.
Study Limitations
Our study did have limitations; we were unable to retrieve detailed data on participant entries for step counts and strength measures from the app to study whether patient-reported improvements correlated with objectively measured study assessments of physical activity and strength. The app had to be deployed via a third-party smartphone, which may have been a limitation in terms of device familiarity and internet connectivity, as some trial participants noted. Our study only tested app usage for 13 weeks, therefore, it is unclear whether there would be continued engagement for longer periods of time. Furthermore, clinical trial participants may not represent the broader population of COPD patients, therefore, our findings cannot be generalized in terms of app acceptability. Finally, we need to evaluate the effectiveness of our app and to compare outcomes with PR in future studies.
Conclusion
In conclusion, Respercise can be successfully deployed in clinical trials, offering the opportunity for standardization of exercise in clinical trials and, with further development, could have wider reach as a home-based intervention for individuals with COPD and other lung diseases. A further opportunity may be to use the app as a maintenance strategy following PR or when exercise facilities are not easily available at a time of social distancing such as during the COVID-19 pandemic which restricted the availability of exercise facilities.
Acknowledgements
Author contributions: JGY contributed to the app conception and design, acquisition of data, and writing of the article; DM contributed to the app conception and design, study design and conduct, acquisition of data, and writing of the article; RTS contributed to the app conception and design, acquisition of data, and writing of the article; AG contributed to the app conception and design and revision of the article; SS contributed to the app conception and design, study design and conduct, acquisition of data, and writing of the article; NKL contributed to the review of the app content and revision of the article.
Data sharing statement: Data relating to GSK study 200182 (NCT03359473) will be available on clinicaltrials.gov and via a separate manuscript. Unpublished data is available upon request by contacting the corresponding author.
Declarations of Interest
JGY is a current employee of and shareholder in GlaxoSmithKline (GSK). DM is a former employee of GSK and current employee and shareholder of Genentech/Roche. RTS and ARG are former employees and current shareholders of GSK and RTS reports personal fees from Immunomet, Ena Respiratory, Teva and Vocalis Health. SS reports grants from Actegy, grants from Pfizer, outside the submitted work; and the University Hospitals of Leicester NHS trust own the IP for the SpaceforCOPD content. NKL has nothing to declare.