Running Head: 25-Hydroxy Vitamin D Levels and COPD Symptoms
Funding Support: RMB is supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) grant F32HL143867. MBD is supported by NIH/NHLBI grant R01HL125432. SPIROMICS was supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), and grants from the NIH/NHLBI (U01 HL137880. U01 HL137880 and U24 HL141762), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune, Bayer, Bellerophon Therapeutics, Boehringer- Ingelheim Pharmaceuticals, Inc., Chiesi Farmaceutici S.p.A.. Forest Research Institute, Inc., GlaxoSmithKline, Grifols Therapeutics, Inc., Ikaria, Inc., Novartis Pharmaceuticals Corporation, Nycomed GmbH, ProterixBio, Regeneron Pharmaceuticals, Inc., Sanofi, Sunovion, Takeda Pharmaceutical Company, Theravance Biopharma, and Mylan.
Date of acceptance: March 31, 2021 │ Published online: April 6, 2021
Abbreviations: chronic obstructive pulmonary disease, COPD; Subpopulations and Intermediate Outcome Measures in COPD Study, SPIROMICS; serum 25-hydroxy, 25-OH; COPD Assessment Test, CAT; St George’s Respiratory Questionnaire, SGRQ; Chronic Respiratory Disease Questionnaire, CRQ; body mass index, BMI; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; body mass index, BMI; American Thoracic Society, ATS; 6-minute walk test, 6MWT; modified Medical Research Council dyspnea scale, mMRC; minimal clinically important difference, MCID; Veteran’s Specific Activity Questionnaire, VSAQ
Citation: Burkes RM, Couper DJ, Barjaktarevic IZ, et al.Age-dependent associations between 25-hydroxy vitamin D levels and COPD symptoms: analysis of SPIROMICS. Chronic Obstr Pulm Dis. 2021; 8(2): 277-291. doi: http://doi.org/10.15326/jcopdf.2020.0180
Online Supplemental Material: Read Online Supplemental Material (589KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is defined as obstruction on spirometry, the presence of clinical risk factors, and symptoms consistent with COPD diagnosis.1,2 Assessment of COPD symptoms is an essential part of the COPD treatment algorithm3 and a clinical endpoint for drug investigation.4-7 COPD symptoms lead to more missed days of work, unemployment, use of health care resources, and financial difficulties.8 Previous studies have shown middle-aged (aged 45-65) persons have worse symptom scores than older (>65 years-old) counterparts independent of common medical and psychological comorbidities.9 Middle-aged COPD patients may be affected by lower symptom tolerance,10 greater perception of and discomfort from increasing ventilatory loads,11 increased prevalence of depression,12 and/or poorer access to medical care due to lack of medical insurance.13 Comorbidities play an important role in the quality of life of patients with COPD and are associated with worse reported symptoms.14 Also, younger persons with alpha-1 proteinase inhibitor deficiency and concomitant COPD were described as having higher rates of depression and poorer quality-of-life scores.12 Younger patients with poorer health status and vitamin D deficiency have been described in other chronic conditions.15,16 To this end, we believe that younger persons with COPD are a population with differential disease expression and identifying risk factors for increased symptoms in this population is potentially beneficial to the approach of younger persons with COPD and/or those with “early” COPD.17
Vitamin D deficiency is present in 40%-70% of COPD patients18,19 and is of particular interest due to the potential effects of vitamin D on muscle strength and physical performance,20 as well as exacerbations21 and lung function decline.22 Higher 25-hydroxy (OH)-vitamin D levels have been associated with improved COPD quality-of-life metrics in a small study.20 However, there was no improvement in Chronic Respiratory Disease Questionnaire (CRQ) with vitamin D supplementation in a randomized controlled trial.6 Other studies describe poorer mobility and gait in vitamin D deficient persons,23 which may potentially be manifested in symptom scores in younger persons with COPD. Response to pulmonary rehabilitation is also thought to be blunted in those with low 25-OH-vitamin D levels.24 The 25-OH-Vitamin D levels may reflect overall poor health status associated with global functional decline in COPD.25 In COPD, lower 25-OH-vitamin D levels present a modifiable risk factor that may be associated with increased symptom burden, potentially more impactful in middle-aged persons with COPD.9 However, to date the association between age, 25-OH-vitamin D levels, and reported COPD symptoms has not been described in the literature.
We have previously shown that 25-OH-vitamin D levels are associated with poor lung function and COPD exacerbation outcomes.22 The analysis presented here extends the exploration of vitamin D in COPD through an evaluation of vitamin D levels and symptoms across age groups, previously unexplored in this well-defined, multicenter COPD cohort. By leveraging the detailed demographic, clinical, and symptoms data from the multicenter, prospective Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS),26 we characterize the independent associations between younger age, 25-OH-vitamin D deficiency, and pulmonary symptoms in persons with COPD. We hypothesize that lower 25-OH-vitamin D levels will be associated with a worse symptoms profile in a young COPD cohort, independent of other comorbidities.
Methods
Study Participants
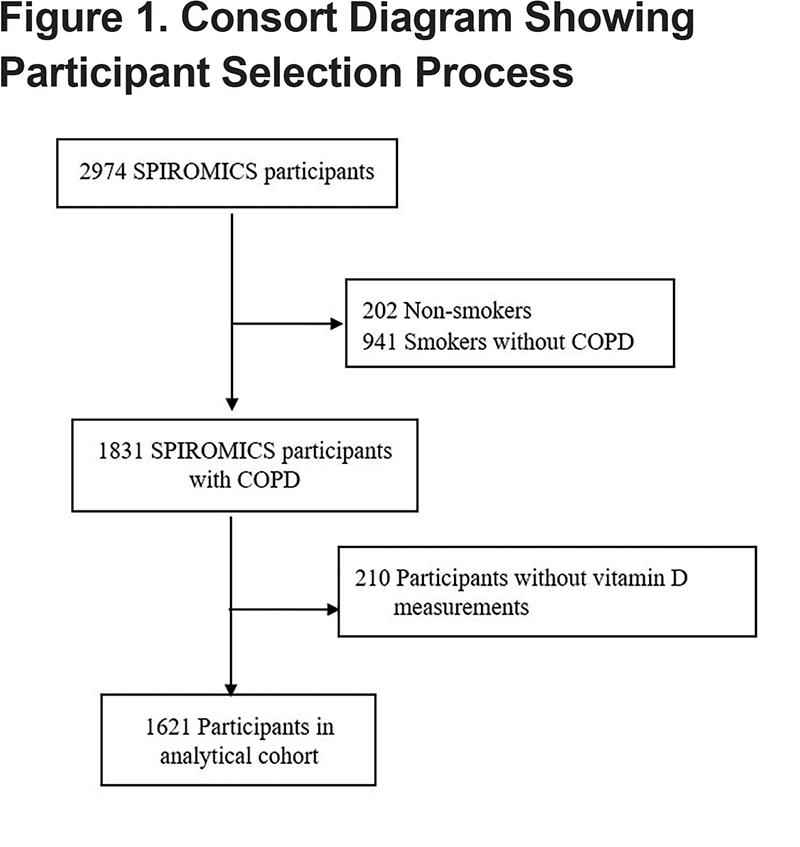
SPIROMICS is a multicenter, observational, prospective, cohort study including participants who are current or former smokers (≥20 pack years) and nonsmoking controls, with or without chronic airflow obstruction, between the ages of 40-80 (n=2974) at recruitment from 2011-2016.26 The analytical cohort includes SPIROMICS participants with spirometry confirmed COPD (post-bronchodilator forced expiratory volume in 1 second to forced vital capacity [FEV1/FVC] <0.70), available clinical data, and serum vitamin D measurements (N=1621) (Figure 1). Institutional review boards at each center approved SPIROMICS and all participants provided informed, written consent.
Data Collection
Demographic data, past medical history, smoking status (current versus former), smoking history (pack years), body mass index (BMI), and participant-reported vitamin D supplementation or multivitamin usage were collected at enrollment (baseline). Participants who reported any type of supplementation that contained vitamin D (e.g., vitamin D, multivitamin with D) were abstracted as reporting vitamin D supplementation. Individuals who reported other forms of supplements without mention of vitamin D (e.g., vitamin use, multivitamin) were not recorded as taking vitamin D supplementation. The dose, frequency, and compliance with supplement was not determined. Pre- and post-bronchodilator spirometry was performed in accordance with the American Thoracic Society (ATS)-European Respiratory Society guidelines27 for the measurement of FEV1 and FVC. The 6-minute walk test (6MWT) conducted in accordance with ATS criteria was completed at the time of enrollment. Frequent exacerbation was defined as 2-or-more reported total exacerbations (any worsening of COPD symptoms requiring therapy and/or unscheduled health care encounters) in the year prior to enrollment. Serum 25-OH-vitamin D levels were measured from stored samples of SPIROMICS participants with an FEV1/FVC <0.70 which were collected at the baseline visit using radioimmunoassay (iDS, Enzyme Immunoassay; intra-assay coefficient of variance: 8.14%, limit of blank: 1.8ng/ml and limit of detection: 3.7ng/ml) as described previously.22
Participant-report outcomes collected at enrollment and incorporated in this analysis include: (1) modified Medical Research Council (mMRC) score (on a 0–4 scale with higher values representing worse breathlessness),28 (2) COPD Assessment Test ([CAT] on a 0-40 scale with higher scores representing worse COPD symptoms with suggested minimal clinically important difference [MCID] of 2),29 and (3) SGRQ (on a 0-100 scale over 3 subdomains [symptoms, activity, impact] with higher scores representing worse COPD symptoms with a 4-point decrement [range between 2-8] generally thought to be MCID).30,31 The Veteran’s Specific Activity Questionnaire ([VSAQ] on a 1-13 scale with higher scores correlating to better patient-reported aerobic capacity) and 6MWT (measured in meters ambulated in 6 minutes) were used to approximate physical functioning of participants.32
Statistical Methods
Descriptive statistics were used to determine the proportion of middle-aged (<65 years-old) versus older (≥65 years-old) participants in the cohort as well as distribution of 25-OH-vitamin D within the cohort. For analyses, vitamin D levels (exposure) were modeled continuously (per 5ng/ml) and dichotomously as sufficient (≥20ng/ml) versus deficient (<20ng/ml). Two-sample t-test or Mann-Whitney U tests for continuous variables (depending on skew of data) or chi-squared tests (for categorical variables) were performed to compare demographic, clinical factors, and symptom scores associated with age group and 25-OH-vitamin D sufficiency. Similar bivariate methods were used to identify differences between middle-aged and older participants as performed by Martinez et al.9 Spearman’s correlation was used to determine the relationship between symptoms and vitamin D levels modeled continuously. The mMRC, CAT, SGRQ total and subdomains scores, and VSAQ outcomes were analyzed as continuous scores in linear regression. The 6MWT distance was modeled continuously as meters walked. Covariates were based on a conceptual framework33 and included age, race, sex, current smoking, pack years smoked, BMI, and FEV1.
Differential outcomes on patient-reported symptom tools in the SPIROMICS cohort between the older and middle-aged have been previously noted.9 Based on this finding, we performed multivariable linear analyses stratified by these 2 age groups. Covariates included sex, race, current smoking, pack years smoked, BMI, and FEV1. As previously described, a score for physical and psychological comorbidities,14 to assess vitamin D deficiency associations independent of comorbid disease, was added in a separate analysis to determine if 25-OH vitamin D levels were associated with poor symptom scores independent of other comorbidities. The comorbidity score is a total count of COPD-related comorbidities (score range 0-10). Sensitivity analyses of statistically significant multivariable models were performed with addition of season of blood draw, use of vitamin D or multivitamin supplementation combined to create a vitamin D use variable, patient-reported per-day time spent outdoors in the year prior to enrollment, and geographic site of blood draw. We also performed the above analyses with the removal of those on vitamin D supplementation (n=180 removed), modeling lung function as FEV1 % predicted (race and sex removed from models to avoid collinearity), and as an interaction term between vitamin D sufficiency and age. As the relationship between vitamin D metabolism and race is differential,34 we also subdivided the middle-aged cohort by race and presented the associations with 25-OH-vitamin D and respiratory symptoms. For all comparisons, P<0.05 was considered significant. Statistical analysis was performed using STATA 15.1 (STATA Corp, College Station, Texas).
Results
Description of Cohort
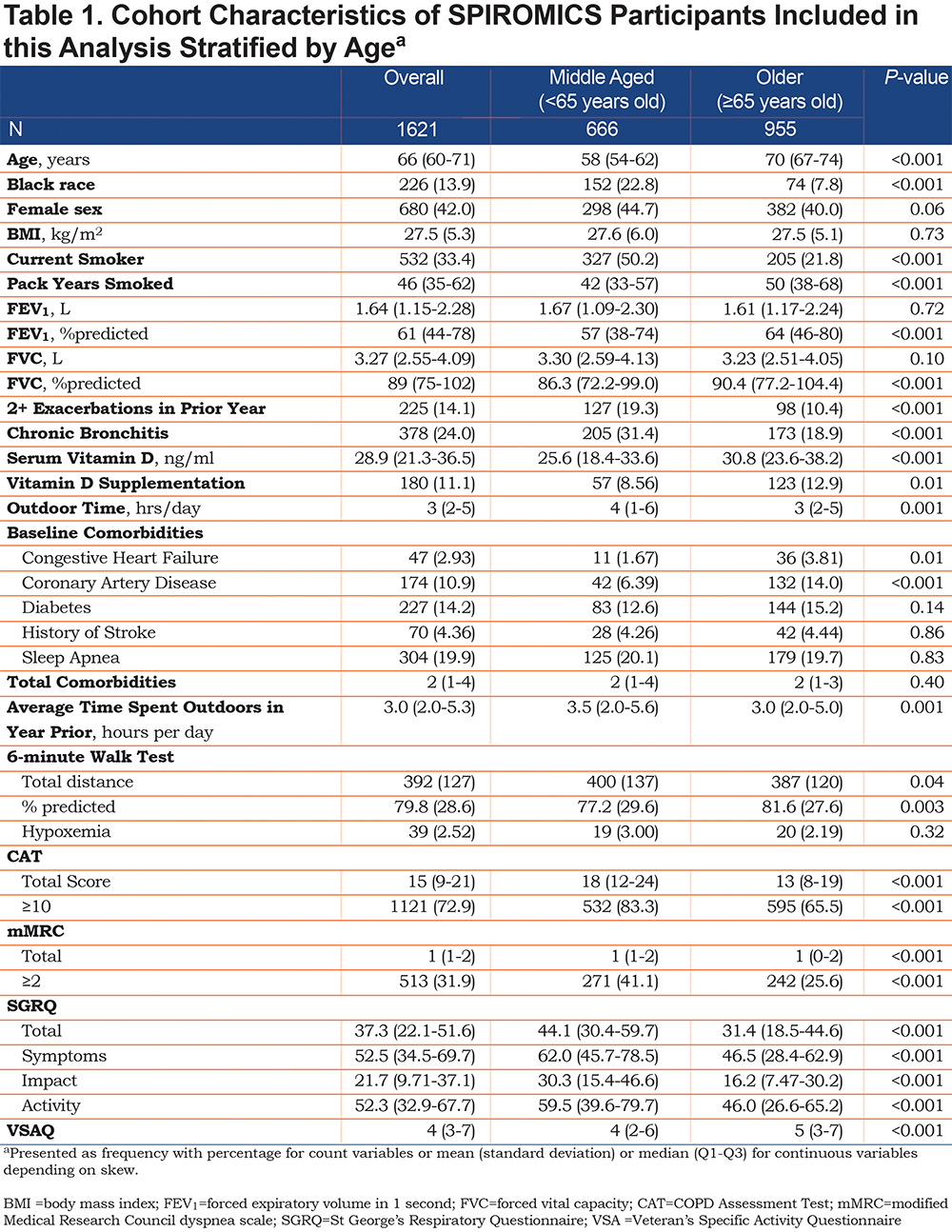
Of the 1621 participants, 995 (59%) were aged 65 years or older (Table 1). The median age in the entire cohort was 66 years-old (Q1–Q3, 60–71 years-old). In the entire cohort, 42% were female and 14% reported Black race. There were 33% current smokers and a median of 46 pack years smoked (Q1–Q3, 35–62 pack years). A total of 14% of the cohort reported 2 or more COPD exacerbations in the year prior to enrollment and 24% of the cohort reported chronic bronchitis. The mean 25-OH-vitamin D level was 30.0 (standard deviation [SD] 12.4), median 28.9 (Q1–Q3, 21.3–36.5), and levels ranged from minimum of 6.59ng/ml to maximum of 106ng/ml (Figure 2). A total of 11% of participants reported either taking vitamin supplementation or a multivitamin. The median comorbidity score in the entire cohort was 2 (Q1–Q3, 1–4). The average reported outdoor time in the year prior to enrollment was 3 hours/day (Q1–Q3, 2–5.3 hours/day). In the total cohort, median mMRC score was 1 (Q1–Q3, 1–2), the median CAT score was 15 (Q1–Q3, 9–21), and median SGRQ total score was 37.3 (Q1–Q3, 22.1–51.6). The mean 6MWT distance was 392 meters (SD 127 meters) with a median VSAQ score of 4 (Q1–Q3, 3–7).
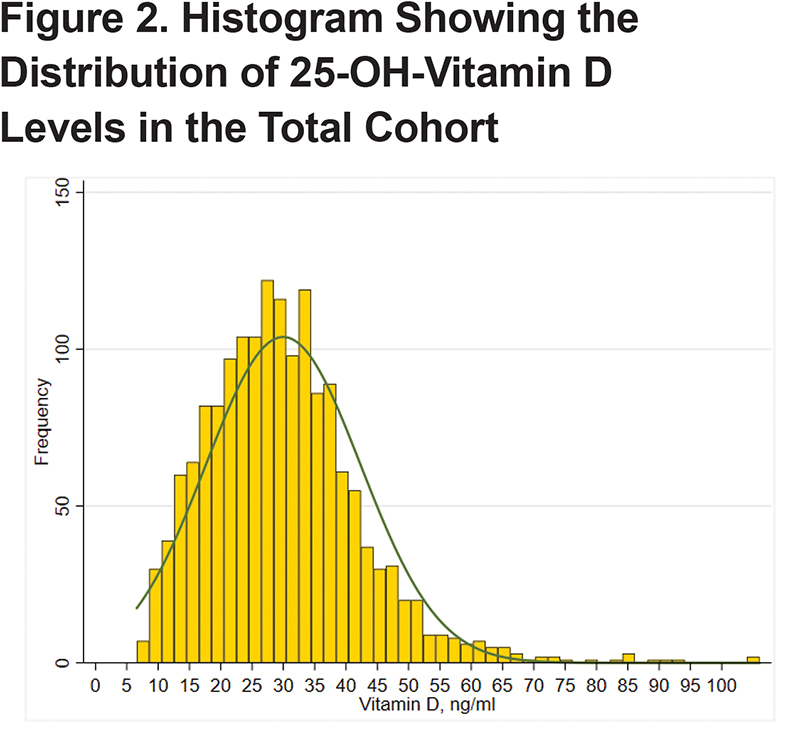
Older participants were less likely to be Black (8% versus 23%, P<0.001), less likely to be current smokers (22% versus 50%, P<0.001), but reported higher pack years smoked (50 pack years versus 42 pack years, P<0.001). Older participants were less likely to report symptoms of chronic bronchitis (19% versus 31%, P<0.001) and to have had 2-or-more exacerbations in the year prior to enrollment (10% versus 19%, P<0.001). The 25-OH-Vitamin D levels were significantly higher in the older participants (30.8ng/ml versus 25.6ng/ml, P<0.001). The older group was more likely to report some form of vitamin D supplementation or multivitamin use (13% versus 9%, P=0.01). Comparing middle-aged to older participants, there was no difference in season of sample draw (P=0.32) or total comorbidity score (2 versus 2, P=0.40). The older age group spent fewer hours-per-day outdoors than the middle-aged group (3 hrs/day versus 3.5 hrs/day, P=0.001). The older age group had a lower (better) median CAT score (13 versus 18, P<0.001), similar median mMRC but more favorable score distribution (median 1; [Q1–Q3, 0-2] versus [Q1–Q3, 1–2], P<0.0010), and a lower (better) total SGRQ score (31.4 versus 44.1, P<0.001). The older age group had a higher (better) VSAQ score (5 versus 4, P<0.001) while the middle-aged group had a longer average 6MWT distance (400 m versus 387 m, P=0.04).
Continuous Vitamin D Serum Levels and Symptoms
Across the entire cohort, Spearman’s correlation showed a statistically significant correlation between better symptom scores and higher 25-OH-vitamin D levels: CAT score (r=-0.138), mMRC (r=-0.103), total SGRQ (r=-0.179), SGRQ subdomains (Symptoms r=-0.134; Impact r=-0.174; Activity r=-0.158), and VSAQ (r=0.120) (P<0.001 for all comparisons).
In the total cohort, using multivariable linear regression adjusting for age, race, sex, smoking status, smoking history, FEV1, and BMI, a 5ng/ml higher 25-OH-vitamin D level was associated with a 0.41 lower (better) total SGRQ score (95% CI -0.79 to -0.04; P=0.03) (Table 2). A 5ng/ml higher 25-OH vitamin D level was also associated with significantly more favorable scores for the SGRQ Impact (-0.40 [95% CI -0.77 to -0.02], P=0.04) and Activity (-0.52 [95% CI -0.99 to -0.05], P=0.03) subdomains. Further, in the total cohort, every 5ng/ml higher level of 25-OH-vitamin D was associated with a statistically better VSAQ (0.06 [95% CI 0.002 to 0.11]; P=0.04); however, there is no MCID reported for VSAQ in COPD patients. A single unit VSAQ increase represents an approximate 3.5 ml/kg/min increase in oxygen consumption, and due to the magnitude of this point estimate, while statistically significant, is likely clinically negligible.35 Higher 25-OH-vitamin D levels were not associated with changes in CAT score, mMRC, SGRQ Symptoms subdomain score, or 6MWT distance. Modeling using linear spline knots at clinical thresholds (e.g., 10ng/ml, 20ng/ml, and 30ng/ml of 25-OH-vitamin) did not identify any inflection points representing statistical differences above and below that threshold.
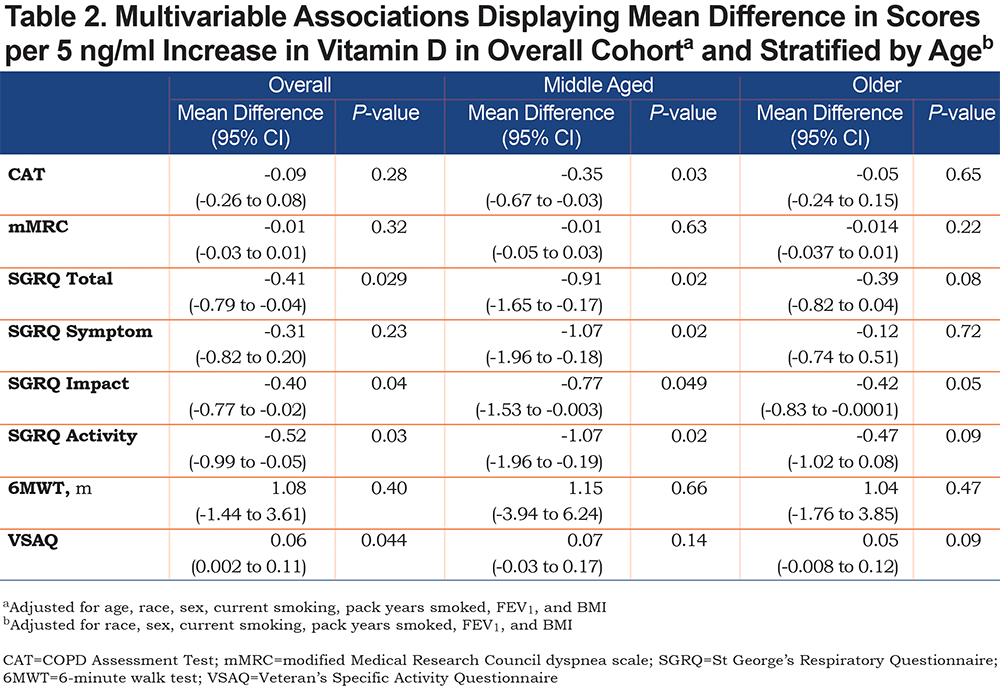
When stratifying the cohort into middle-aged and older participants, after adjustment for race, sex, current smoking, pack years smoked, BMI, and FEV1, in the middle-aged group, every 5ng/ml higher 25-OH-vitamin D level was associated with a significantly lower total SGRQ (-0.91 [95% CI -1.65 to -0.17]; P=0.02), and SGRQ subdomains of Symptoms (-1.07 [95% CI -1.96 to -0.18]; P=0.02), Impact (-0.77 [95% CI -1.53 to -0.003]; P=0.049), and Activity (-1.07 [95% CI -1.96 to -0.19]; P=0.02) (Table 2). There was also an association with better CAT score (-0.35 [95% CI -0.67 to -0.03]; P=0.03). The mMRC, VSAQ, and 6MWT distance were not significantly associated with vitamin D levels in the middle-age group. The only significant association observed in the older age group was the SGRQ Impact score (-0.42 [95% CT -0.83 to -0.0001]; P=0.049). Predicted scores for CAT and SGRQ stratified by age based on 25-OH-vitamin D level are shown in Figure 3 and Figure 4, respectively.
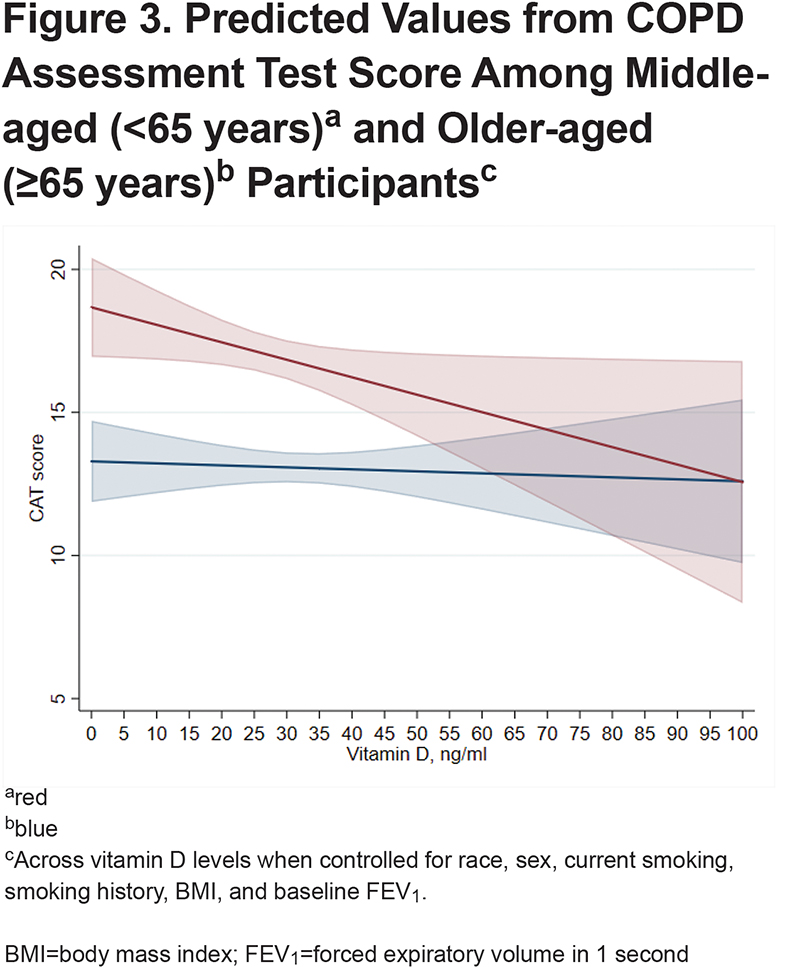
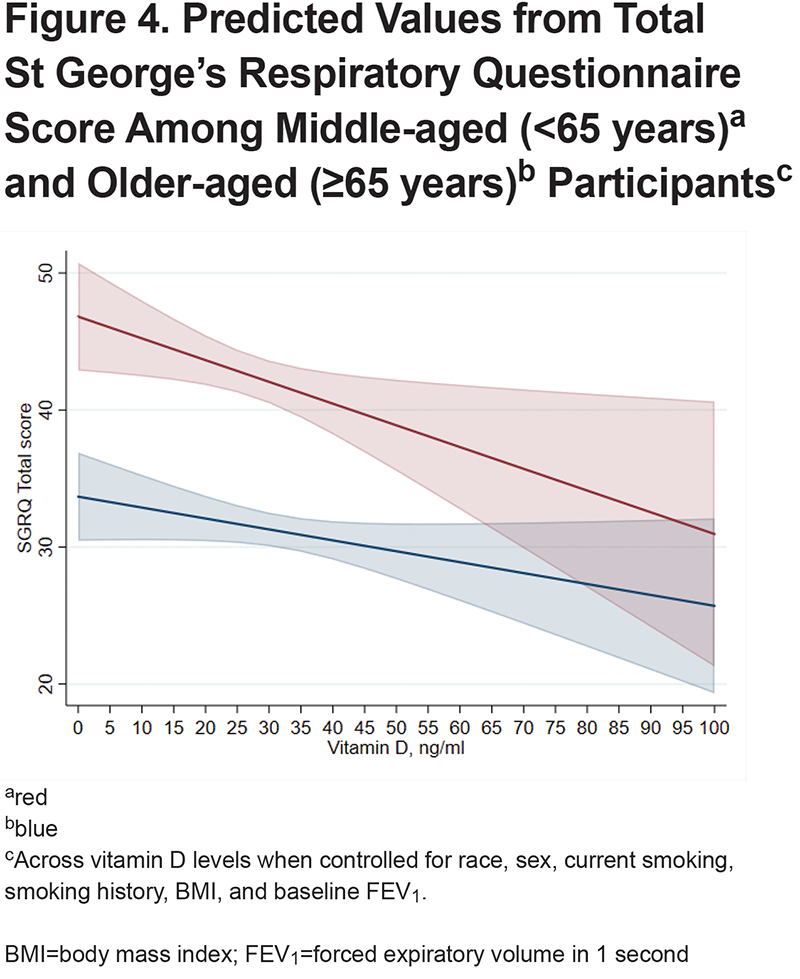
Impact of Comorbidity Score on Vitamin D Level-Symptoms Associations
Several factors that could impact vitamin D levels and symptom relationships were considered. Older participants had more frequent coronary artery disease (14% versus 6%; P<0.001) and congestive heart failure (4% versus 2%; P=0.01), but not other comorbidities or total comorbidity count (Table 1). Comorbidity score was added to the continuous 25-OH-vitamin D multivariable models described previously. In the overall cohort, significant associations were not attenuated (Supplementary Table 1 in the online supplement) when comorbidity score was added to the models. When stratifying by age, CAT (-0.39 [95% CI -0.70 to -0.07]; P=0.02) and SGRQ total (-0.99 [95% CI -1.70 to -0.29]; P=0.02) remained statistically significant in the middle-aged cohort after adjustment for comorbidity. Inclusion of comorbidity score did not attenuate the relationships between SGRQ subdomains and continuous 25-OH-vitamin D in middle-aged participants.
Sensitivity Analysis
Season of blood draw, participant-reported use of vitamin D or a multivitamin, and total participant-reported outdoor time were added to the above statistically significant age-stratified multivariable models (Supplementary Table 2 in the online supplement). Only the SGRQ Impact score relationship was attenuated in the middle-age group (-0.72 [95% CI -1.54 to 0.03], P=0.06) when the season of blood draw was added. The addition of study site (as an adjustment for latitude) did not attenuate independent associations between 25-OH-vitamin D levels and symptom scores in the middle-aged cohort. Further, when 25-OH-vitmain D levels in the entire cohort were stratified into <10ng/ml, 10–20ng/ml, 20–30ng/ml, and >30ng/ml, the median SGRQ Total values were 56 versus 42 versus 37 versus 33 (analysis of variance P=0.03). Due to the relatively low number of participants with 25-OH-vitamin D levels <10ng/ml we were unable to evaluate for statistical relationships in multivariable analysis.
Alternative Modeling of Age, Vitamin D, and Symptoms
We examined the relationship between age, vitamin D levels, and symptoms by alternative approaches. The relationship between 25-OH-vitamin D and symptoms in the middle-aged cohort was attenuated when an interaction term for middle-age and 25-OH-vitamin D levels was added. For the interaction term among those who were classified as middle-aged and with 25-OH-vitamin D levels <20ng/ml: CAT (-0.17 [95% CI -0.47 to 0.14]; P=0.29) and SGRQ Total (-0.39 [95% CI (-1.10 to 0.32); P=0.29]). Modeling lung function by %predicted FEV1 did not attenuate relationships between 5ng/ml increases in 25-OH-vitamin D and CAT or SGRQ total in the whole cohort. Further, when those receiving some form of vitamin D supplementation were removed from the cohort the relationship with CAT score was not attenuated and there was a notable increase in the change per 5ng/ml 25-OH-vitamin D increase and SGRQ Total score (-1.05 [95% CI -1.43 to -0.67]; P<0.001). In the age-stratified cohort, when excluding those who reported some form of vitamin D supplementation, CAT (-0.30 [95% CI -0.59 to -0.03]; P=0.034) and SGRQ Total (-0.92 [95% CI -1.58 to -0.26]; P=0.006) had a continued statistically significant improvement with every 5ng/ml increase in 25-OH-vitamin D.
The Relationship Between 25-Hydroxy Vitamin D, Middle Age, and Race
Given the increased prevalence of Black race in the middle-aged cohort, we have subdivided the middle-aged cohort by race and have assessed the independent associations between a 5ng/ml increase in 25-OH-vitamin D and respiratory symptoms when controlled for sex, current smoking, pack years smoked, and absolute FEV1. In White (-0.40 [95% CI -0.69 to -0.12]; P=0.006) but not Black (-0.10 [95% CI -0.78 to 0.57]; P=0.77) participants there was an association with a 5ng/ml increase in 25-OH-vitamin D and CAT score. For SGRQ Total, White (-1.10 [95% CI -1.80 to -0.40]; P=0.002) but not Black (-0.54 [95% CI -1.96 to 0.88]; P=0.45) participants had a significant association between increases in 25-OH-vitamin D by 5ng/ml and more favorable scores.
Vitamin D Sufficiency and Symptoms
In this cohort of 1621 participants, 1282 (79%) were vitamin D sufficient (vitamin D ≥20ng/ml) (Supplementary Table 3 in the online supplement). Vitamin D sufficient participants were more likely to be older (67 years versus 62 years; P<0.001), less likely to be Black (10% versus 29%; P<0.001), less likely to be current smokers (29% versus 48%; P<0.001), and more likely to report vitamin D supplementation (12% versus 6%; P=0.001). Vitamin D sufficient participants had higher FEV1 and FVC. Vitamin D sufficient participants had more favorable CAT scores (14 versus 17; P<0.001), SGRQ totals (34.8 versus 42.9; P<0.001), and VSAQ scores (5 versus 4; P<0.001).
In the overall cohort, after controlling for age, race, sex, BMI, FEV1, current smoking, and pack years in multivariable modelling, 25-OH-vitamin D level >20ng/ml was not associated with more favorable CAT scores, mMRC, total SGRQ and subdomains, VSAQ score, or 6MWT distance (Supplementary Table 4 in the online supplement). Further, there were no multivariable associations between vitamin D sufficiency and symptom outcomes observed when stratified by age.
Discussion
In this analysis of 1621 SPIROMICS participants, higher vitamin D levels when modeled continuously were associated with better CAT scores, SGRQ total, and SGRQ subdomain scores in middle-aged but not older age groups. These associations persisted after accounting for comorbidity burden. In the middle-aged group, an approximate 20ng/ml higher vitamin D level was associated with a minimal clinically meaningful difference in total SGRQ (4 units). Further, associations were not attenuated by the addition of vitamin D supplementation, outdoor time, and season of blood draw. In addition, when the middle-aged subgroup was further divided by race, the association between symptoms and vitamin D levels was restricted to non-Black participants, informing the need to explore the role of vitamin D in pulmonary health across demographic groups. These findings suggest that lower vitamin D levels may have a meaningful association with worse symptom scores in middle-aged COPD patients. However, based on current Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines,36 vitamin D supplementation can be considered in deficient persons of all age groups to prevent poor COPD outcomes.
Associations between vitamin D levels and subjective symptoms may reflect overall poor health and/or effect of vitamin D on the musculoskeletal system.37 COPD patients with sufficient vitamin D levels show better muscle strength,20,38 and may accrue greater benefit from pulmonary rehabilitation. Vitamin D deficiency at enrollment in pulmonary rehabilitation is associated with poor health status and increased dropout.24 The median age of participants in these studies tended to be in the late-60s. Other studies have not described an increase in physical performance with improvement of nutritional status in muscle-wasted COPD patients.39 Also, vitamin D supplementation has not been shown to improve CRQ scores in 1 study.6 Participants in these studies also had a median age in the 60s. Our study adds to the body of literature by describing associations in a well-defined cohort and illustrating the potential importance of relatively young-aged COPD individuals in the vitamin D-symptoms relationship. The mechanism driving the relationship between vitamin D levels and worse symptoms remains to be determined. Further studies evaluating the role of persons with very deficient vitamin D levels (<10ng/ml) and studies enriched to specifically determine associations in racially diverse groups, may be warranted to further understand this relationship.
Differential COPD symptom burden between age groups was observed in a prior SPIROMICS analysis.9 The relationships were independent of physical function and comorbidities. A separate cohort study further suggests that those developing COPD at a younger age have worse psychological and reported well-being outcomes.12 The response of symptoms and physical performance to vitamin D supplementation in middle-aged COPD participants is not well-studied. However, studies of vitamin D supplementation in younger participants with other chronic diseases have been performed. A cohort of participants (mean age 64) with osteoarthritis and an average increase in 25-OH-vitamin D from 20ng/ml to 32ng/ml with supplementation showed an improvement in health-related quality of life and grip strength.16 Further, supplementation in younger versus older volunteers with Parkinson’s Disease (raising serum levels by an average 30ng/ml) has been associated with significantly better balance scores.15 COPD studies with older participants have not shown supplementation to be beneficial to symptom scores.6 These findings, combined with observations, suggest that low vitamin D levels may be of particular concern in younger persons with COPD. The role of the aging process, including cognitive decline40 and lack of response to bronchodilators with age,41 may make the factors driving increased symptom perception in older persons different from younger persons. Also, there may be a “survivor” effect in the cohort, where the older group has more stable disease that has allowed for a longer life. The associations between the middle-age group, vitamin D levels, and COPD symptoms may describe a phenotype of younger, more symptomatic COPD patients who potentially represent a target for further intervention trials of the impact of vitamin D supplementation on COPD symptoms.
Independent associations between vitamin D levels and symptom scores persist when controlled for a validated comorbidity score.14 Comorbid conditions increase morbidity and mortality in COPD.14,42-46 We observe that the association between serum vitamin D and symptom scores is independent of comorbidity burden. This finding suggests low serum vitamin D levels may represent a distinctly important comorbidity along with the other known long-term health issues that interface with the COPD disease state. Vitamin D metabolites may have a potential relationship with certain immune processes47-49 and 25-OH-vitamin D deficiency is associated with decreased musculoskeletal performance,23,25,37,50 illustrating a potentially multifaceted mechanism by which vitamin D deficiency negatively affects persons with COPD. Deficiency of 25-OH-vitamin D has been hypothesized to promote chronic bronchitis. A study in the COPD Genetic Epidemiology cohort described similar improvement in symptom scores with increasing 25-OH-vitamin D levels and also a higher prevalence of radiographic findings associated with chronic bronchitis.51 However, a previous study conducted by our group22 did not find radiographic differences between those with 25-OH-Vitamin D levels ≥20 and <20. Further study incorporating vitamin D supplementation, measurement of muscular mass and function, markers of inflammation, and longitudinal outcomes could further define vitamin D deficiency as an important and modifiable comorbidity in COPD or if lower levels are simply a marker of poorer health status.
Associations between 25-OH-vitamin D and symptoms in the middle-aged group are seen when 25-OH-vitamin D is modeled continuously, but not by thresholds of sufficiency. The serum level at which 25-OH-vitamin D is associated with better outcomes may vary across diseases,52 and patients with very low 25-OH-vitamin D levels (<10ng/ml) potentially represent the group who have worse outcomes.6 Further, the response to supplementation on vitamin D levels may play a role in physical and symptom response, although the literature contains conflicting results.20,38,53-55 Participant-reported vitamin D supplementation did not impact the observed associations between 25-OH-vitamin D levels and symptom scores. Additionally, supplementation was not independently associated with better symptom scores in our study, however, the ascertainment of vitamin D supplementation, including dose, frequency, and compliance with therapy, was poor in SPIROMICS. These findings add to the importance of describing the 25-OH-vitamin D level at which supplementation will provide the most benefit and determining the importance of magnitude of response to supplementation versus merely achieving vitamin D sufficiency.
Better SGRQ total and subdomain scores in the middle-aged group are associated with higher vitamin D levels in this study. A large analysis of drug trial participants has associated poorer total SGRQ with worse COPD outcomes, including exacerbations.56 High physical and psychological comorbidity burden affects SGRQ scores in COPD patients. Literature suggests the SGRQ mainly assesses subjective symptoms and impairment and may not be an accurate indicator of physical functioning,57 explaining the lack of associations seen between vitamin D levels and 6MWT or VSAQ. Further, in the middle-aged group, there was not an association in Black participants when analyses were further subdivided by race. This may be due to differential vitamin D metabolism34 and represents an area in need of further investigation. Stronger associations seen between vitamin D levels and SGRQ scores as opposed to those observed with the CAT score, as seen in the analysis of the total cohort, have been noted elsewhere58 and may be due to the SGRQ's more granular nature.
This study has limitations. The associations presented are cross-sectional and do not prove causality between low vitamin D and increased symptoms. While the middle-aged cohort reported more time outdoors, this does not necessarily prove better overall health in this group. We do not have available data for recent pulmonary rehabilitation attendance among participants. Evaluating measures of sarcopenia would further enrich the analysis and better define phenotypes for future studies. Some important covariables including medication usage, time spent outdoors, and comorbidities were collected by patient report, introducing the potential for recall bias. Also, doses of vitamin D supplementation and whether the participants were compliant with this therapy, was not validated by the study. While modeling our continuous exposure as per 5ng/ml 25-OH-vitamin D increase, we did not find differences in CAT and SGRQ Total scores that met MCID, however, these thresholds are reached at a 20ng/ml change in 25-OH-vitamin D. The low number of participants with 25-OH-vitamin D <10ng/ml limits our ability to accurately assess for threshold effects at that level which would otherwise be informative. We elected to not adjust for multiple comparisons given the controversy of using correction techniques when assessing significance using a universal mull not fitting the conceptual framework.59 Importantly, given the uniform nature of data collection in SPIROMICS, we would not expect these biases to differentially impact younger or older age groups.
In conclusion, increasing levels of vitamin D, are associated with improved respiratory-specific symptoms, most notable in middle-aged participants. These relationships persisted when accounting for important confounders. These findings highlight the need to explore mechanisms more robustly by which vitamin D may improve symptoms. Additionally, study of the role of vitamin D supplementation in the symptom burden of younger COPD patients is needed.
Acknowledgements
Author contributions: RMB and MBD conceptualized the study and produced the original manuscript. RMB performed data analysis. LRL reviewed the statistical approach and manuscript critically and revised the manuscript. All other coauthors collected study data, critically assessed methodological and statistical approach, critically reviewed manuscript, and revised the manuscript.
Data sharing: More information about the study and how to access SPIROMICS data is at www.spiromics.org
The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible. We would like to acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E. Alexis, MD; Wayne H. Anderson, PhD; Mehrdad Arjomandi, MD; Igor Barjaktarevic, MD, PhD; R. Graham Barr, MD, DrPH; Lori A. Bateman, MSc; Surya P. Bhatt, MD; Eugene R. Bleecker, MD; Richard C. Boucher, MD; Russell P. Bowler, MD, PhD; Stephanie A. Christenson, MD; Alejandro P. Comellas, MD; Christopher B. Cooper, MD, PhD; David J. Couper, PhD; Gerard J. Criner, MD; Ronald G. Crystal, MD; Jeffrey L. Curtis, MD; Claire M. Doerschuk, MD; Mark T. Dransfield, MD; Brad Drummond, MD; Christine M. Freeman, PhD; Craig Galban, PhD; MeiLan K. Han, MD, MS; Nadia N. Hansel, MD, MPH; Annette T. Hastie, PhD; Eric A. Hoffman, PhD; Yvonne Huang, MD; Robert J Kaner, MD; Richard E. Kanner, MD; Eric C. Kleerup, MD; Jerry A. Krishnan, MD, PhD; Lisa M. LaVange, PhD; Stephen C. Lazarus, MD; Fernando J. Martinez, MD, MS; Deborah A. Meyers, PhD; Wendy C. Moore, MD; John D. Newell, Jr, MD; Robert Paine, III, MD; Laura Paulin, MD, MHS; Stephen P. Peters, MD, PhD; Cheryl Pirozzi, MD; Nirupama Putcha, MD, MHS; Elizabeth C Oelsner, MD, MPH; Wanda K. O’Neal, PhD; Victor E. Ortega, MD, PhD; Sanjeev Raman, MBBS, MD; Stephen I. Rennard, MD; Donald P. Tashkin, MD; J. Michael Wells, MD; Robert A. Wise, MD; and Prescott G. Woodruff, MD, MPH. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Lisa Postow, PhD, and Lisa Viviano, BSN; SPIROMICS was supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), and a grant from the NIH/NHLBI (U01 HL137880), grants from the NIH/NHLBI (U01 HL137880 and U24 HL141762), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer- Ingelheim Pharmaceuticals, Inc.; Chiesi Farmaceutici S.p.A.; Forest Research Institute, Inc.; GlaxoSmithKline; Grifols Therapeutics, Inc.; Ikaria, Inc.; Novartis Pharmaceuticals Corporation; Nycomed GmbH; ProterixBio; Regeneron Pharmaceuticals, Inc.; Sanofi; Sunovion; Takeda Pharmaceutical Company; and Theravance Biopharma and Mylan.
Declaration of Interest
RMB Received Grants from the National Institutes of Health (NIH) during the conduct of this study. DJC received grants from the NIH and the COPD Foundation during the conduct of this study. IZB reports grants from the National Heart, Lung, and Blood Institute (NHLBI) during the conduct of the study; grants and personal fees from Theravance and Mylan, grants from Amgen, personal fees from Astra Zeneca, GlaxoSmithKline, Boehringer Ingelheim, Verona Pharma, and Grifols outside the submitted work. CBC reports grants from the NIH/NHLBI, grants from the Foundation of the NIH, and grants from the COPD Foundation during the conduct of the study; personal fees from PulmonX, NUVAIRA and MGC Diagnostics and other from GlaxoSmithKline, outside the submitted work. MKH reports personal fees from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Mylan, Merck, and Verona, and other from Novartis and Sunovion, outside the submitted work. PGW reports personal fees from Regeneron, Sanofi, Theravance, NGM, Glenmark, Genentech, and Amgen, outside the submitted work. SCL reports grants from the NIH. RP III reports grants from the NHLBI and grants from COPD Foundation, during the conduct of the study; grants from the Department of Veterans Affairs, and personal fees from Partner Therapeutics, outside the submitted work. APC reports grants from the NIH, during the conduct of the study; grants from the NIH, non-financial support from VIDA, and personal fees from GlaxoSmithKline, outside the submitted work. NP reports grants from the NIH. RAW reports grants and personal fees from AstraZeneca / Medimmune / Pearl, grants and personal fees from Boehringer Ingelheim, grants and personal fees from GlaxoSmithKline, personal fees from Contrafect, Roche, Merck, Circassia, Pneuma, Verona, Mylan/Theravance, AbbVie, ChemRx, Propeller Health, Kiniksa, Bristol Myers Squibb, Galderma, and Kinevant and grants from Sanofi-Aventis, outside the submitted work. MBD reports grants from the NIH and the NHLBI during the conduct of the study; personal fees from Boehringer-Ingelheim, GlaxoSmithKline, AstraZeneca, Mylan-Theravance, Parion, Midmark, and Phillips and grants from the Department of Defense outside the submitted work. WWL, TMP, RPB, LRL and TTB have nothing to disclose.