Running Head: Cathelicidin and Vitamin D
Funding Support: Research reported in this publication was supported by National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL125432. JRM is supported by NIH/NHLBI grant R01HL152077.
Date of acceptance: May 14, 2021 | Published online: May 21, 2021
Abbreviations: bronchoalveolar lavage, BAL; 25-hydroxy vitamin D, 25-OH vitamin D; chronic obstructive pulmonary disease, COPD; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; glomerular filtration rate, GFR; enzyme-linked immunosorbent assay, ELISA; limit of detection, LOD; coronavirus disease 2019, COVID-19; interquartile range, IQR; airway surface liquid, ASL
Citation: Sanders EC, Burkes RM, Mock JR, et al. Bronchoalveolar lavage and plasma cathelicidin response to 25-hydroxy vitamin D supplementation: a pilot study. Chronic Obstr Pulm Dis. 2021; 8(3): 371-381. doi: http://doi.org/10.15326/jcopdf.2021.0220
Introduction
Chronic obstructive pulmonary disease (COPD) is currently the fourth leading cause of death in the United States and worldwide.1,2 COPD is defined by obstructive patterns on spirometry, along with the presence of symptoms and risk factors.3 Multiple risk factors can contribute to the development of COPD, including active smoking, smoke exposure, environmental exposures, and airway infections.4 Vitamin D deficiency has also been associated with decreased lung function and more rapid lung function decline in active smokers and patients with COPD,5,6 and is a prevalent nutritional deficiency in this population.7 Despite the epidemiological associations between vitamin D deficiency and adverse pulmonary outcomes, randomized clinical trials of vitamin D deficiency have failed to demonstrate consistent improvements in lung function, COPD exacerbations, or symptom burden.8-10 Investigating the underlying mechanisms of vitamin D supplementation in individuals at-risk or with COPD can provide insight into this potential therapy.
Vitamin D impacts lung function as a result of its interaction with human innate immunity.11 Vitamin D is converted to the major circulating form, 25-hydroxy vitamin D (25-OH vitamin D), and then to 1,25-dihydroxyvitamin D, which acts at target cells via signaling cascades as a direct inducer of antimicrobial peptide gene expression.12,13 Specifically, the vitamin D signaling pathway upregulates the expression of cathelicidin (often measured as the antibacterial C terminus, LL-37, of human cationic antimicrobial protein 18), an antimicrobial peptide expressed in granules of immune cells and epithelium.12 Cathelicidin has anti-bactericidal, anti-antiviral, and anti-endotoxic properties along with chemoattractant activity and promotes local tissue healing.12,14-17 Previous studies have shown that low blood cathelicidin levels in smokers and patients with COPD are associated with low forced expiratory volume (FEV1) as well a higher prevalence of reported bacterial pneumonia.18,19 The efficacy of vitamin D to increase lung cathelicidin levels provides a potential therapeutic intervention for individuals at-risk or with lung function impairment, respiratory infections, and COPD exacerbations.
Despite the established relationship between vitamin D and cathelicidin, there is limited evidence evaluating the impact of vitamin D supplementation on lung or blood cathelicidin levels. Prior studies have shown that vitamin D can be measured in BAL fluid, which correlates with serum levels20 and an increase after allergen challenge.21 To assess this relationship, we performed a prospective pilot study to evaluate the effect of oral vitamin D supplementation on plasma and bronchoalveolar lavage (BAL) cathelicidin and 25-OH vitamin D levels in vitamin D-deficient active smokers with and without COPD. We hypothesized that supplementation of vitamin D would increase measured cathelicidin levels in the blood and BAL.
Methods
Study Population
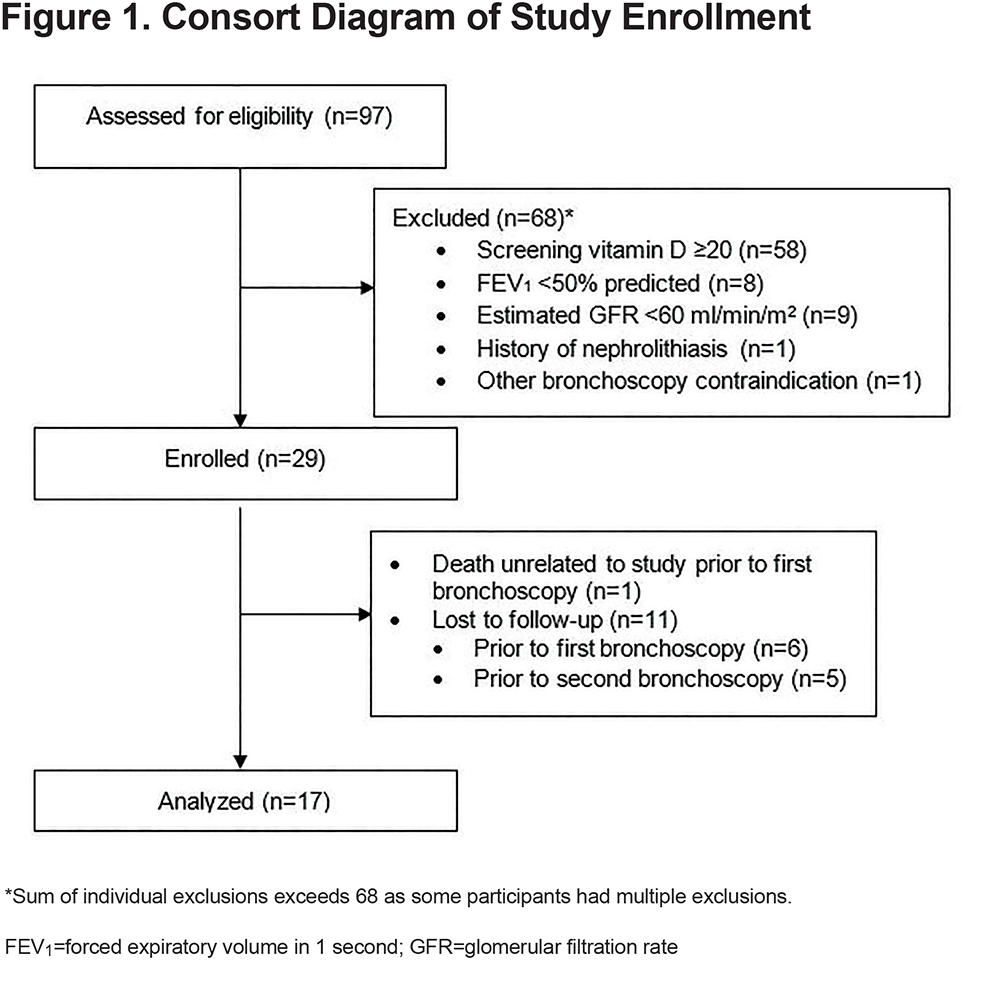
This study was a prospective pilot study to determine the effects of 8 weeks of oral vitamin D supplementation on cathelicidin levels in the blood and BAL. Eligible participants included 2 cohorts: (1) current smokers without COPD and (2) current or former smokers with spirometry-confirmed COPD. Despite former smoker eligibility among the COPD cohort, the study failed to recruit any former smokers. Therefore, the analytical cohort for this study included 17 participants who were current smokers with vitamin D deficiency (25-OH vitamin D <20ng/ml) recruited from 2 centers (N=9 with COPD; N=8 without COPD). COPD was defined as the presence of post-bronchodilator FEV1 to forced vital capacity (FVC) ratio <0.70. Exclusion criteria included 25-OH vitamin D >20ng/mL, serum calcium ≥10.5ng/dl, baseline FEV1 less than 50% (due to increased procedural risk with bronchoscopy), oxygen use >2L/min at rest, pregnancy or current breast-feeding, self-reported HIV positive serostatus (given potential impact on BAL 25-OH vitamin D levels),20 glomerular filtration rate (GFR) < 60mL/min or history of nephrolithiasis. This study was approved by the institutional review boards of the University of North Carolina at Chapel Hill and Johns Hopkins University, and all participants provided written informed consent. Results of this trial have been filed with clinicaltrials.gov (NCT02464059).
Measurements and Intervention
At an initial enrollment visit, after informed consent was obtained, baseline demographic information was collected. Smoking patterns were obtained at enrollment through participant self-report. Current smoking status was defined as current history of smoking at least 5 cigarettes daily. Blood samples were drawn for measurement of plasma cathelicidin and serum 25-OH vitamin D at baseline. Eligible participants underwent baseline bronchoscopy with BAL of the right middle lobe (2 aliquots of 50 ml each) within 30 days of the screening visit. After bronchoscopy was completed, participants returned for weekly in-person visits for 8 weeks for directly observed administration of vitamin D3 (cholecalciferol) 50,000 IU orally (BioTech Pharmacal, Inc., Fayetteville, Arkansas). This dosing and duration were selected based on prior studies showing the ability to correct vitamin D deficiency in patient populations.22-25 Blood samples were collected at week 1 (safety), week 4 (safety, vitamin D), and week 8 (safety, vitamin D, cathelicidin). Bronchoscopy was repeated within 30 days of the final oral vitamin D supplementation. This window was selected given the stability of blood vitamin D over time given the 4-week half-life of serum 25-OH vitamin D.26,27 Plasma and BAL cathelicidin was measured using commercially available enzyme-linked immunosorbent assay (ELISA) (Hyacult Biotech Inc; limit of detection [LOD]: 0.14ng/ml, standard range: 0.14ng/ml to 100ng/ml). BAL cathelicidin levels were examined with both raw values and corrected for BAL albumin levels. Serum 25-OH vitamin D was measured using commercially available radioimmunoassay (RIA, Immunodiagnostic Systems). BAL 25-OH vitamin D was measured after extraction/concentration from 4 ml aliquots of BAL fluid by previously published methods21 involving methanol precipitation, concentration by solid-phase extraction on octadecylsilyl silica cartridges, and assay using the same RIA used for the serum measurements. BAL 25-OH vitamin D data are presented as the concentration per ml of BAL fluid by dividing by the mls of BAL fluid initially extracted (i.e., 4ml). For the BAL 25-OH vitamin D levels, 6/34 (18%) of samples were undetectable, and 5/34 (15%) were below the LOD of 3nmol/L (or 1.2ng/ml) provided by the manufacturer. For statistical comparisons, the 11 samples that were below 50% of the LOD were all assigned a value of 50% of the LOD (e.g., 0.6ng/ml).
Sample Size Determination
The sample size for this pilot study was calculated based upon the minimal detectable % change and intraperson correlation. This pilot study's targeted enrollment was 40 participants. Assuming intraperson correlation of 0.7 between measures, with a sample size of N=40 the study would be powered to detect a 39% change in BAL cathelicidin after vitamin D supplementation. Bhan et al28 demonstrated that oral supplementation with 50,000U Vitamin D3 every other day x 5 days increased plasma cathelicidin 26%. Due to a higher than anticipated screen failure rate (largely due to lower prevalence of vitamin D deficiency) and impact of the coronavirus disease 2019 (COVID-19) pandemic on study-related procedures, the study was only able to enroll 29 participants and complete all study procedures on 17 participants during the funding period.
Statistical Analyses
Descriptive statistics were used to describe differences in clinical and demographic factors within the cohort. Continuous variables were presented with median and Q1-to-Q3 ranges. Frequency and % were reported for categorical variables. Participants were dichotomized based on the presence or absence of COPD, and differences between these subsets were determined using Mann-Whitney U testing (for continuous variables) or chi-squared testing (for categorical variables). For comparisons of values before and after vitamin D supplementation, mean differences are presented and, due to skew of data, statistical significance between values at both time points are determined by Wilcoxon paired values, signed rank testing. Spearman correlations were performed to examine the correlation between measurements, and coefficients are presented as ρ. For all comparisons p<0.05 was considered significant. The analysis was performed using STATA software (STATA Corp, College Station, Texas).
Results
Participant Characteristics
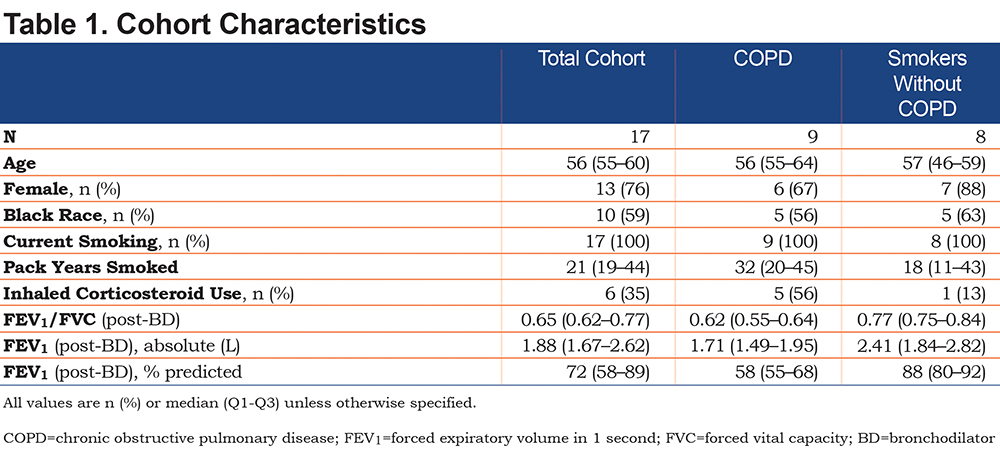
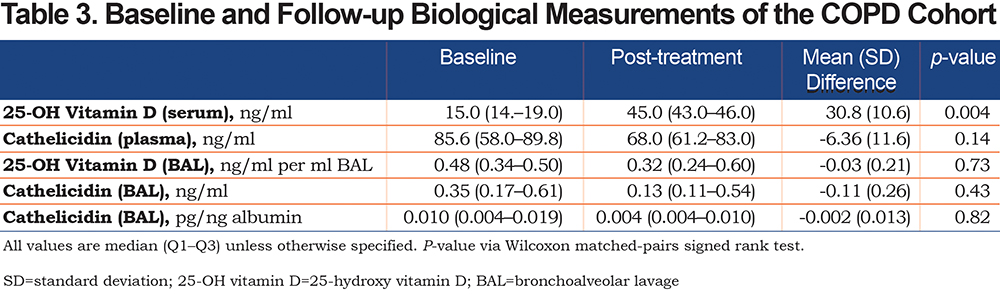
A total of 97 participants were screened, 29 enrolled, and 12 lost to follow-up after enrollment (Figure 1). The final analytical cohort of 17 participants was 76% female, 59% African American, with a median age of 56 years (Table 1). All participants were active smokers, with a median pack-year history of 21 (interquartile range [IQR], 19–44 pack years). Of the 17 participants, 6 reported current use of inhaled corticosteroids. At baseline, the median post-bronchodilator FEV1 was 1.88L (IQR, 1.67–2.62L) and median FEV1% predicted was 72% predicted (IQR, 58%–89% predicted). The median FEV1/FVC ratio was 0.65 (IQR, 0.62–0.77). Median plasma cathelicidin measurement was 85.6ng/ml (IQR, 59.0–102ng/ml), and there was no difference in cathelicidin levels comparing participants with COPD to those without COPD. Specifically, the median cathelicidin was 85.6ng/ml (IQR, 58.0–89.8ng/ml) among those participants with COPD and 84.8ng/ml (IQR, 60.4–102ng/ml) for participants without (p=0.19).
Effects of Vitamin D Supplementation on Blood 25-OH vitamin D and Cathelicidin
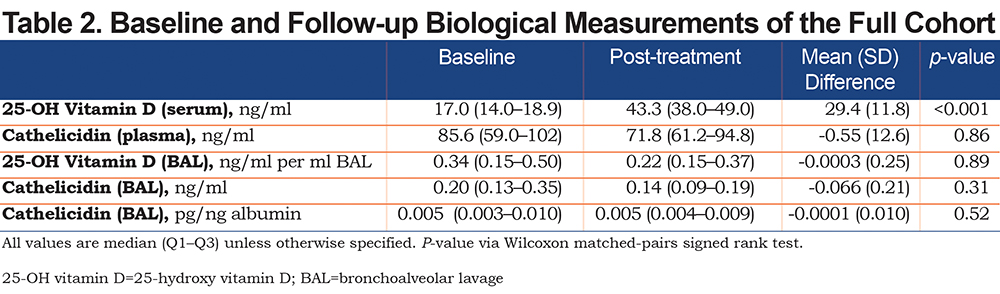
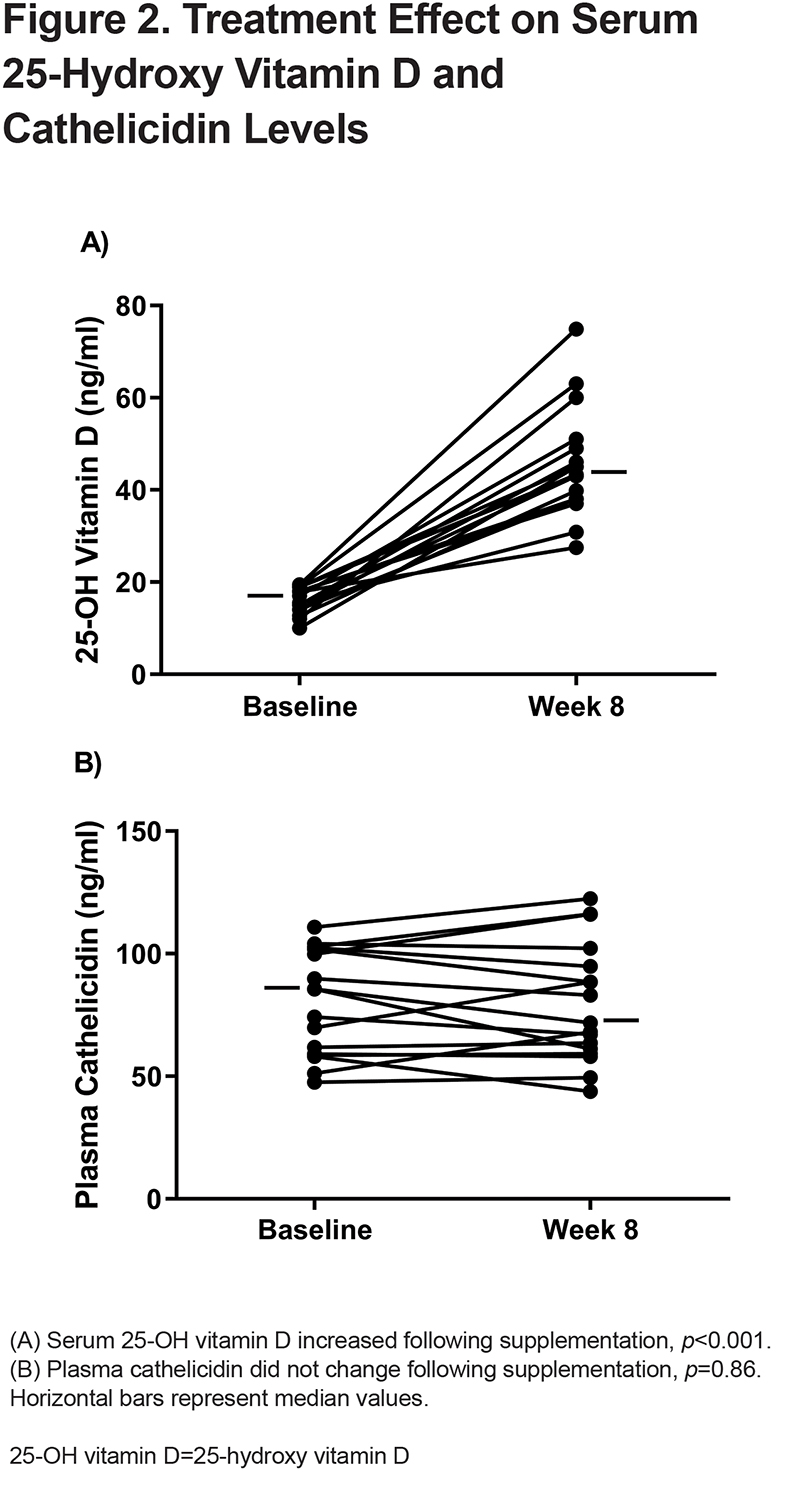
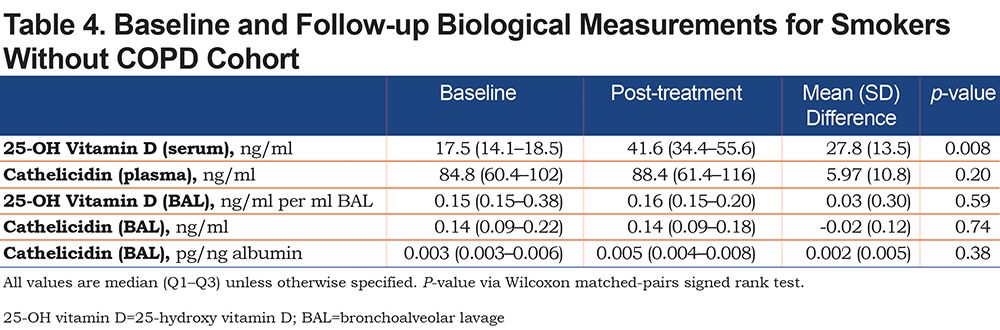
At baseline, the median serum 25-OH vitamin D level was 17ng/ml (IQR, 14.0–18.9ng/mL) (Table 2). Following the 8-week vitamin D supplementation period, the median measured 25-OH vitamin D level increased to 43.3ng/ml (IQR, 38.0–49.0ng/ml), representing a statistically significant increase in serum 25-OH vitamin D level (mean difference 29.4ng/ml, p <0.001) (Figure 2A). At baseline, the median plasma cathelicidin level was 85.6ng/ml (IQR, 59.0–102ng/ml) (Table 2). Following 8 weeks of vitamin D supplementation, the median plasma cathelicidin level was 71.8ng/ml (IQR, 61.2–94.8ng/ml). There was no change in plasma cathelicidin levels with vitamin D supplementation (mean difference -0.55, p=0.86) (Figure 2B). When stratifying by COPD status, similar effects of vitamin D supplementation on serum 25-OH vitamin D and cathelicidin levels were observed (Table 3 and Table 4).
Correlation Between Blood Vitamin D and Cathelicidin
Serum 25-OH vitamin D level and plasma cathelicidin levels were not correlated. When analyzed as a full cohort (n=17), there was no correlation at baseline (Spearman correlation coefficient ρ=0.29) or following supplementation period (ρ= -0.02). Similarly, when the cohort was stratified based on COPD diagnosis, there was no correlation between serum 25-OH vitamin D and plasma cathelicidin. Participants with COPD showed no correlation (baseline Spearman correlation coefficient ρ=0.45, post-supplementation ρ=0.10) and smokers without COPD similarly showed a lack of correlation (at baseline ρ=0.13, post-supplementation ρ=-0.24).
Effects of Supplementation on BAL 25-OH vitamin D and Cathelicidin
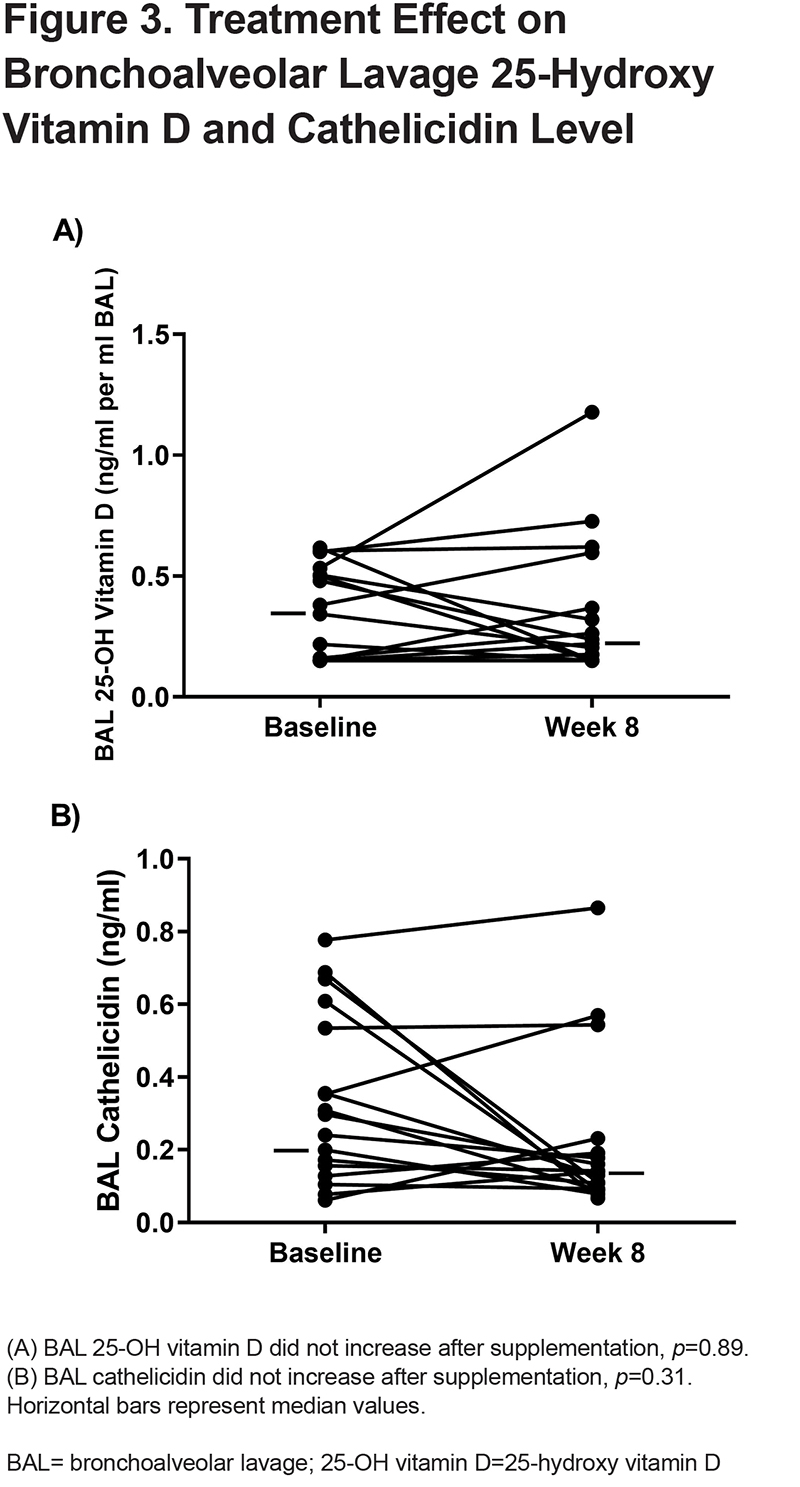
At baseline, the median BAL 25-OH vitamin D level was 0.34ng/ml per ml BAL fluid (IQR 0.15–0.50ng/ml per ml BAL fluid). (Table 2). After the 8-week vitamin D supplementation period, the median BAL 25-OH vitamin D level was 0.22ng/ml per ml BAL fluid (IQR 0.15–0.37ng/ml per ml BAL fluid) (mean difference of -0.0003ng/ml per ml BAL fluid, p=0.89). (Figure 3A). At baseline, the median BAL cathelicidin level was 0.20ng/ml (IQR 0.13-0.35ng/ml) (Table 2). After the 8-week supplementation with vitamin D, the BAL cathelicidin level was 0.14ng/ml (IQR 0.09–0.19ng/ml) (mean difference 0.066ng/ml, p=0.31) (Figure 3B). Similar findings were observed after adjustment for sample volume by albumin (Table 2). There was no difference in any BAL measures post-supplementation when stratifying by COPD versus non-COPD status (Table 3 and Table 4). There were no consistent differences in BAL cathelicidin (baseline or 8-week) between participants using inhaled corticosteroids compared to those not using inhaled corticosteroids.
Correlation Between Plasma and Lung Cathelicidin
Plasma and lung cathelicidin levels were not correlated in either pre- or post-treatment measurements. In the full cohort of 17 participants, there was appreciably no correlation at baseline (ρ=-0.24) or following supplementation (ρ=-0.10), and a similar lack of correlation was seen when the cohort was separated on the basis of obstructive lung disease (COPD subgroup baseline ρ=0.10 and post-supplementation ρ=0.20; smokers without COPD baseline ρ=-0.24 and post-supplementation ρ=-0.42).
Discussion
To our knowledge, this is the first study to prospectively examine the impact of vitamin D supplementation on BAL and plasma cathelicidin levels. Despite significant increases in serum 25-OH vitamin D levels, vitamin D supplementation over an 8-week period among vitamin D-deficient individuals was not associated with increases in BAL or plasma cathelicidin levels. Moreover, there was no change in BAL 25-OH vitamin D levels with oral vitamin D supplementation. There was also no correlation between plasma and lung cathelicidin levels at either pre- or post-supplementation measurement. The results of this study suggest that 8weeks of oral vitamin D supplementation does not lead to a measurable rise in cathelicidin levels in either the lung or peripheral blood compartments.
There has been conflicting data regarding vitamin D supplementation and blood cathelicidin levels. Cross-sectional analysis of an urban observational cohort showed higher cathelicidin levels associated with self-reported multivitamin use.19 In that study, participants reporting multivitamin use were more likely to have sufficient levels of serum vitamin D, suggesting an indirect correlation between vitamin D and cathelicidin. However, a separate observational, longitudinal cohort study found no association between vitamin D and plasma cathelicidin levels over 4 years of the study,29 similar to our findings. Studies in ulcerative colitis30 and sepsis31 do show a relationship between epithelial 25-OH vitamin D exposure and an increase in cathelicidin level from both epithelia and in peripheral blood. Separately, children with 25-OH vitamin D deficiency were found to not increase urinary cathelicidin concentrations in response to a clinical urinary tract infection.32 In a human model of allergic inflammation in the lung induced by segmental allergen challenge, late-phase increases of 25-OH vitamin D and metabolite in BAL correlated directly with increases in cathelicidin.21 These discrepant findings across studies highlight that the relationship between serum 25-OH vitamin D and plasma cathelicidin is complex and potentially confounded by factors that may influence levels of both molecules.18,29 It is possible that associations between 25-OH vitamin D and cathelicidin may be strongest in those with very severe (<10ng/ml) 25-OH vitamin D deficiency.19 In our cohort, no participant had severe 25-OH vitamin D deficiency at baseline. Further insight may be gained with a focus on cohorts enriched for very severe 25-OH vitamin D deficiency.
We also found no increase in BAL cathelicidin after supplementation. The lack of increased BAL cathelicidin with vitamin D supplementation observed in this study may be explained by the absence of an increase in 25-OH vitamin D concentration measured in the lung compartment despite increases in the peripheral compartment. Other studies have reported a correlation between serum and BAL vitamin D20 as well as airway surface liquid (ASL) vitamin D.33 Failure to observe increased BAL vitamin D levels may also be due to insufficient dosing of supplementation, failure of vitamin D to engage putative mechanism for increasing cathelicidin levels or related to vitamin D binding to other proteins in circulation, preventing penetration into the lung compartment. Active cigarette smoking also impacts cathelicidin activity. The literature presents conflicting results on the response of the 1,25-OH-vitamin D-cathelicidin pathway to smoke exposure in vitro.34,35 In prior studies of active smokers, increased antimicrobial activity of ASL cathelicidin was not observed.33 While no difference in BAL or plasma cathelicidin with supplementation was observed in our study, we cannot exclude an effect of concurrent smoking present in all of our participants. Studies assessing cathelicidin antimicrobial activity in participants with differential smoking status may yield different results.
Despite the lack of increase in blood or BAL cathelicidin with vitamin D supplementation, cathelicidin in both compartments was remarkably stable. Levels of cathelicidin may act as an acute phase reactant,30,36 with similar trajectories across compartments when measured in samples of acutely ill participants,37 that may not be reflected in this study of participants with stable COPD. Similar levels across time in stable COPD participants suggest longitudinal assessment of cathelicidin levels measured in stable participants and COPD outcomes may be feasible and informative. Our study showed no correlation between measured BAL and serum cathelicidin levels. This suggests that static measurements of cathelicidin in one compartment may not reflect levels in a separate compartment.
While not observing an increase in cathelicidin level in either the blood or BAL compartments, this does not exclude possible augmentation in cathelicidin activity with vitamin D supplementation. While the antimicrobial role of cathelicidin and its association with vitamin D levels have been described,31 it is unclear whether measured cathelicidin level predicts antimicrobial activity. Following conversion to the active metabolite, 1,25-OH vitamin D, local 1,25-OH vitamin D levels upregulate transcription of the cathelicidin gene.17,38,39 After 90 days of vitamin D supplementation, the antimicrobial activity of cathelicidin was increased in ASL.33 Studies in other patient populations suggest vitamin D supplementation may increase the activity of cellular immunity.40 For example, in the urogenital system, bladder cells treated with vitamin D had increased antibacterial activity against uropathogenic E. coli without a concomitant measurable rise in epithelial cathelicidin concentrations.41 Moreover, our current study design does not evaluate the effect of vitamin D supplementation on cathelicidin levels during acute injury or infection. Liu and colleagues have previously shown that both vitamin D metabolites and cathelicidin increase after segmental allergen challenge.21 In their study, normalization to albumin suggested plasma exudation as a mechanism for the increased levels. Additional studies are needed to understand the impact of vitamin D supplementation on lung cathelicidin activity in steady state and during acute injury or infection.
This study has limitations. As an exploratory study with a relatively low number of participants, it is likely underpowered to detect some differences. The study only achieved 63% of targeted enrollment. With this sample size, the mean percentage change we observed from the data was only 2%. Assuming similar variance, even with N=80 and the observed correlation of 0.6, we would be powered to detect a minimal detectable percentage change of 31%, still well beyond our observed 2% change. Another limitation relates to generalizability, as the majority of participants were female (75%) and African American (59%), with 100% current smokers. As highlighted earlier, it is possible that former smokers may have different lung responses to vitamin D supplementation. The ELISA assay used in this study measured combined human cathelicidin/LL-37, but not cleaved (active) cathelicidin. It is possible vitamin D supplementation may have augmented this more functionally important form of the protein. It is also unclear whether bronchoalveolar lavage sampling is an accurate reflection of relevant lung cathelicidin levels, as other studies have used ASL to quantify cathelicidin. However, despite these limitations, our study brings additional information to the discussion of cathelicidin and its relationship to vitamin D.
In conclusion, despite the relationship between vitamin D and its role in the upregulation of cathelicidin gene transcription, supplementation of vitamin D in deficient smokers was not associated with cathelicidin increases in either the blood or alveolar compartment in this pilot study despite significant increases in serum 25-OH vitamin D level. Additionally, we found that 8 weeks of oral vitamin D supplementation did not augment lung vitamin D levels. While lung and plasma cathelicidin levels were not correlated in either pre- or post-supplementation measurements, plasma cathelicidin level was stable over the 8-week time course, which may further support its role as a stable, informative biomarker for longitudinal outcomes. Future studies are needed to determine the effects of vitamin D supplementation on lung and blood functional activity.
Acknowledgements
Authors were involved in conception and study design (TB, RW, NH, ML and BD), acquisition of data (RW, ML, MD), data analysis and interpretation (ES, RB, JR, TB, RW, NH, ML, MD), writing the article (ES, RB, MD) and substantial involvement in revisions (ES, RB, JM, TB, RW, NH, ML, MD). De-identified participant data will be shared by request through the corresponding author. Study protocols and statistical analysis plans are available at clinicaltrials.gov (https://clinicaltrials.gov/ct2/show/NCT02464059).
Declaration of Interest
No authors report conflicts of interest related to this work.