Running Head: Rural Challenges to COPD Care
Funding support: This study was funded by the National Institutes of Health (HL129938-03) and by the reserve account for author RWV.
Date of acceptance: May 6, 2021 │ Published online date: May 28, 2021
Abbreviations: chronic obstructive pulmonary disease, COPD; advance practice providers, APPs; rural practice-based research networks, PBRNs; state offices of rural health, SORHs; nurse practitioner, NP; physician assistant, PA; COPD Assessment Test, CAT; modified Medical Research Council, mMRC; Research Electronic Data Capture, REDCap; Federally Qualified Health Centers, FQHC; rural health clinics, RHC; Global initiative for chronic Lung Disease, GOLD; American Thoracic Society, ATS; European Respiratory Society, ERS; coronavirus disease 2019, COVID-19
Citation: Diaz del Valle F, Koff PB, Min SJ, et al.Challenges faced by rural primary care providers when caring for COPD patients in the western United States. Chronic Obstr Pulm Dis. 2021; 8(3): 336-349. doi: http://doi.org/10.15326/jcopdf.2021.0215
Online Supplemental Material: Read Online Supplemental Material (1404KB)
Introduction
In the United States, chronic obstructive pulmonary disease (COPD) is the 4th leading cause of death, affects over 15 million people,1-3 and is nearly twice as common in rural versus urban areas (i.e., 8.2% versus 4.7%).2,4-6 The problem is even worse in economically distressed areas where the prevalence of COPD climbs7 to as high as 15.7%. The higher prevalence of smoking in rural areas is undoubtedly one of the prime causes for this urban/rural disparity.8,9 But in rural America, COPD is also 34% more common among never smokers, suggesting that exposure to fine particulates from other sources (e.g., secondhand cigarette smoke and dusts from agricultural, industrial, and biomass fuels) plays an important role in disease pathogenesis.7
COPD takes a greater toll in rural areas of the country where it results in more exacerbations10 and hospitalizations,2 worse quality of life,11 and higher mortality.2,12 In contrast to other major causes of death, COPD-related mortality continues to rise in the rural United States at the same time that it is falling in urban areas.13 The reasons why rural outcomes for COPD are worse than urban are complex and likely to include ongoing occupational and home exposures, as well as barriers to access and delivery of medical care.13-20
Nationwide, approximately 80% of COPD patients are under the care of a primary care medical provider.21 Given the paucity of pulmonary specialists in rural areas,16,17 the role of rural primary care medical providers in COPD care is likely to be even more important.20,22 Studies have explored the barriers faced by primary care providers when caring for COPD patients nationally.23-26 But to our knowledge, limited attention has been given to the challenges faced by rural medical providers,20 and a comprehensive study has not been performed. A group of pulmonary specialists, rural primary care providers, and a rural community advisory council that included lay members created “The Rural Medical Provider COPD Survey.” The survey was administered by email to rural medical providers in 7 states through rural practice-based research networks (PBRNs) at the University of Colorado and the University of Kansas, and through state offices of rural health (SORHs) in Nevada, Utah, Wyoming, North Dakota, and Arizona.27
Methods
Study Design
The Rural Medical Provider COPD Survey received exempt status by the Colorado Multiple Institutional Review Board (COMIRB Protocol 19-2016) and was distributed to rural medical providers from September 10,2019 through April 2, 2020.
Participants were identified through PBRNs affiliated with the State Networks of Colorado Ambulatory Practices and Partners at the University of Colorado (i.e., High Plain Research Network, Colorado Research Network, and Partners Engaged in Achieving Change in Health Network) and the University of Kansas (i.e., Kansas Patients and Providers Engaged in Prevention Research). They were also identified through SORHs in Nevada, Utah, Wyoming, North Dakota, and Arizona.27 Participants were included in the study if they identified themselves as a rural medical provider and were excluded if they did not. We asked participating organizations to send the survey to physicians with an MD or DO degree or to advanced practice providers (APPs) with a nurse practitioner (NP) or physician assistant (PA) degree. Participants were invited to take part in the study through newsletters and emails sent by each organization.
Survey
The Rural Medical Provider COPD Survey contained 26 questions and sub-questions (see online supplement) that were designed to collect demographic data and to determine rural medical providers’ satisfaction with their practices’ ability to diagnose, assess, and treat COPD, and to access ancillary services. After confirming rurality by self-report, questions 1–5 asked about the use of COPD guidelines, diagnosis of COPD, and how participants prefer to access educational information. Questions 1–4 were taken directly from 2 national surveys of primary care providers by Yawn and colleagues.23,24 Questions 6–9 asked about providers’ satisfaction with their practices’ ability to assess and treat COPD patients, access ancillary services, and whether their practice measured alpha-1 antitrypsin levels or used guideline-recommended assessment tools, such as the COPD assessment test (CAT) or the modified Medical Research Council (mMRC) Dyspnea Scale. The remaining questions collected demographic information related to providers, their practice, and the presence of an office spirometer.
Survey Administration
PBRNs and SORHs were contacted and asked to distribute the survey to rural medical providers within their network with 1 reminder email. PBRNs in Colorado and Kansas delivered the survey to providers in their network via newsletters and email. SORHs delivered surveys via emails. The newsletter or email included a summary of the survey goal, a consent statement along with information on how to connect electronically with the survey by hyperlink, web address, or QR code. Participants were also provided with an electronic copy of the survey to complete and mail in, but this method was not utilized. Participants were not compensated for their time. The Research Electronic Data Capture (REDCap) platform was used to collect survey data for analysis.28
Statistical Methods
Descriptive statistics were reported as frequencies and percentages. Differences between subgroups were compared with chi-square tests (or Fisher Exact Tests when cell counts were small). A significance level of 0.05 was used for all tests. SAS v9.4 (SAS Institute, Cary, North Carolina) was used for analysis.
Subgroup Analyses
The study performed analyses to determine whether there were survey response differences based upon training (i.e., physicians versusAPPs), practice location (i.e., Colorado and Kansas versus Other States), medically underserved communities (i.e., Federally Qualified Health Centers [FQHC] and rural health clinics [RHC]) versusprivate or hospital-based), or practice size (i.e.,≤5 providers versus >5 providers).
Sample Size Calculations
Approximately 1200 providers were available for the survey, with potential response rates ranging from 3%–10% for an email survey with no incentives.29 Descriptive statistics were utilized to compile and analyze the survey results. National studies of primary care physicians and AAPs show that 45%–50% use COPD guidelines.23,24 Based on these data, N=110 participants would be needed to show a ±10 percentage point accuracy using 95% confidence intervals of (35.2%, 54.8%) to (40.02%, 59.8%), or N=410 participants to show a ±5 percentage point accuracy using 95% confidence intervals of (40.1%, 49.9%) to (45.04%, 54.96%).
Results
Of the estimated 1018 rural medical providers that had access to the survey through email and newsletters, 8% responded (n=77). A total of 71 identified themselves as rural medical providers and were included in the analysis. Some results were condensed to simplify figures and tables.
Demographics
Providers in the Total Cohort tended to be younger (i.e., < 50 years old) and to have been in practice for fewer years (i.e., ≤ 15 years) (Table 1). Providers were divided by degree into physicians with an MD or DO, and APPs with an NP or PA. We included 2providers in the APP groups who chose “other” for their highest degree, because they identified themselves as a rural medical provider and indicated that their highest degree was a Masters. Half of the providers were physicians, and the other half were APPs, comparably split between NPs (51%) and PAs (46%). Practices were also evenly divided between those with ≤5 and >5 providers per practice. Sixty-two percent of providers practiced in RHCs (39%), FQHCs (21%) or in the Indian Health Service (1%). The other 38% of providers practiced in private (20%) or hospital/health system (18%) settings. Forty-nine percent of providers surveyed practiced in Colorado, 24% in Kansas, and 27% in the Other States group, including Nevada, Utah, Wyoming, North Dakota, and Arizona.
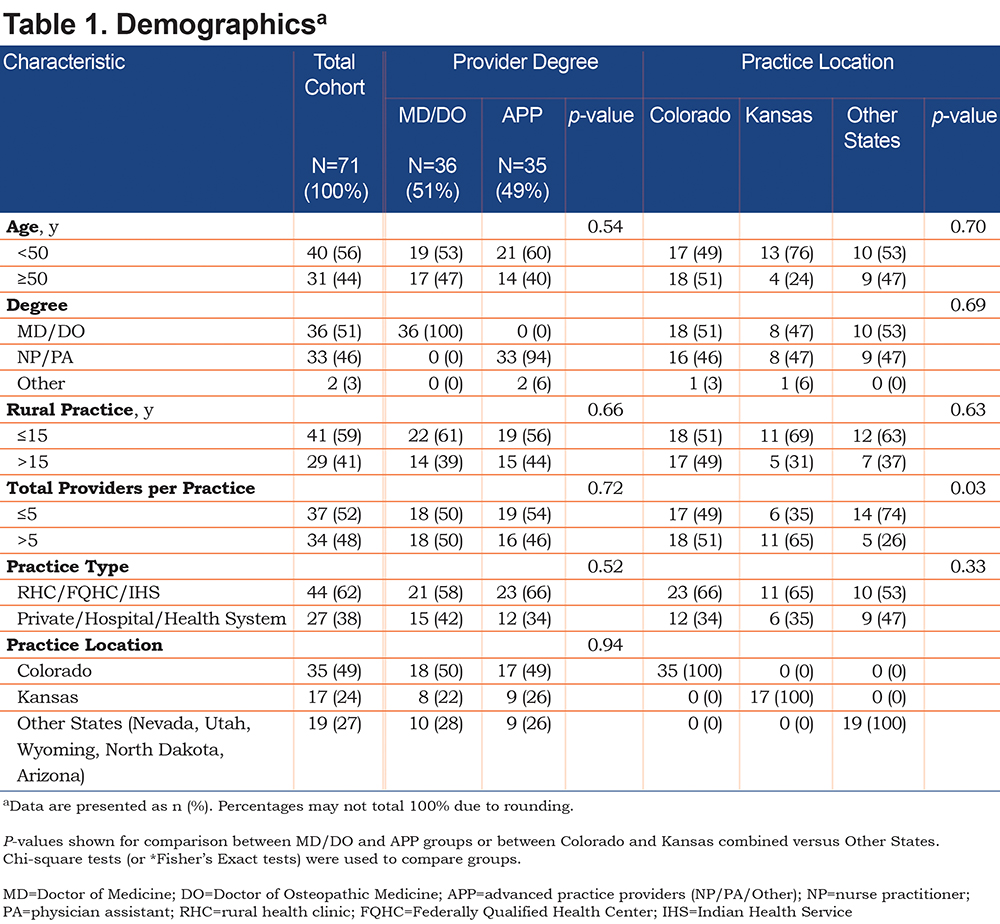
COPD Guidelines and Diagnosis
Figure 1 and Supplementary Table 1 (in the online supplement) include responses to questions aimed at determining provider use of COPD guidelines, barriers to the diagnosis of COPD, and how providers typically make the diagnosis of COPD. Sixty-one percent of providers reported using Global initiative for chronic Obstructive Lung Disease (GOLD)30 (55%) or American Thoracic Society (ATS)/European Respiratory Society (ERS)31 (6%) COPD guidelines as a resource to diagnose and manage COPD. The remaining 39% reported that they used COPD guidelines, but could not remember their name (14%), did not use guidelines (17%), did not know there were any guidelines (4%), or mistakenly used asthma guidelines instead (4%) (Figure 1A and Supplementary Table 1 [in the online supplement]). Seventy-six percent of providers reported that electronic sources were the best way for them to access information to guide treatment of COPD patients, including online medical resources (48%), electronic health record-based guideline prompts (15%), and smartphone applications (13%) (Figure 1B and Supplementary Table 1 [in the online supplement]). Only 24% of providers preferred non-electronic sources of information, such as printed guideline summaries (17%), standing orders (3%), or other sources (4%).
Providers face many barriers to diagnosing COPD, but the most prominent are the presence of multiple chronic conditions, patient failure to recognize and report symptoms, and lack of easy access to spirometry (Figure 1C and Supplementary Table 1[in the online supplement]). Eighty-nine percent of rural providers were aware that spirometry was needed to diagnose COPD, but they also relied upon a history of smoking, dyspnea at rest, dyspnea with activity, or the presence of a smoker’s cough (Figure 1D and Supplementary Table 1[in the online supplement]). Spirometry was the most common test used to diagnose COPD, but a chest X-ray, trial of an inhaled bronchodilator or inhaled corticosteroid were also used (Figure 1E and Supplementary Table 1[in the online supplement]).
Assessment
Complete survey results for assessments are shown in Supplementary Table 2 (in the online supplement). Eighty-seven percent of providers were satisfied with their practices’ ability to assess symptoms yet only 11% used a validated assessment tool, such as the CAT or the mMRC dyspnea scale (Figure 1F–G). On the other hand, only 62% of providers were satisfied with their practices’ ability to access spirometry and 66% had a spirometer in their office (Figure 1H –I). Screening for alpha-1 antitrypsin deficiency is recommended for everyone with airflow limitation by spirometry.30,32-34 The survey showed that only 1% of rural practices routinely test for alpha-1 antitrypsin, 58% occasionally test, and 41% never test (Figure 1J).
Treatment and Prevention
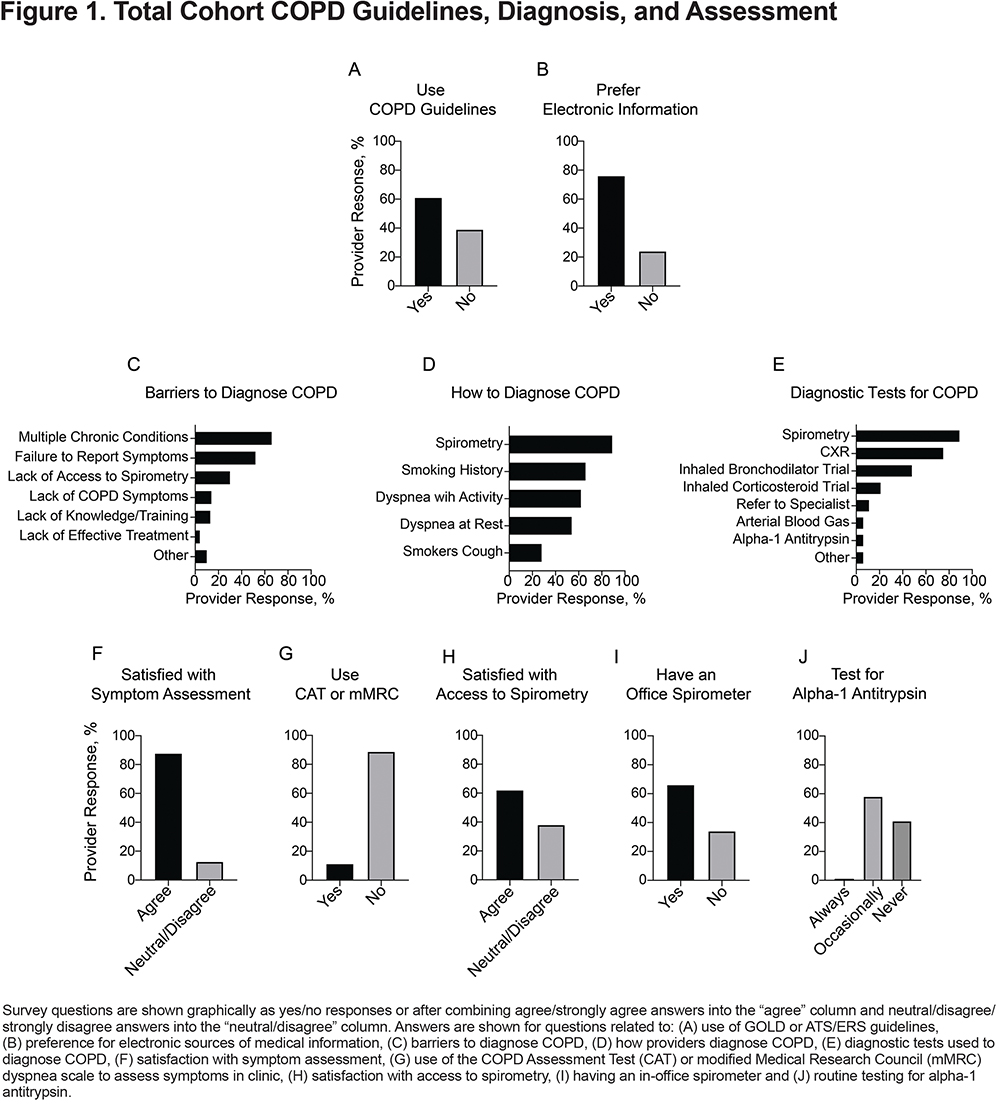
A high proportion of providers were satisfied with their practices’ ability to deliver smoking cessation counseling and inhaler education and treat symptoms and exacerbations, but fewer (66%) were satisfied with their practices’ ability to prevent exacerbations (Figure 2A–E and Supplementary Table 3 [in the online supplement]).
Access to Supportive Services
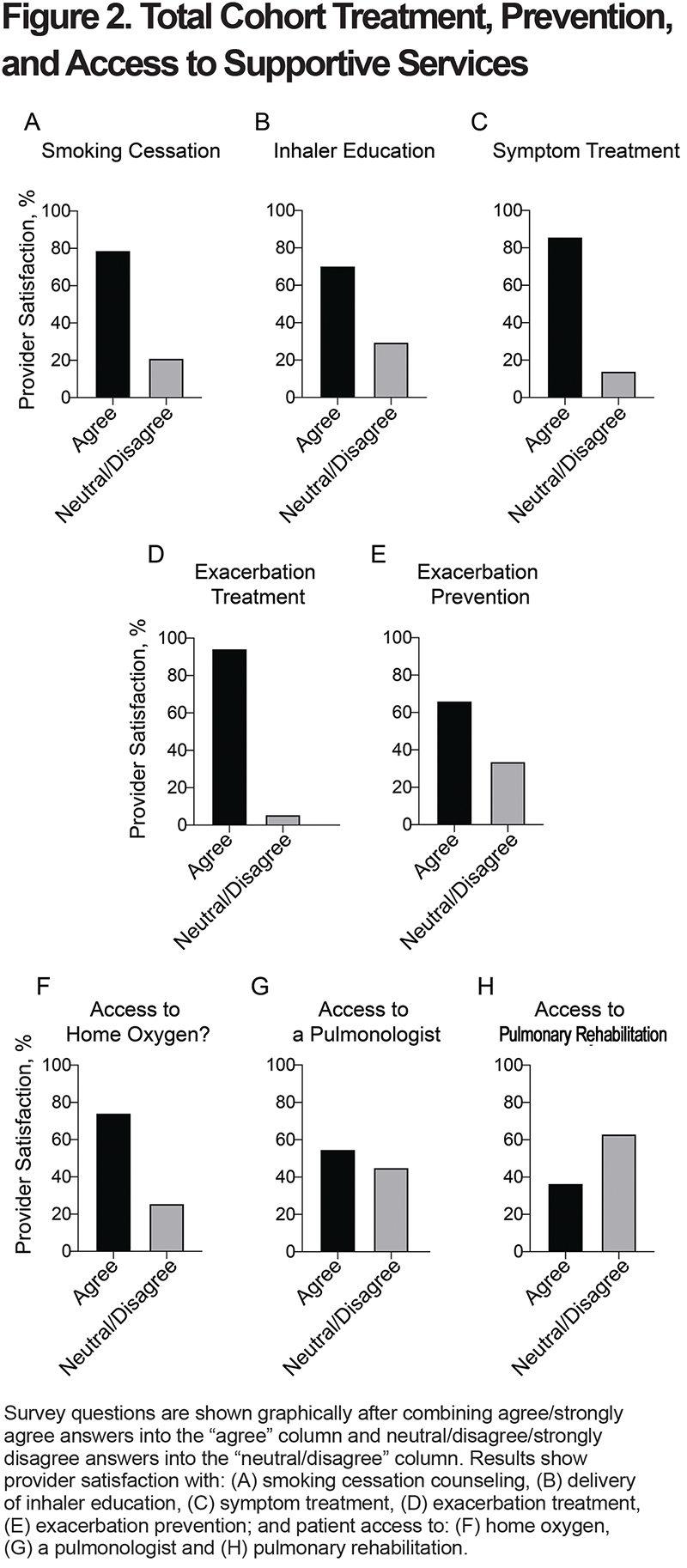
Most providers felt that their COPD patients had access to home oxygen therapy if needed (Figure 2F and Supplementary Table 4 [in the online supplement]). In contrast, only 55% of providers agreed that their patients had access to a pulmonologist for consultation, and only 37% agreed that their patients had access to pulmonary rehabilitation (Figure 2G–H and Supplementary Table 4 [in the online supplement]).
Subgroup Analyses
Provider Training: To determine if professional training impacted survey answers, providers were divided and analyzed by training: physicians (MD or DO) and APPs (NP or PA). Although physicians were significantly more likely than APPs to feel that multiple chronic conditions were a barrier in the diagnosis of COPD (p=0.01), APPs tended to feel that lack of knowledge/training was a barrier in COPD diagnosis (p=0.08) (Figure 3A–B). Physicians were more likely to use dyspnea with activity (p=0.02) or dyspnea at rest (p=0.08) to diagnose COPD, compared to APPs (Figure 3C–D). Otherwise, physicians and APPs were similar in terms of demographics and responses to survey questions (Table 1 and Supplementary Tables 1–4 in the online supplement).
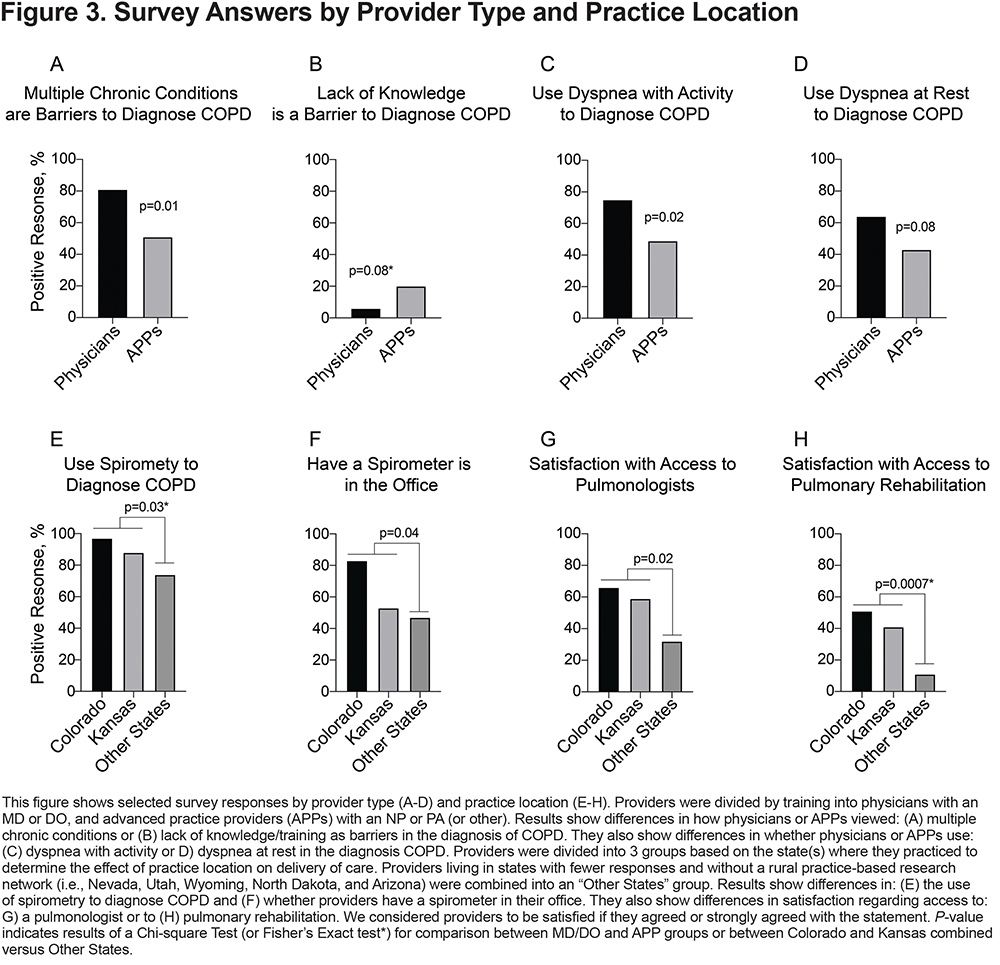
Practice Location: To determine the effect of practice location on barriers to care delivery, providers were divided into 3 groups based on their state designation. Compared to providers in Colorado and Kansas, providers in the Other States group were more likely to have smaller practices (p=0.03) (Table 1). Their practices also used less spirometry to diagnose COPD (p=0.03) (Figure 3E), and fewer had a spirometer in their office (p=0.04) (Figure 3F). Providers in the Other States group also felt that their patients had less access to pulmonologists (p=0.02) or pulmonary rehabilitation (p=0.0007), compared to providers in Colorado and Kansas (Figures 3G–H). Responses to the remainder of the survey questions were similar (Table 1 and Supplementary Tables 1–4 in the online supplement).
Practice Population and Size: To determine whether practice population or size impacted survey answers, the total cohort was divided into those targeted to medically underserved areas or populations (i.e., FQHCs, RHCs and Indian Health Service) or not (i.e., private/hospital clinics), and small (i.e., ≤5 providers) versus larger (i.e., >5 providers) practices. Sixty-two percent of providers worked in an FQHC or RHC and 38% worked in a private/hospital clinic. Fifty-two percent of providers worked in a smaller practice and 48% worked in a larger practice. Neither of these analyses revealed differences in provider demographics or responses (data not shown).
Discussion
Primary care providers are on the front line of care for COPD patients nationally.35 Given the paucity of pulmonary specialists in rural areas,16,17 the role of the primary care provider for rural COPD patients is paramount.34 Outcomes for rural COPD patients, such as mortality and hospitalizations, are worse than their urban counterparts in many respects.2,10-12 This is likely due to ongoing exposures (e.g., cigarette smoke, secondhand smoke, and agricultural dusts, fumes, and pesticides),7,9,35-39 as well as unique barriers that impede access to care.15,16 Poor outcomes may also be due to obstacles that prevent optimal delivery of care, but these barriers have received much less attention.20 Our study found significant challenges to diagnosis, assessment, prevention, and access to ancillary services that were affected by provider type and practice location, but not by the patient population served or practice size.
GOLD and ATS/ERS COPD guidelines are the primary sources of evidence-based information for the diagnosis and management of stable COPD and COPD exacerbations.30,31 Yawn et al assessed the use of GOLD or ATS/ERS guidelines in a group of mostly urban primary care medical providers attending continuing medical education conferences in 2014, and found that approximately 48% used COPD guidelines but that approximately 29% were not aware of them.24 This study asked the same question and found that 61% of rural providers used GOLD or ATS/ERS guidelines and that 4% were unaware of them, suggesting that progress is being made.
Up to 60% of people with low lung function are unaware that they have obstructive lung disease, indicating that COPD is under-diagnosed in the United States.40 Under-diagnosis of COPD may be due to suboptimal communication between patients and providers, or inadequate knowledge about how to diagnose COPD.20 This survey showed that the ability of rural providers to diagnose COPD was impaired by the presence of multiple chronic conditions and the inability of patients to recognize and report symptoms, both of which make diagnosis more difficult. Almost all rural providers understand that spirometry is necessary to diagnose COPD, but it appears that access to spirometry is still a problem for many.
GOLD Guidelines recommend regular assessments of symptoms with the CAT or mMRC dyspnea scale because they relate to risk of exacerbation, acute deterioration in health status, depression, and death, and can be used to guide therapy.30,41 They can also be used to capture symptoms that may not be recognized or reported otherwise.30,42 The CAT is the preferred assessment tool because it assesses respiratory and non-respiratory symptoms, while the mMRC only assesses dyspnea.30 Study findings indicate that only 11% of rural providers use the CAT or the mMRC dyspnea scale to assess symptoms, but that 87% were satisfied with their ability to assess symptoms. The reason(s) that the CAT or mMRC have not been integrated into routine assessment is not known, but possibilities include the perception that it is difficult to incorporate an assessment tool into a busy practice, providers may not be aware of this recommendation, or providers may trust their ability to assess symptoms already. In this context, implementation of the CAT or mMRC as a routine clinic measurement may help providers identify patients who fail to recognize and report symptoms, which was noted to be a barrier to COPD diagnosis by 52% of providers.
The study revealed that 58% of providers occasionally test for alpha-1 antitrypsin deficiency and that 41% never test. In Europe, approximately 24% of general practitioners, 34% of internists and 73% of pulmonologists surveyed said that they “currently test for alpha-1 antitrypsin deficiency,”43 and testing was directly related to knowledge about the disease.43,44 Alpha-1 antitrypsin deficiency has been recognized as a risk factor for COPD since 196345,46 and is present in 1%–2% of COPD patients within the United States.47,48 Identification that a person is deficient allows for smoking cessation efforts, identification of affected family members, and replacement therapy in select patients.47 The World Health Organization, the ATS/ERS, and GOLD guidelines recommend that all patients with fixed airflow obstruction (e.g., COPD and asthma) or unexplained liver disease be tested for alpha-1 antitrypsin deficiency, with or without the presence of an additional COPD risk factor such as smoking.30,32-34 Despite these recommendations, diagnosis is frequently delayed and >85% are estimated to have not yet been recognized.47,49 Novel approaches to increase alpha-1 antitrypsin screening would identify increased numbers of deficient patients and provide new avenues for treatment of patients and knowledge for families.50-52
Rural COPD patients have decreased access to pulmonary specialists and pulmonary rehabilitation,4,5,17,25 but less is known about whether rural providers perceive this deficiency. This is an important distinction, because many rural providers have multidisciplinary training in Family Medicine, are comfortable caring for complex patients, and may not feel the need for these services. Our survey found that 45% of rural providers were dissatisfied with their patients’ access to pulmonologists. Croft et al examined geographic access to pulmonologists and found that 65% of rural COPD patients do not have access to a pulmonologist within a 10-mile buffer distance.17 Therefore, it appears that provider dissatisfaction with access to a pulmonologist (i.e., 45%) is roughly similar to the geographic lack of access for patients (i.e., 65%). Kimet al also demonstrated a link between pulmonary specialty care and reduced hospital/emergency department visits for COPD.16 Novel mechanisms to move knowledge to rural primary care providers instead of moving patients,4,53,54 and/or to increase remote access to pulmonary specialty care, might help to alleviate this gap in rural settings.55-58
The study also found that only 37% of rural providers were satisfied with access to pulmonary rehabilitation. This contrasts with the National Heart, Lung, and Blood Institute national survey which found that 74% of non-urban primary care physicians reported that rehabilitation programs were “available” to their patients.20 Our results suggest that availability of pulmonary rehabilitation may be worse in the western United States where distances are greater than they are nationwide. Difficulty with transportation, distance, and location of programs have been identified as major factors with both attendance and completion of pulmonary rehabilitation.59,60 Increasing community-19 and home-based pulmonary rehabilitation programs61,62 or remote programs that have components of pulmonary rehabilitation55-58 may help to overcome these hurdles.
To determine whether provider responses varied by location, participants were separated into 3 groups based on their residence in Colorado, Kansas, or “Other States” that individually had a lower number of survey responses and were not associated with a rural PBRN. Providers in the Other States group worked in smaller practices, used less spirometry to diagnose COPD, were less likely to have a spirometer in their office, and had less access to pulmonologists and to pulmonary rehabilitation compared to providers in Colorado and Kansas. Smaller practices, worse access to ancillary services, and lack of statewide PBRNs to bind practices together,63 suggest that providers in these states may be less supported.
This study has several limitations, some with potential importance for future, rural clinical studies. First, only 77 providers responded to the survey out of approximately 1018 who had access to the survey through newsletters and emails without incentives, resulting in an 8% response rate. This response rate is low but is similar to the 8%–9.7% response rate for surveys sent to pharmacists by email only.29 Eberth et al also obtained a 13% response rate from a national cancer screening survey that was sent to primary care physicians by 3 mailings, 1 email, follow-up and incentives.64 Because distribution methods for our survey utilized newsletters, we can only estimate the number of providers that had access to the survey and not the number who saw the survey, which could make the responses appear artificially low. The response rate may also be low because of the coronavirus disease 2019 (COVID-19) pandemic. Non-PBRN providers were contacted from February through April of 2020, as the COVID-19 pandemic took hold in the United States. The pandemic prevented recruitment of additional PBRNs to the study and may have decreased responses from providers, especially those living in non-PBRN states. An estimated 110 responses were needed to yield a ±10 percentage point accuracy in estimation of the percentage of rural providers using GOLD or ATS/ERS COPD guidelines. The Rural Medical Provider COPD Survey showed that 61% of rural medical providers use COPD guidelines, which was 13 percentage points higher than a national sample of primary care providers from 2014, which exceeded our estimates.24
Second, survey distribution methods might have impacted results and numbers of responders. Seventy-three percent of providers were recruited from rural primary care PBRNs in Colorado and Kansas, while 27% were recruited primarily through SORHs in Nevada, Utah, Wyoming, North Dakota, and Arizona.27 Fewer survey responses were noted from non-PBRN states (3.5%) versusPBRN states (11%). Delivery of the survey though PBRNs versus SORHs could have influenced both distribution numbers and provider type. These results could also reflect real differences in states with different levels or types of support for providers. Third, the low number of responses is a concern for generalizability, but it is reassuring that the pattern of responses to questions 1–4 was similar to identical questions included in 2 national surveys of primary care providers by Yawn et al.23,24
Additional limitations might be related to: the self-identification of rural and non-rural providers causing surveys to be inadvertently sent to non-rural providers, no financial compensation mechanism, and small response numbers not adequately representing rural providers in each state. Because survey data was self-reported, results were also subject to social demand and recall biases. Selection bias may have occurred, because only electronic surveys were received. The survey did not address barriers or challenges related to access to health insurance, geographic barriers, or ability to afford medications. It also did not address barriers to care faced by COPD patients in rural areas, which is an equally important question to address.
COPD in rural areas of the United States is plagued by high prevalence, poor outcomes, and excess mortality compared with urban areas of the country.2,4-13 The reasons for these disparities are complex, but likely relate in part to important challenges faced by rural medical providers as they work to deliver the best care to COPD patients.18-20 The Rural Medical Provider COPD Survey identified bright spots in the increased, but not yet optimal, use of COPD guidelines to manage COPD and the high use of spirometry to diagnose COPD. But it also identified deficits in the assessment of symptoms and alpha-1 antitrypsin, prevention of exacerbations, and access to pulmonary specialty care and pulmonary rehabilitation that could be targeted for improvement. The survey also presents intriguing data suggesting that there may be regional/state differences, which could relate to expansive geography and/or the presence of a rural PBRN. It is also possible that the presence or absence of a PBRN is a marker for variable levels of provider support. Ultimately, as articulated by Westfall et al, rural primary care practices are important laboratories to ask fundamental questions about how best to implement recommendations derived from academic studies.63 In this context, it is important to remember that “the final crucial step in clinical care is the delivery of recommended care to the right patient at the right time, resulting in improvement in that patient’s health,”63 and this is not always easy or straightforward. The results of this survey create opportunities for improvement through action.
Acknowledgements
We would like to extend our deep appreciation to the rural providers who participated in this study, to the PBRNs in Colorado and Kansas, and to the SORHs in Nevada, Utah, Wyoming, North Dakota, and Arizona for their help recruiting rural medical providers. We are also grateful to lay members of the Community Advisory Council in Colorado who helped design the survey.
Author contributions: RWV, FDV, PBK, and SJM had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. RWV, FDV, PBK, KAG, TKO, LZ, DHF, AN, DEN, EJM, CG, and JLN contributed to the study concept. FDV, PBK, LZ, TKO, LZ, DHF, AN, KMT, DEN, EJM, KAG, and RWV contributed to acquisition of data. FDV, PBK, SJM, TKO, LZ, KMT, AN, DEN, EJM, KAG, and RWV contributed to analysis and interpretation of data. FDV, PBK, SJM, TKO, LZ, KMT, JLN, CG, KAG, and RWV drafted the manuscript. FDV, PBK, JKZ, TKO, LZ, KMT, DHF, AN, DEN, EJM, JLN, CG, KAG, and RWV contributed to critical revision of the manuscript for important intellectual content. SJM, FDV, and RWV performed statistical analysis. FDV, PBK, JKZ, and RWV provided administrative, technical, or material support. RWV supervised the study.
Declaration of Interest
The authors have nothing to declare.