Running Head: Excessive Mucous Production in COPD Gene cohort
Funding support: This work was supported by funds from RespirTech to HealthPartners Institute.
Date of acceptance: May 12, 2021 | Published online: June 10, 2021
Abbreviations: chronic obstructive pulmonary disease, COPD; COPD Genetic Epidemiology study, COPDGene®; confirmed presence of bronchiectasis, BE+; confirmed absence of bronchiectasis; BE-; confirmed presence of chronic bronchitis, CB+; confirmed absence of chronic bronchitis, CB-; St George’s Respiratory Questionnaire, SGRQ; Global initiative for chronic Obstructive Lung Disease, GOLD; odds ratio, OR; Body mass index-Obstruction-Dyspnea-Exercise index, BODE; confidence interval, CI
Citation: Bohn O, Xi M, Woodruff NK, Hansen GL, McEvoy CE; COPDGene investigators. Chronic bronchitis in COPD patients creates worse symptom burden regardless of the presence of bronchiectasis in the COPDGene cohort. Chronic Obstr Pulm Dis. 2021; 8(3): 350-359. doi: http://doi.org/10.15326/jcopdf.2021.0202
Introduction
Note: An abstract of this work was presented at the 2017 CHEST conference.
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality globally1 In the United States, COPD ranks fourth in causes of mortality with over 150,000 deaths annually.2 An estimated 15.5 million people had diagnosed COPD in 2015,3 and an estimated 12 million potential cases remain undiagnosed (44%).4 The estimated total annual cost of COPD for 2010 was $49.9 billion.5
Chronic bronchitis symptoms related to COPD often bring patients into both primary and specialty care clinics.6,7 The presence of chronic bronchitis in persons with COPD is associated with negative patient outcomes such as more severe airflow obstruction, diminished quality of life, high rate of hospitalization, and early mortality.8-13 Despite the significant impact of chronic bronchitis symptoms on patients, there are very few management strategies available to patients who present with this disease phenotype.14
Another condition that is often associated with COPD is bronchiectasis, a pulmonary disorder characterized by permanent bronchial dilatation with associated inflammation, chronic cough, and recurrent exacerbations.15 There is substantial overlap between the populations with chronic bronchitis and bronchiectasis,16,17 but the direct effect of bronchiectasis on COPD patients with and without the symptoms of chronic bronchitis has not been adequately explored. Several past studies have examined the relationship between chronic bronchitis and symptoms using the COPD Genetic Epidemiology Study (COPDGene®) cohort, a large multicenter observational study designed to analyze genetic and clinical factors associated with COPD. Kim et al found the presence of chronic bronchitis symptoms was associated with higher St George’s Respiratory Questionnaire (SGRQ) “Total” and “Respiratory” scores.13 This study did not report other SGRQ domain sub-scores such as “Activity” and “Impact” and did not distinguish between patients with and without bronchiectasis. A subsequent abstract by Stewart et al examined COPDGene data based on the presence of bronchiectasis (BE+), finding that the BE+ phenotype was associated with more severe airflow obstruction, higher Body mass index-Obstruction-Dyspnea-Exercise (BODE) index, and more exacerbations.18 They did not stratify the bronchiectasis results by the presence of chronic bronchitis symptoms. Accordingly, this study estimates the full symptomatic burden of patients with COPD, stratified by the presence of both chronic bronchitis and bronchiectasis.
Methods
Data used in this study were extracted from the COPDGene cohort (ClinicalTrials.gov: NCT00608764). Characteristics of this cohort are published elsewhere.19 Succinctly, it includes over 10,000 persons with a history of smoking, Black and non-Hispanic White race, and aged 40 to 80 years. All participants undergo computed tomography imaging as well as extensive symptom characterization. The data also include self-reported information on cough and phlegm production, and chronic bronchitis, defined as the presence of cough and phlegm production for 3 months of the year for at least 2 consecutive years. The presence of bronchiectasis was identified via independent scoring by 2 chest radiologists. Bronchiectasis was defined as 1 or more of the following: (1) airway dilation, with the airway diameter greater than the adjacent pulmonary vessel, (2) abnormal airway tapering, or (3) location of a bronchus within 1 cm of the pleura. These criteria were assessed by multiple independent readers, who coded the presence of bronchiectasis as “yes,” “no,” or “equivocal.”20 This study met all criteria for institutional review board approval (HealthPartners Institute IRB #A07-127).
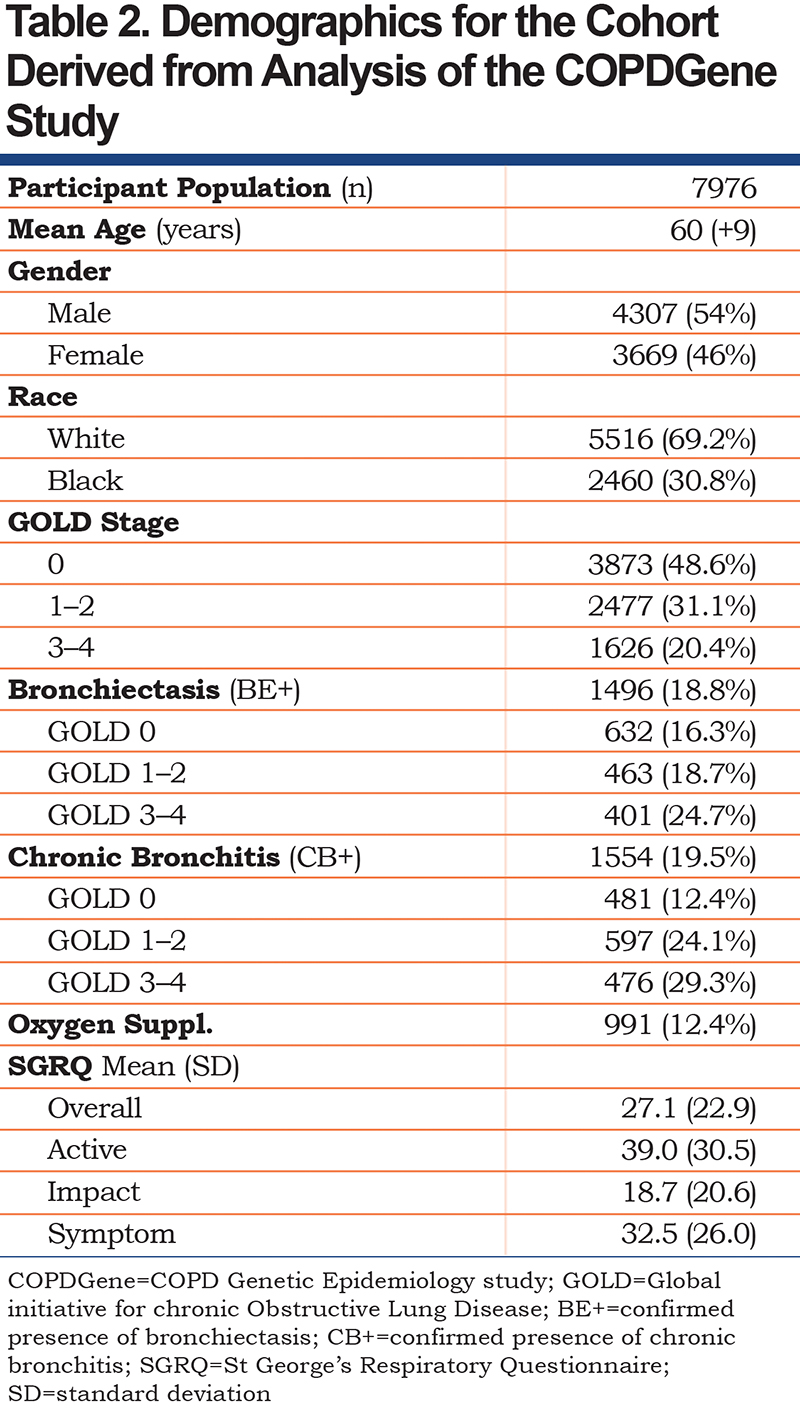
For this analysis, participants in the COPDGene cohort were divided into groups based on the presence or absence of chronic bronchitis (CB+/CB-) and bronchiectasis (BE+/BE-). The control cohort included those without either finding. Of the 10,729 persons in the full cohort,21 we identified 7976 participants for whom relevant imaging and symptom data were available, including some patients without identifiable obstruction (“GOLD 0”).22 To assess the impact of chronic bronchitis on our participants, we then compared the available data regarding their COPD Global initiative for chronic Obstructive Lung Disease(GOLD) stages,23 the presence of radiological bronchiectasis, and quality of life as measured by the SGRQ.24 The latter included total scores as well as individual components: “Activity,” “Psychosocial Impact,” and “Symptom” sub-scores. The Symptom sub-score was left out of further analysis because its questions closely resembled the questions used to identity chronic bronchitis; comparing the 2 metrics would be tautological.
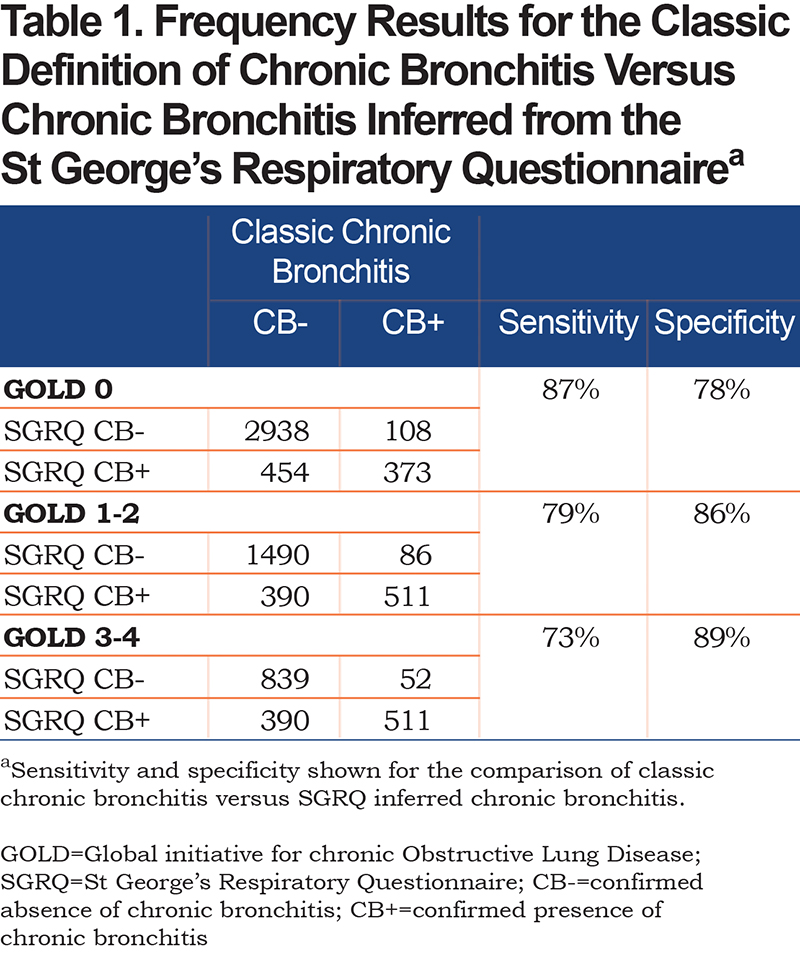
In order to provide an independent assessment of the presence of chronic bronchitis for the various patient populations, we compared the results for the “classic” American Thoracic Society definition used in this study25 to similar criteria found in the SGRQ, following the method of Kim.26 SGRQ chronic bronchitis was assigned if patients answered “almost every day” or “most days a week” to the following questions: “Over the last 4 weeks, I have coughed” and “Over the last 4 weeks, I have brought up phlegm (sputum).” The sensitivity for an SGRQ CB+ result given a classic CB+ result varies between 73% and 87% depending on the GOLD stage, while the corresponding specificity varies between 78% and 89% depending on the GOLD stage (Table 1), which demonstrates a close correspondence between the 2 methods.
Statistical Analysis
Differences between SGRQ scores for various combinations of bronchiectasis and bronchitis status were evaluated using the Mann-Whitney test for non-normal data and the t-test if data were normally distributed. Odds ratios (95% confidence interval [CI]) were calculated for the frequency of bronchiectasis and bronchitis for different GOLD stages and was calculated using a 2x2 contingency table. All statistical analyses were conducted using the statistical software SAS v9.4 (SAS Inc.).
Results
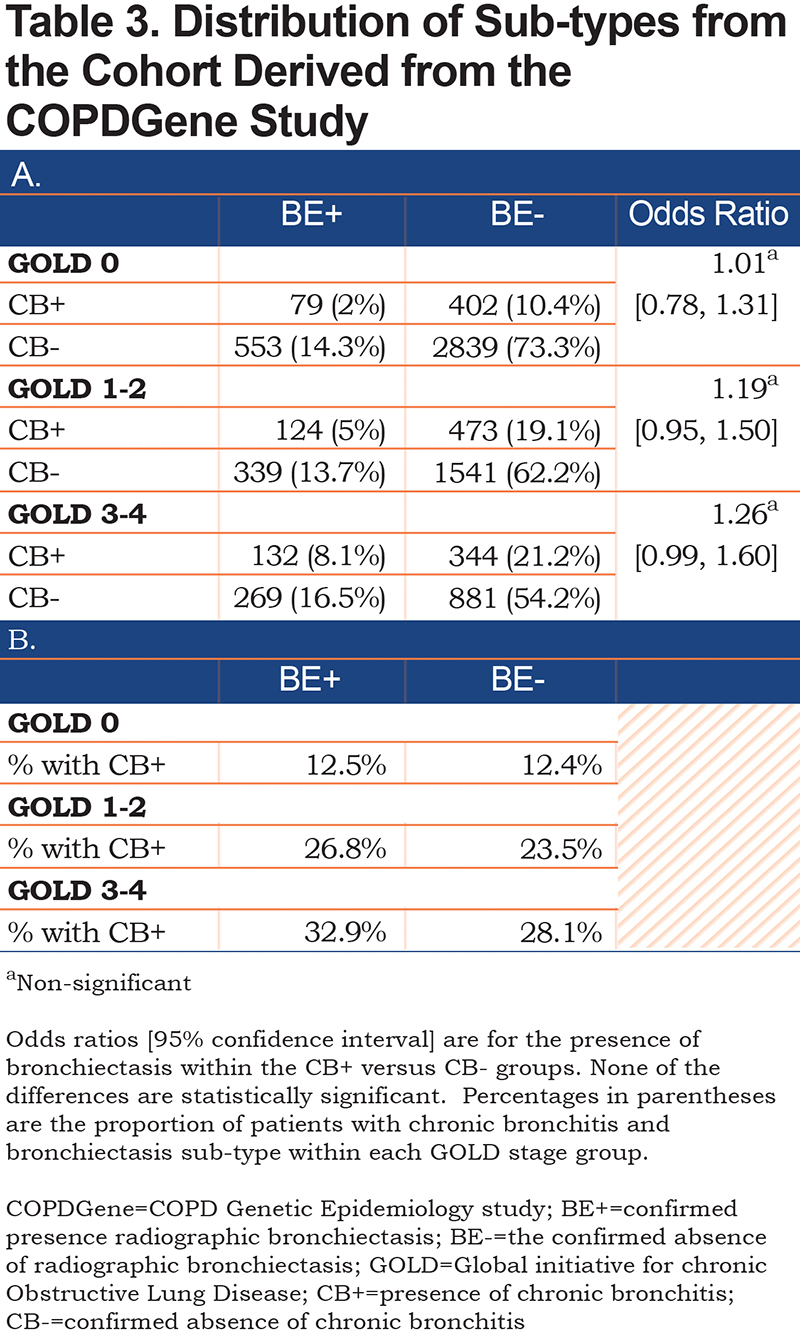
Participants included in our analysis had a mean age of 60 (+ 9 years), were 54% male, and 69% self-identified as White versus 31% as Black. Additional demographics are shown on Table 2. The data were segregated into 3 cohorts: GOLD stage 0 (3873, 48.6%), GOLD stage 1–2 (2477, 31.1%), and GOLD 3–4 (1626, 20.4%). Almost equal portions of the overall participant population had bronchiectasis (18.8%) and/or chronic bronchitis (19.5%). Within these subgroups, bronchiectasis (BE+) was found in higher proportions among progressively higher GOLD stages (19.5% in GOLD 0, 23.0% in GOLD 1–2, and 32.7% in GOLD 3–4). Similarly, chronic bronchitis (CB+) was also found in higher proportions among progressively higher GOLD stages (14.2% in GOLD 0, 31.8% in GOLD 1–2, and 41.4% in GOLD 3–4). The study cohort included participants who had combined chronic bronchitis and bronchiectasis (CB+/BE+), chronic bronchitis only (CB+/BE-), bronchiectasis only (CB-/BE+) and a control group with neither condition (CB-/BE-). Data show that the presence or absence of bronchiectasis was not associated with a higher frequency of chronic bronchitis (GOLD 0 group OR 1.01 [0.78,1.31], GOLD 1–2 group OR 1.19 [0.95, 1.50], GOLD 3–4 group OR 1.26 [0.99, 1.60]) (Table 3).
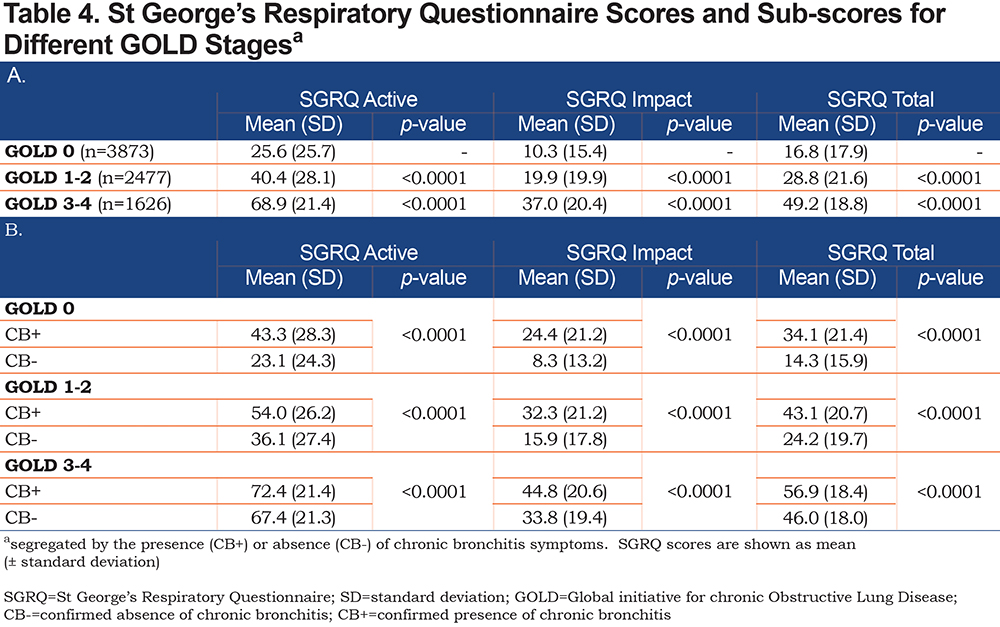
Individuals with progressively higher GOLD stages had overall higher SGRQ scores (Table 4A). Additionally, the data indicated that SGRQ scores were markedly higher among participants with chronic bronchitis symptoms (CB+) when compared to CB- participants within the same GOLD group; all differences were statistically significant (Table 4B).
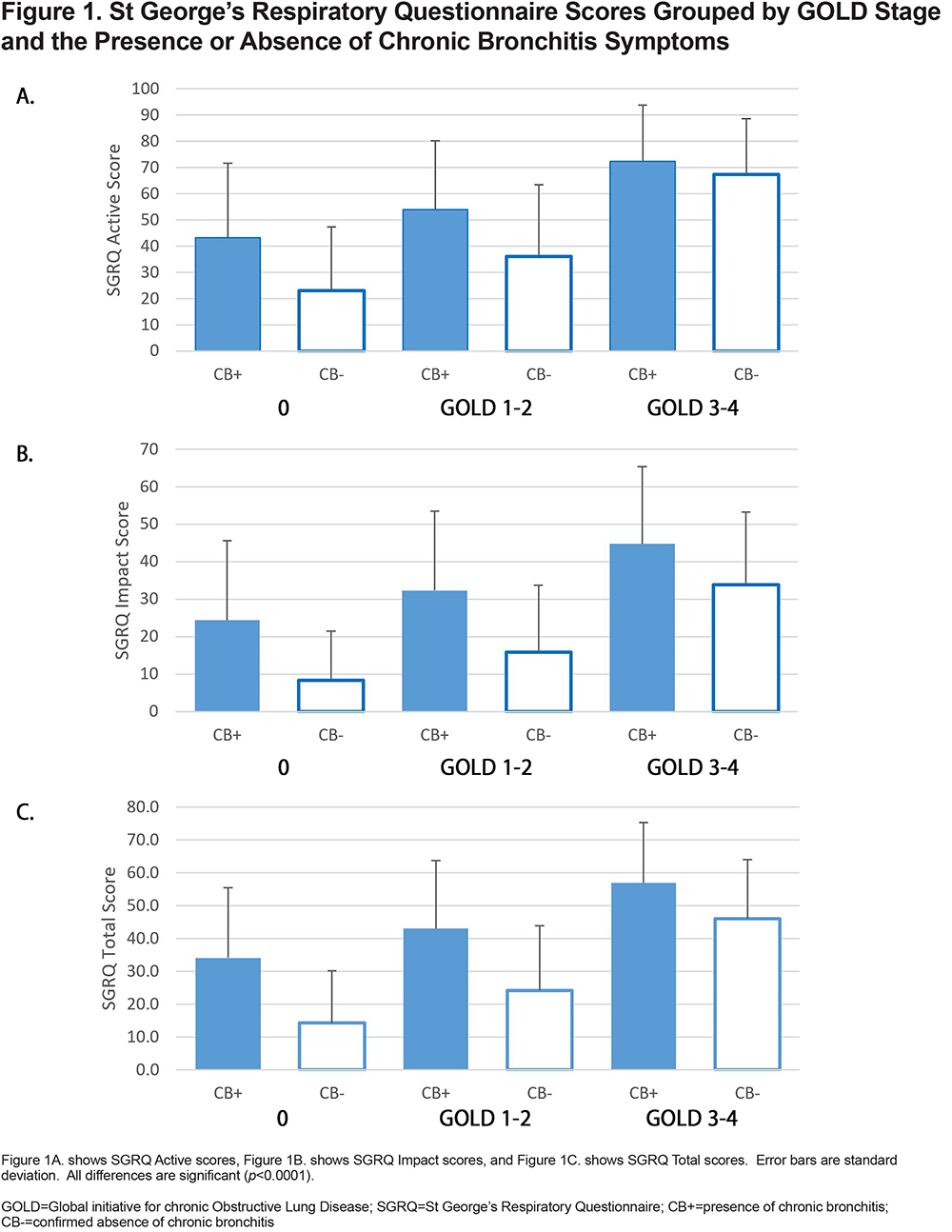
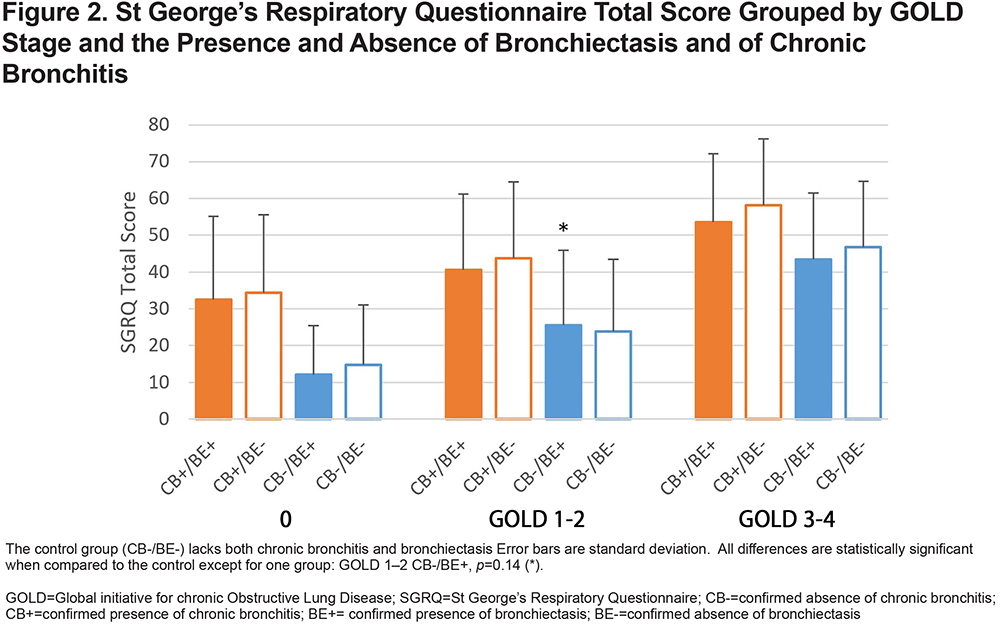
Using these data, it was possible to compare the effect of bronchiectasis on SGRQ scores for patients with and without symptoms of chronic bronchitis (Figure 1). The results showed higher SGRQ Total scores among individuals with higher COPD GOLD stages and among CB+ participants across all GOLD stage groups and regardless of the presence of bronchiectasis (Figure 2). In all cases, the CB+/BE- groups had significantly elevated symptom scores while the BE+/CB- groups were similar to the control group (CB-/BE-).
Discussion
In the COPDGene cohort, individuals with chronic bronchitis had significantly poorer quality of life across all GOLD stages compared to those who do not. Our analysis of 7976 participants identified 1554 persons with symptoms of chronic bronchitis, representing 19.5% of our study cohort. This made chronic bronchitis a condition affecting a significant proportion of COPDGene population. The proportion of participants with chronic bronchitis was roughly similar to participants with radiographic evidence of bronchiectasis (18.8%), although it is notable that only 4.2% of our study population exhibited both bronchiectasis and chronic bronchitis, whereas 15.3% exhibited chronic bronchitis alone. Moreover, the data consistently demonstrated a negative impact of chronic bronchitis on the affected participants. Persons with chronic bronchitis reported higher SGRQ scores (i.e., worse) across all studied categories regardless of their COPD GOLD stage when compared to controls without chronic bronchitis. This is consistent with the results of Kim,13 who also found this relationship however, our study extends the results to the SGRQ “Impact” and “Active” scores, suggesting an even broader degradation in quality of life for these patients.
Furthermore, our data showed the presence of bronchiectasis had little impact on the patients’ SGRQ scores, while the presence of chronic bronchitis had a major detrimental impact. Notably, the great majority of patients with bronchiectasis/COPD overlap did not have chronic bronchitis as defined by conventional criteria. Like the study of Stewart,18 our results suggest that bronchiectasis alone does not have a significant impact on participants’ quality of life, and that bronchiectasis and bronchitis is not more common in CB+ and CB- groups. We find that that chronic bronchitis may be a distinct phenotype that affects the SGRQ scores of persons with COPD regardless of any underlying structural disease. This result may be surprising in light of past work that shows a significant impact of bronchiectasis on other clinical variables.27 We propose that excess sputum production is the primary factor affecting quality of life in persons with COPD, and that the presence of bronchiectasis, while it may contribute to retention of mucus, is a minor contributor. That is, patient-reported outcomes/symptoms better inform treatment options than radiographic reported structural changes.
This study was limited by the subjective nature of SGRQ score reporting. However, the data consistently show that persons with chronic bronchitis consistently exhibit more burdensome respiratory symptoms than controls at a similar GOLD stage. This work was also limited by the lack of clinical context behind the chronic bronchitis determination, including sputum color and quantity, any specific treatments the patient may have received, and the past record of exacerbations.
Current pharmaceutical treatments for COPD focus on improving forced expiratory volume in 1 second, dyspnea, and exacerbations, while there are few options specifically for chronic bronchitis, and these are often costly and not well-tolerated.28 At the same time, recent research suggests that reduction of symptoms is associated with fewer exacerbations and reduced exacerbation rates in patients with bronchiectasis.29 Patients with symptoms of chronic bronchitis, with or without bronchiectasis, should receive appropriate treatment for their symptoms, which may include mucoactive therapies,28,30-32 inhaled corticosteroids,14 macrolides,14 bronchodilators,14 and airway clearance.14,33-35 In addition, there is a clear need for further research concerning the treatment of symptoms in this population, particularly with quality of life as an outcome.
Conclusion
Across all GOLD stages, chronic bronchitis symptoms are associated with worse pulmonary symptoms and significant impairment in quality of life. For patients with chronic bronchitis, the presence or absence of bronchiectasis is not associated with a significant difference in SGRQ symptom scores. Symptoms of chronic bronchitis impose a heavy burden on patients and should be treated regardless of the presence or absence of underlying bronchiectasis.
Acknowledgments
The authors would like to acknowledge the assistance of the COPDGene data center.
Authors’ Contributions: Dr. McEvoy takes responsibility for the content of this manuscript including the data and analysis. Substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; has drafted the submitted article or revised it critically and substantially for important intellectual content; has provided final approval of the version to be published; has agreed to be accountable for all aspects of the work: OB, MX, SK, NW, GH, CM.Analysis and interpretation: OB, MX, SK, NW, GH, CM.Data acquisition: SK, NW.Drafting the manuscript for important intellectual content: OB, GH, CM.
Declaration of Interest
Gary Hansen is employed by RespirTech. Charlene McEvoy is a clinical advisor to RespirTech. No other authors have a conflict related to this manuscript.