Running Head: Polycythemia and Severe COPD Exacerbations
Funding support: SPIROMICS was supported by contracts from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), grants from the NIH/NHLBI (U01 HL137880 and U24 HL141762), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune, Bayer, Bellerophon Therapeutics, Boehringer-Ingelheim Pharmaceuticals, Inc., Chiesi Farmaceutici S.p.A., Forest Research Institute, Inc., GlaxoSmithKline, Grifols Therapeutics, Inc., Ikaria, Inc., Novartis Pharmaceuticals Corporation, Nycomed GmbH, ProterixBio, Regeneron Pharmaceuticals, Inc., Sanofi, Sunovion, Takeda Pharmaceutical Company, and Theravance Biopharma and Mylan. AF is supported by NIH/NHLBI K23HL151758
Date of acceptance: June 2, 2021 │ Published online: June 30, 2021
Abbreviations: chronic obstructive pulmonary disease, COPD; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; SubPopulations and InteRmediate Outcomes Measures In COPD Study, SPIROMICS; Hounsfield units, HU; acute exacerbation of COPD, AECOPD; long-term oxygen therapy, LTOT; complete blood counts, CBC; COPD Assessment Test, CAT; modified Medical Research Council, mMRC; 6-minute Walk Test, 6MWT; St George’s Respiratory Questionnaire, SGRQ; computed tomography, CT; total lung capacity, TLC; Hospital Anxiety and Depression Scale, HADS; standard deviation, SD; patient-reported outcomes, PROs; adjusted incidence rate ratio, aIRR
Citation: Fawzy A, Woo H, Balasubramanian A, et al. Polycythemia is associated with lower incidence of severe COPD exacerbations in the SPIROMICS study. Chronic Obstr Pulm Dis. 2021; 8(3): 326-335. doi: http://doi.org/10.15326/jcopdf.2021.0216
Introduction
While several studies have highlighted the association of anemia with adverse outcomes in chronic obstructive pulmonary disease (COPD),1-5 secondary polycythemia has also been recognized as a consequence of chronic pulmonary disease and hypoxemia for over a century.6,7 Polycythemia has been associated with lower mortality and fewer hospitalizations among individuals with COPD-prescribed long-term oxygen therapy (LTOT),8,9 while other studies found no association between polycythemia and respiratory outcomes.10,11 The implications of polycythemia in a more contemporary and representative sample of individuals with COPD is unclear. Furthermore, beyond hypoxemia, the phenotypic features of polycythemic individuals with COPD remains unexplored. This analysis of the well phenotyped, longitudinal SubPopulations and InteRmediate Outcome Measures In COPD Study (SPIROMICS) investigates the association of polycythemia with COPD severity, phenotypic features, and respiratory exacerbations.
Methods
Current and former smokers with COPD (FEV1/FVC<70%) without a history of hematologic/oncologic disorders were selected for this analysis from SPIROMICS.12 Baseline complete blood counts (CBC) were performed at certified laboratories at the respective sites. Polycythemia was defined as a hemoglobin concentration (Hgb)≥15.0g/dL for women or ≥17.0g/dL for men.9,10,13 Participants with anemia (Hgb<12.0g/dL for females, <13.0g/dL for males) were excluded since results have been reported previously.5
Baseline evaluations included percent predicted FEV1, the COPD Assessment Test (CAT) score,14 the modified Medical Research Council (mMRC) questionnaire score,15 the 6-minute walk test in meters (6MWT),16 and the St George’s Respiratory Questionnaire (SGRQ) score.17 Structured telephone questionnaires were conducted quarterly to ascertain acute exacerbation of COPD (AECOPD). AECOPDs were defined as respiratory symptoms treated with antibiotics or oral corticosteroids or requiring unscheduled health care utilization. Severe AECOPD included the subset requiring an emergency department visit or hospitalization. Whole lung, multi-detector row computed tomography (CT) scans were performed at baseline with breath hold at total lung capacity (TLC) and residual volume.18 Emphysema-like lung was calculated based on percentage of total lung voxels <-950 Hounsfield units(HU) at TLC, and its distribution was characterized on a whole lung and lobar basis.
Linear or logistic regression comparing participants with baseline polycythemia to those with normal Hgb was used for cross-sectional outcomes. The association of polycythemia with AECOPD was investigated using zero-inflated negative binomial models (follow-up time as offset, FEV1 as zero-inflation factor). Models were adjusted for age, sex, race, educational achievement, smoking status, altitude of clinical site (Colorado [5280 feet] or Utah [4226 feet] versus sea level [all other sites]), comorbidity count,19 platelet count,20 and body mass index. Models investigating AECOPD were additionally adjusted for percent predicted FEV1, and cross-sectional outcomes were performed with and without percent predicted FEV1 as it may mediate some of the outcomes. CT scanner model or self-reported AECOPD in the prior 12 months (yes/no) was added to models evaluating emphysema and AECOPD, respectively. Effect modification by sex, smoking status, oxygen use, and hypoxemia (oxygen saturation <88% or <92%) at rest or following the 6MWT (performed with supplemental oxygen for participants with usual use) were evaluated. All analyses were performed using SAS 9.4 (Carey, North Carolina). SPIROMICS was approved by institutional review boards at each center and participants provided written informed consent.
Results
Among 1845 SPIROMICS participants with COPD, 1829 had a CBC performed at baseline of whom 161 (8.8%) were excluded for anemia. Of the remaining 1668 participants, 33 (2%) did not accrue follow-up time, 91 (5.5%) were excluded for missing covariates, and 283 (17%) were excluded for self-reported history of hematologic/oncologic disorder. Among the 1261 eligible participants, 11.7% had polycythemia. Individuals with polycythemia were more likely female, current smokers, underweight, and living at elevation (Table 1). Notably 16.9% of individuals with polycythemia did not have any of the traditional risk factors for secondary polycythemia such as resting or ambulatory hypoxemia (<88%), supplemental oxygen use, living at elevation, or current smoking status. After adjusting for covariates, compared to normal Hgb, polycythemia was associated with significantly lower percent predicted FEV1, lower resting oxygen saturation, and increased upper to lower lobe ratio of emphysema (Table 2). A significant association between polycythemia and higher percentage of emphysema was completely attenuated with addition of percent predicted FEV1 to the multivariable model (Table 2). There was no association of polycythemia with patient-reported outcomes (PROs) or exercise tolerance (Table 2).
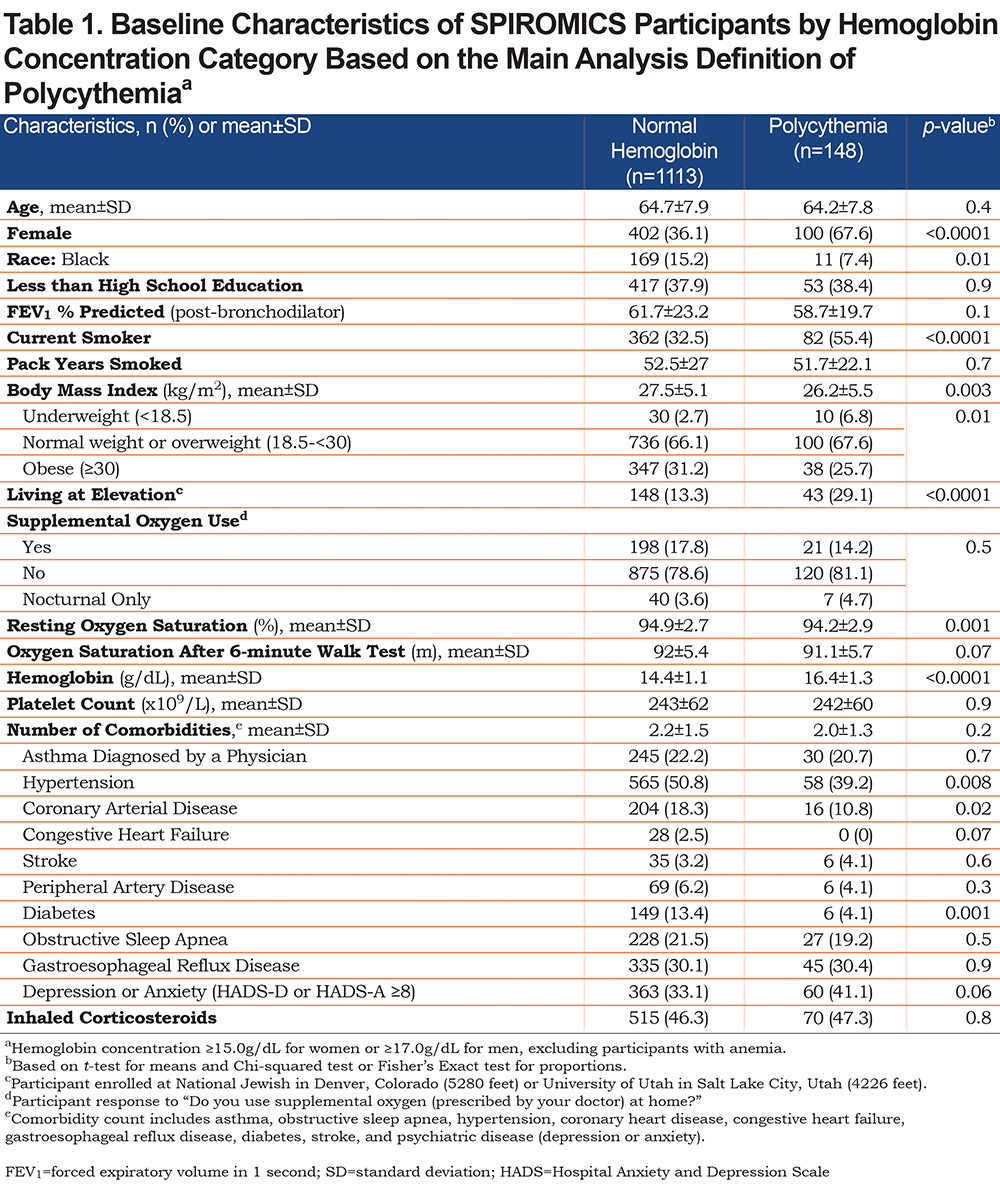
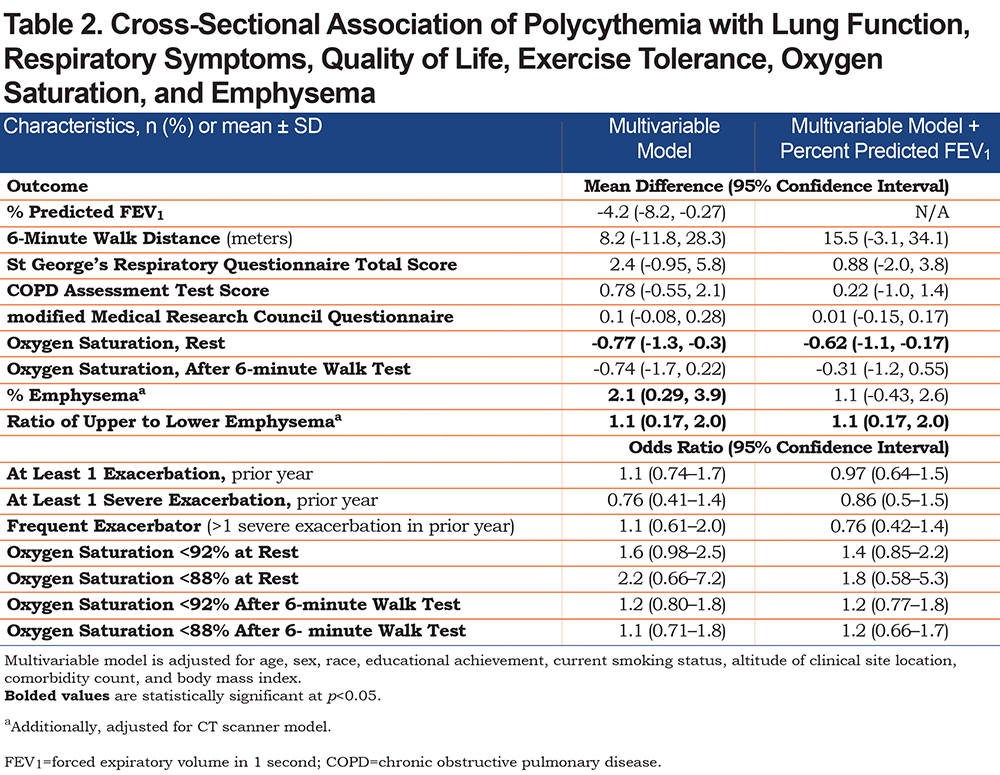
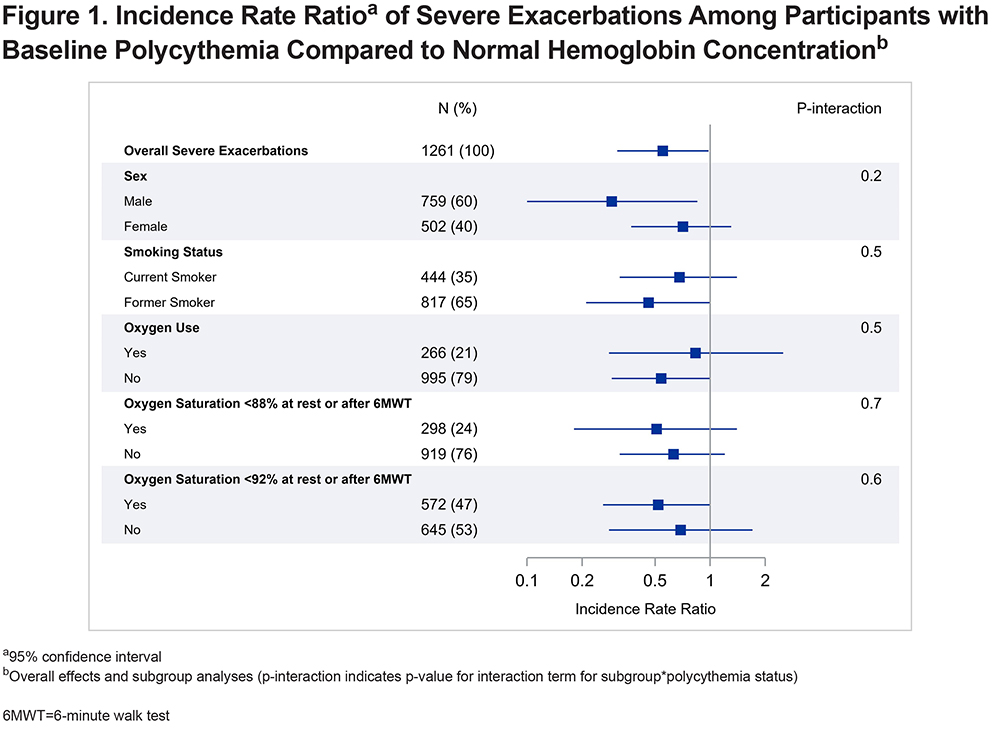
Participants were followed up for 4.2±1.7 years, which was similar across Hgb categories (p=0.2). Compared to normal Hgb, polycythemia was associated with a reduced rate of severe AECOPDs (adjusted incidence rate ratio [aIRR] 0.57, 95% CI: 0.33–0.98). The incidence rate of total AECOPD among participants with polycythemia was not significantly different than among participants with normal Hgb (aIRR 0.79, 95% CI: 0.55-1.1). There was no significant effect modification of the association between polycythemia and severe exacerbations by sex, smoking status, oxygen use, or hypoxemia (Figure 1).
Discussion
In a contemporary, heterogeneous, and well phenotyped sample of individuals with COPD, polycythemia was associated with a reduced rate of severe AECOPD but no difference in total AECOPDs. Despite an association of polycythemia with worse disease severity, increased emphysema, and a higher ratio of upper to lower lobe ratio of emphysema, there was no independent association between polycythemia and PROs or exercise tolerance. This adds to the recent study in the same cohort showing that individuals with COPD and anemia had more severe disease, worse exercise capacity, and greater dyspnea.5 These findings replicate similar results in individuals with advanced COPD prescribed LTOT without evidence of effect modification by presence of hypoxia or oxygen use.8,9 Past studies reporting no association between polycythemia and severe AECOPD had notable limitations including a dearth of female participants or low polycythemia prevalence.10,11 A historical study suggesting improved lung capacity and hemodynamic parameters related to venesection in a small group of individuals with emphysema and polycythemia included individuals with much higher average hematocrit levels than was observed in this modern cohort,21 while another study demonstrated no difference in the hemodynamic status or pulmonary function of individuals with emphysema and polycythemia after repeated phlebotomies that reduced hematocrit to normal levels.22 Furthermore, although polycythemia was associated with less favorable physiologic parameters such as worse lung function, more emphysema, and greater ratio of upper to lower lobe emphysema, which has been linked to worse quality of life and greater FEV1 decline,23,24 there was no independent association of polycythemia with more symptoms or worse quality of life.
The polycythemia prevalence of 11.7% in the SPIROMICS cohort is consistent with prior estimates of 5.9–18.1%, although definitions are inconsistent.8-10 Polycythemia was more prevalent among current smokers and individuals living at altitude and was associated with lower resting but not exertional oxygen saturation. However, not all individuals with polycythemia in this study had one of the traditional risk factors identified (living at elevation, oxygen use, smoking, or hypoxemia) implying that nearly one fifth of individuals with secondary polycythemia may have unrecognized risk factors including awake or nocturnal hypoxemia.25 Thus, presence of polycythemia without clearly identified risk factors should prompt additional investigation such as ambulatory or nocturnal oximetry which may identify clinically significant hypoxemia warranting intervention that could potentially improve quality of life, exercise tolerance, or avert cognitive impairment.26-28
There are limitations to this study. Changes in Hgb concentration during follow-up were not captured and history of kidney disease, which impacts erythropoiesis and Hgb, was not collected. Moderate AECOPDs represent a more heterogenous outcome and are prone to misclassification compared with severe exacerbations that result in an emergency department visit or hospitalization, which may have attenuated any association between polycythemia and total AECOPDs. Inferences regarding associations between hemoglobin and ambulatory oxygen saturation are limited due to variability in administration of the 6MWT and variable use of home oxygen with ambulation. Ultimately, these findings support previous literature describing improved outcomes among individuals with COPD and polycythemia. Future studies should explore the potentially protective role of increased Hgb beyond the correction of anemia.
Acknowledgements
Author Contributions: AF and NP contributed to the conception and design of the study and drafted the manuscript; IB, RGB, RPB, APC, CBC, DC, GJC, MTD, MKH, EAH, REK, JAK, FJM, RP, SP, PGW, and NNH contributed to the acquisition of the data. AF and HW analyzed the data; AF, NNH, and NP contributed to data interpretation; all authors contributed to revisions of the manuscript, reviewed, and approved the final manuscript. AB, MM, RW, and all other authors contributed to revisions of the manuscript and reviewed and approved the final manuscript.
The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible. More information about the study and how to access SPIROMICS data is available at www.spiromics.org. The authors would like to acknowledge the University of North Carolina at Chapel Hill BioSpecimen Processing Facility for sample processing, storage, and sample disbursements (http://bsp.web.unc.edu/).
We would like to acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E. Alexis, MD; Wayne H. Anderson, PhD; Mehrdad Arjomandi, MD; Igor Barjaktarevic, MD, PhD; R. Graham Barr, MD, DrPH; Patricia Basta, PhD; Lori A. Bateman, MSc; Surya P. Bhatt, MD; Eugene R. Bleecker, MD; Richard C. Boucher, MD; Russell P. Bowler, MD, PhD; Stephanie A. Christenson, MD; Alejandro P. Comellas, MD; Christopher B. Cooper, MD, PhD; David J. Couper, PhD; Gerard J. Criner, MD; Ronald G. Crystal, MD; Jeffrey L. Curtis, MD; Claire M. Doerschuk, MD; Mark T. Dransfield, MD; Brad Drummond, MD; Christine M. Freeman, PhD; Craig Galban, PhD; MeiLan K. Han, MD, MS; Nadia N. Hansel, MD, MPH; Annette T. Hastie, PhD; Eric A. Hoffman, PhD; Yvonne Huang, MD; Robert J. Kaner, MD; Richard E. Kanner, MD; Eric C. Kleerup, MD; Jerry A. Krishnan, MD, PhD; Lisa M. LaVange, PhD; Stephen C. Lazarus, MD; Fernando J. Martinez, MD, MS; Deborah A. Meyers, PhD; Wendy C. Moore, MD; John D. Newell Jr, MD; Robert Paine, III, MD; Laura Paulin, MD, MHS; Stephen P. Peters, MD, PhD; Cheryl Pirozzi, MD; Nirupama Putcha, MD, MHS; Elizabeth C. Oelsner, MD, MPH; Wanda K. O’Neal, PhD; Victor E. Ortega, MD, PhD; Sanjeev Raman, MBBS, MD; Stephen I. Rennard, MD; Donald P. Tashkin, MD; J. Michael Wells, MD; Robert A. Wise, MD; and Prescott G. Woodruff, MD, MPH. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Lisa Postow, PhD, and Lisa Viviano, BSN
Declaration of Interest
IB has received grants from Theravance, Mylan, Amgen and GE Healthcare and has consulted for Theravance, Mylan, AstraZeneca, GlaxoSmithKline, Grifols, Verona Pharma and GE Healthcare. RGB reports grants from the National Institutes of Health (NIH) and COPD Foundation pertaining to the current work. RW reports grants and personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, personal fees from Contrafect, grants and personal fees from GlaxoSmithKline, personal fees from Merck, grants and personal fees from Verona, personal fees from Mylan/Theravance, personal fees and non-financial support from Propeller Health, personal fees from Novartis, personal fees from Chmerix, personal fees from FSD Pharma, personal fees from AbbVie, personal fees from Bristol Myers Squibb, personal fees from Puretech, personal fees from Galderma, personal fees from Chiesi ,outside the submitted work. PGW has received grants from the NIH and the COPD Foundation to support the cohort on which this work is based and grants from Genentech and personal fees from Glenmark Pharmaceuticals, Theravance, WSAAI, Sanofi, Regeneron, GlaxoSmithKline, and NGM Pharma outside the submitted work. NP has received grant funding from NIH for the work under consideration. NP has received grant funding from NIH and personal fees from CSL Behring and Pharmacosmos not related to the current work. EAH is a founder and shareholder of VIDA Diagnostics, a company commercializing lung image analysis software developed, in part, at the University of Iowa. JAK received research funding from NIH/National Heart, Lung, and Blood Institute (NHLBI), Sergey Brin Family Foundation, and Patient-Centered Outcomes Research Institute (PCORI), and consulting fees for serving on an advisory board with GlaxoSmithKline about monoclonal antibodies to treat SARS-CoV-2 infection. FJM has participated in COPD advisory boards supported by AstraZeneca, Sanofi/Regeneron, Csl Behring, Gala, Sunovion, Teva, GlaxoSmithKline, Novartis, Polarean and Pulmonx. He has participated in COPD Study Steering Committees of work supported by AstraZeneca, Chiesi, GlaxoSmithKline, and Sanofi/Regeneron. He has presented disease state presentations for AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline. FJM also serves on adjudication committee for study sponsored by Medtronic. MCM has disclosed receiving royalties from UpToDate and consulting fees from Aridis outside of the scope of the published work. RP discloses grant support related to this work from the NHLBI and the COPD Foundation. He has received support for activity unrelated to the current work from the Department of Veterans Affairs (grant support) and Partner Therapeutics (consultancy). AC has received Grant money from the NIH. CBC reports grants from NIH/NHLBI, grants from Foundation for the NIH, grants from the COPD Foundation, during the conduct of the study; personal fees from MGC Diagnostics, NUVAIRA and GlaxoSmithKline, outside the submitted work; and is a consultant for GlaxoSmithKline, Astra Zeneca and VIDA. DC reports grants from NHLBI and the COPD Foundation related to the work in this manuscript. MTD reports grants from the NIH related to this work, grants from DOD and ALA unrelated to this work and personal fees from AstraZeneca, GlaxoSmithKline, Pulmonx and Teva unrelated to this work. MKH has received research support from Novartis and Sunovion. She has conducted industry sponsored studies with funds paid to the institution by Sanofi. She has consulted for GlaxoSmithKline, Boehringer Ingelheim, AZ, Cipla, Chiesi, Teva, Sanofi and Novartis. All other authors have nothing to disclose.