Running Head: Endothelial and Platelet Microparticles and COPD
Funding Support: National Institutes of Health T35 Federal Research Grant
Date of acceptance: May 26, 2021 │ Published online: June 1, 2021
Abbreviations: endothelial microparticles, eMPs; platelet microparticles, pMPs; chronic obstructive pulmonary disease, COPD; Body mass index-airflow Obstruction-Dyspnea-Exercise index, BODE; Global initiative for chronic Obstructive Lung Disease, GOLD; microparticles, MPs; platelet endothelial cell adhesion molecule, PECAM; Ulex europeus 1 lection, ULEX; diffusing capacity of the lungs for carbon monoxide, DLCO, 6-minute walk test, 6MWT; health-related quality of life, HRQoL; St George’s Respiratory Questionnaire, SGRQ; pulmonary function tests, PFTs; platelet rich plasma, PRP; platelet-poor plasma, PPP; fluorescein isothiocynate, FITC; monoclonal antibody, mAb; Multi-Ethnic Study of Atherosclerosis COPD study, MESA
Citation: Lascano J, Katz J, Cearras M, Campos M.Association of systemic endothelial-derived and platelet-derived microparticles with clinical outcomes in chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2021; 8(3): 382-395. doi: http://doi.org/10.15326/jcopdf.2021.0211
Introduction
Microparticles (MPs) are 0.1 to 1µm membrane-bound vesicles present in circulating blood shed from cells during activation, injury, or apoptosis.1,2 They encase and transfer molecules such as mRNAs, microRNAs, pro-inflammatory cytokines, and/or proteins and are thought to be important mediators of cell-to-cell communication.2,3 MPs contain an externalized phosphatidylserine at their surface and can be differentiated based on the presence of specific transmembrane proteins characteristic of their originating cells. For example, platelet-derived microparticles (pMPs) contain CD31 (platelet endothelial cell adhesion molecule [PECAM]), CD41 (the IIb moiety of glycoprotein IIb/IIIa) and CD62P (P-selectin) and endothelial-derived microparticles (eMPs) contain the Ulex europeus I lectin (ULEX), CD31, CD51 (Integrin α V), CD54 (ICAM-1) or CD62E (E-selectin).4-6 MPs that contain Annexin V bind to phosphatidylserine and reflect cellular apoptosis regardless of the originating cell.7,8
Furthermore, quantitative ratios of certain endothelial microparticles (i.e., CD 62E+/CD31+ hereby referenced as apoptotic ratio) have been shown to be a simple, yet robust way to distinguish processes that are activating (i.e., increasing MP shedding, increasing cellular interactions, etc.,) or apoptotic.9,10 Specifically, a decreased ratio corresponds to apoptosis while an increased ratio corresponds to activation. The role of each specific MP is still being defined, but in general they are thought to actively participate in endothelial homeostasis and regulation of coagulation activation.11
There is growing evidence that circulating MP levels correlate with clinical disease, in particular, prothrombotic states and conditions associated with endothelial dysfunction. Elevations of specific MPs have been described in atherosclerosis, acute coronary disease, cerebrovascular accidents, uncontrolled arterial hypertension, diabetes, the metabolic syndrome, end-stage renal disease, and pulmonary arterial hypertension.1,12-16 In this latter condition, elevations of MP 62E has also been linked to increased mortality.13
Chronic obstructive pulmonary disease (COPD) is one condition in which MP level analysis may offer clues into its complex, multi-system pathophysiologic mechanism, which has not been fully elucidated. It has been shown that noxious stimuli like cigarette smoke is associated with alveolar epithelial damage and apoptosis.17 In addition to the endothelium, platelet dysfunction may play a role in COPD. While recent data suggests that COPD Global initiative for chronic Obstructive Lung Disease (GOLD)18 stages 2–4 patients do not have a statistically significant difference in platelet counts compared to age/gender matched controls,19 other studies have shown that increased platelet counts are linked to mortality in this condition.20 There is substantial evidence showing COPD patients having many cardiovascular comorbidities,21 perhaps offering insight into some studies that have even shown a mortality benefit from anti-platelet therapy.22
Based on these observations, in this work we explore the potential role of eMPs and pMPs as prognostic biomarkers in COPD by correlating them with widely used clinical parameters of COPD including GOLD stage, diffusing capacity of the lungs for carbon monoxide, (DLCO), 6-minute walk test (6MWT), health-related quality of life (HRQoL) and lastly Body mass index-airflow Obstruction-Dyspnea-Exercise (BODE) index. We hypothesized that patients with clinically manifested COPD should have significantly different MP levels compared to controls and that MPs correlate with clinical markers of disease severity.
Methods
Study Population
This was a case-control study that also utilized paired comparisons. Prospective participant enrollment occurred on a convenience basis at the Miami Veterans Administration Medical Center. Participants were recruited from pulmonary clinics (stable COPD participants), emergency department and inpatient wards (acute exacerbations) and through local advertisements (non-COPD controls). COPD was defined as individuals with a history of smoking (defined as >10 pack years) with a forced expiratory volume in 1 second to forced vital capacity ratio < 0.70 after administration of a short-acting bronchodilator.23 Controls were participants with a history of smoking (either current or former) and preserved lung function. All participants provided written informed consent. The protocol and the informed consent process were reviewed and approved by the University of Miami and the Miami VA institutional review boards. All relevant ethical guidelines set forth by these institutions were strictly followed.
Protocol
Data collection included a baseline questionnaire (demographics, smoking and occupational history, medications, comorbidities) and a HRQoL via the St George’s Respiratory Questionnaire (SGRQ).24 For accuracy and to limit recall bias, additional information of medications and comorbid conditions were obtained from review of electronic medical records. All participants, including controls and COPD participants when stable, underwent complete pulmonary function tests (PFTs), including DLCO and a 6MWT. (BODE scores were calculated. COPD patients were considered stable if they were at their baseline state without signs of exacerbation. COPD participants recruited during an acute exacerbation, defined as hospitalization due to worsening of their respiratory symptoms and requiring escalation of respiratory therapy,25 were followed at least a month after discharge for reassessment. Exclusion criteria included patients with conditions known to increase independently eMP or pMP levels, such as the presence of an acute coronary syndrome, malignant hypertension, recent cardiopulmonary bypass, heparin-induced thrombocytopenia, antiphospholipid syndrome, thrombotic thrombocytopenic purpura, a transient ischemic attack or new cerebrovascular accident, or diagnosis of multiple sclerosis. In addition, patients with solid organ transplantation, neutropenia, known metastatic or hematologic malignancies, or the inability or unwillingness to perform the 6MWT or PFTs were excluded. Blood samples to measure MPs were drawn in each patient encounter. Specifically, a single draw for non-COPD controls, a single draw for stable COPD patients, and a single draw for acute exacerbators were completed. Thus, an individual patient received 2 blood draws and subsequent MP level analysis if they belonged in the exacerbator cohort (n=17). For stable patients, samples were obtained before any testing and at rest, to avoid changes that may be due to acute exertion.
Laboratory Methods
Blood samples were collected from peripheral venipuncture with a butterfly, in two 3.2% buffered Na citrate tubes (Becton Dickinson). Samples were kept at room temperature and were processed within 2–3 hours of collection at the Coulter Laboratory at the University of Miami, which specializes in MP measurements. The methods of eMP and pMP assay have been previously thoroughly described.5,6,9 In brief, samples from patients and controls were centrifuged at 160g for 10 min to obtain platelet-rich plasma (PRP). The PRP was then centrifuged for 8 minutes at 1000g (4000 RPM, Shelton microcentrifuge)26 to obtain platelet poor plasma (PPP). After this, a 1.0 um filter was used to ensure extraneous proteins and larger vesicles from plasma were removed. Then 50µL of the PPP was incubated with 4µL of each respective monoclonal antibody (mAb) for 20 minutes with gentle shaking using an orbital shaker at 120 rpm. The mAbs used include anti-CD31 (phycoerythrin-conjugated, Pharmingen), anti-CD41 (fluorescein isothiocynate [FITC] conjugated, Pharmingen), anti-CD54 (FITC conjugated, Pharmingen), anti-CD 62E (FITC conjugated, Pharmingen), anti-CD51 (FITC, Pharmingen), biotinylated-Ulex (Vector Laboratories), and anti-Annexin V (FITC conjugated, BD Biosciences). After this, 1ul of 0.2um filtered phosphate-buffered saline was added, allowing the sample to be ready for flow cytometry. For the Annexin V measurements, 5mM of CaCl2 was also present.
Because CD31 occurs on both eMPs and pMPs but CD41 occurs only on platelets, pMPs were defined as CD31+CD41+ events and eMPs as CD31+CD41- events. Events were counted by triggering on the red fluorescence signal of phycoerythrin, above background noise on the y-axis of the dot-plot, whereas the green signal of CD41 was on the x-axis (4-decade log scales, both x and y). Fluorescence minus 1 control was used for accurate gating purposes. Microparticles were analyzed on a Coulter EPICs XL (Beckman Coulter, Miami, Florida) flow cytometer medium flow rate with a 30-second stop time. The next task was to convert flow cytometer counts to the absolute value of eMPs per microliter of plasma. This was accomplished by using standard counting beads in which during a 30-second run time, 18µL of sample was actually aspirated on medium setting. Therefore, since 50µL of PPP was utilized per run, a conversion factor calculated was 1178 (F=[1.06ml/0.018ml] [1.0ml/0.05ml]).
Values are reported as counts/µL of plasma. CD31 occurs also on some leukocyte subsets, but as previously observed using PE-Cy5-labeled CD45 4 leukocyte MPs contribute only a very small fraction of total CD31+CD41-(<7%) in both controls and diseased participants and was therefore, neglected. To further validate this procedure, known amounts of pure pMPs and eMPs were prepared in vitro and mixed in various proportions to be then measured by this method; results were essentially the same as measuring the pure components individually. The eMPs 51, 54, and 62E are not present in platelets in a detectable amount and were measured separately without co-incubation with CD41 antibody. Also, ULEX, CD 62P, and Annexin V were measured in a similar fashion. In each case, the detection of particles was set to trigger by fluorescence signal greater than noise. Fluorescent particles were further separated on another histogram by size by forward light scatter. Particles smaller than 1.5µm from the prior histogram were then analyzed on a third histogram, with those results recorded as the eMP count/µl. Due to the very high counts, to make more accurate measurements, ULEX samples were diluted 1:20. The resulting counts were multiplied by 20 to reflect the count/µL.
Statistical Analysis
A liberal strategy was employed to identify possible associations between MP levels and markers of COPD. Specifically, this meant attempting to elucidate a link between MP concentration levels and apoptotic ratio and any and all of the following: COPD status, GOLD stage (classification based on airflow limitation severity),18 DLCO, HRQoL questionnaire, BODE score, and acute exacerbations. Control participants were assigned a GOLD stage of 0 for these purposes.
Unadjusted MP levels (and the apoptotic ratio, defined as eMP 62E divided by eMP 31) were considered, along with 2 adjusted levels. These adjusted levels reflected, for each participant, deviations from MP levels expected from conditions other than COPD, as determined by linear regression. One regression included as covariates age, gender, body mass index, smoking status, statin use, atherosclerosis or history of acute coronary syndrome, congestive heart failure, chronic kidney disease, connective tissue disease, dementia, diabetes with or without end organ damage, HIV infection, liver disease (mild, moderate, or severe), cancer with or without metastasis, peripheral artery disease, and peptic ulcer disease (Charlson score). The other regression included a pooled category of vascular disease and eliminated any covariates with fewer than 5 cases to dampen outlier effects.
Associations between adjusted and unadjusted MP levels and GOLD stage were tested for using standard analysis of variance, linear test of trend, and 2 contrast factors. This included comparing COPD patients to controls as well as COPD patients by stage compared to controls.
Associations between adjusted and unadjusted MP levels and the DLCO, SGRQ score, and the multidimensional BODE score were evaluated using the coefficient of determination (R2).
All analyses were performed with NCSS 2004 (NCSS, LLC, Kaysville, Utah). Linear regression was performed on the subgroup of Stage 4 patients to correlate BODE score with MP levels.
Results
Demographic Differences Between COPD Participants and Control Participants
MP levels were measured in 19 control participants and 58 stable COPD participants. As expected, participants with COPD were older, had a higher pack-years smoking history, and lower lung function parameters. Not unsurprisingly, COPD participants also presented with more comorbidities than their controls (i.e., higher Charlson score) and had a higher percentage of statin and oxygen use (Table 1).
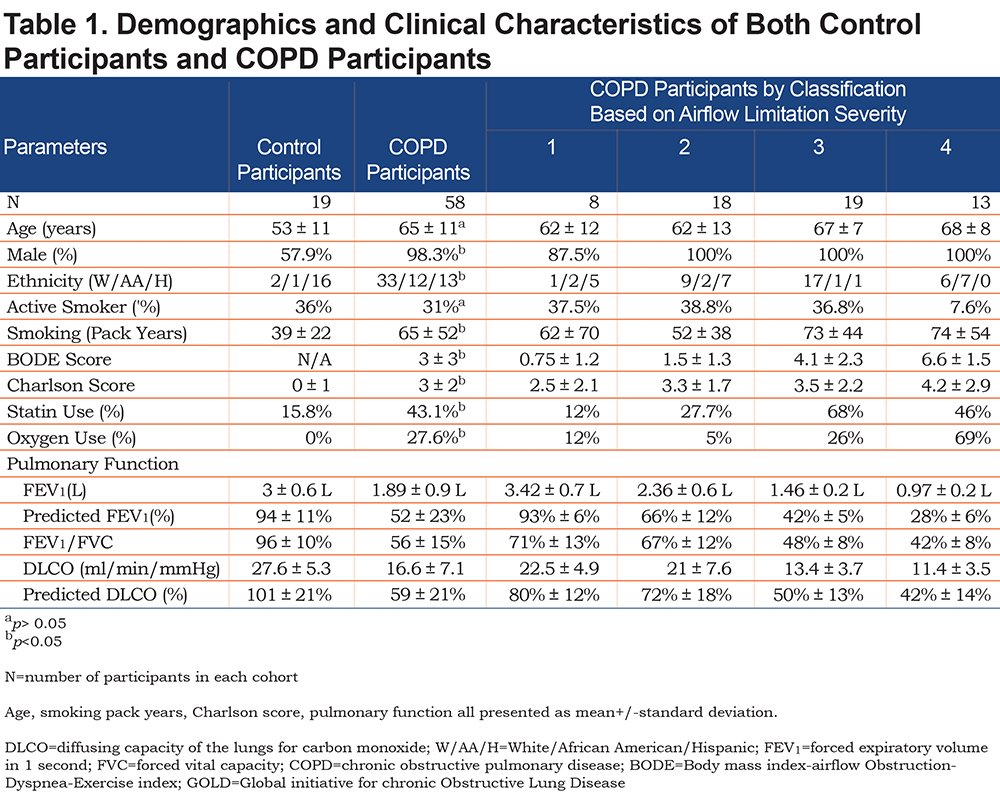
Microparticle Levels in Control Participants versus COPD Participants
The unadjusted values in control participants versus COPD participants show similar medians for all MP levels and the apoptotic ratio. All comparisons were not statistically significant even when adjusted for age, gender, BMI, smoking status, statin use, and comorbidities (Figure 1).
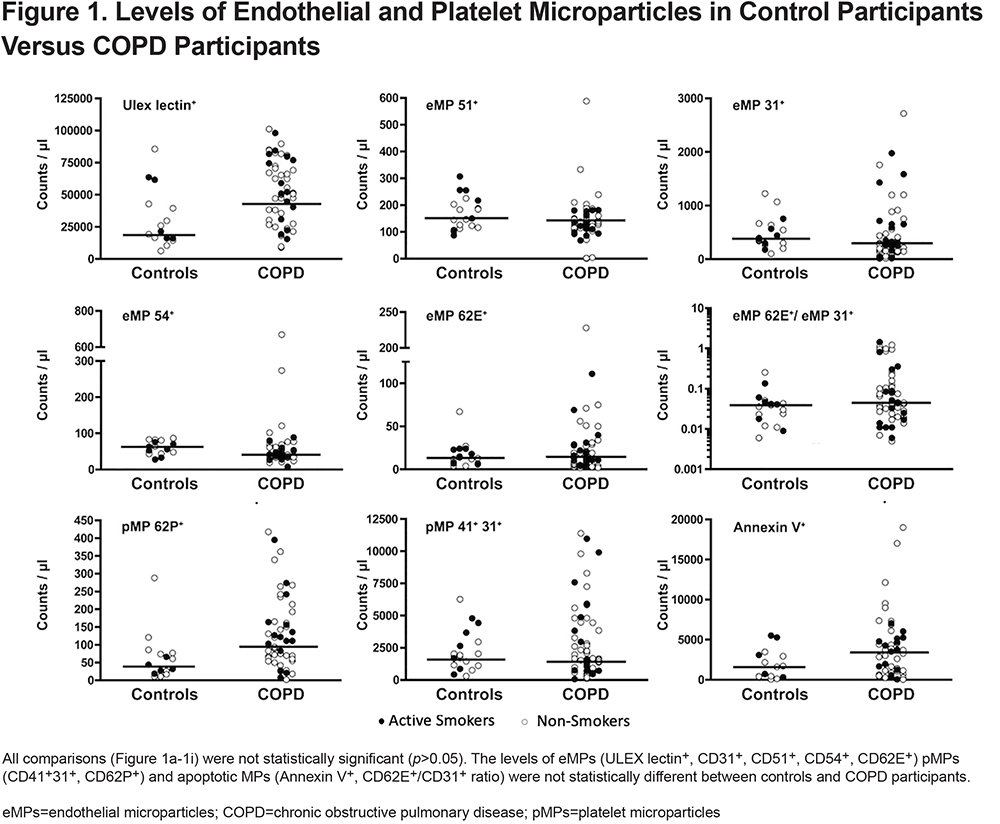
Microparticle Levels in Control Participants versus COPD Participants by GOLD Stage
MP levels in control participants versus COPD participants by GOLD stage show similar medians for most MP levels (Figure 2). However, there was a statistically significant increase in ULEX and pMP 62P+ among COPD stage 3 participants compared to controls (p=0.01, Figures 2a and 2c).
Interestingly when we compared the apoptosis level by eMP 62E+/eMP 31+ ratio we noticed that there was a statistically significant (p < 0.05) increase in apoptotic ratio among stage 2 and stage 3 COPD participants compared independently to either controls or stage 1 COPD participants (Figure 2e). This suggests that non-COPD and less severe COPD individuals may actually exhibit increased apoptosis compared to more severe COPD individuals.
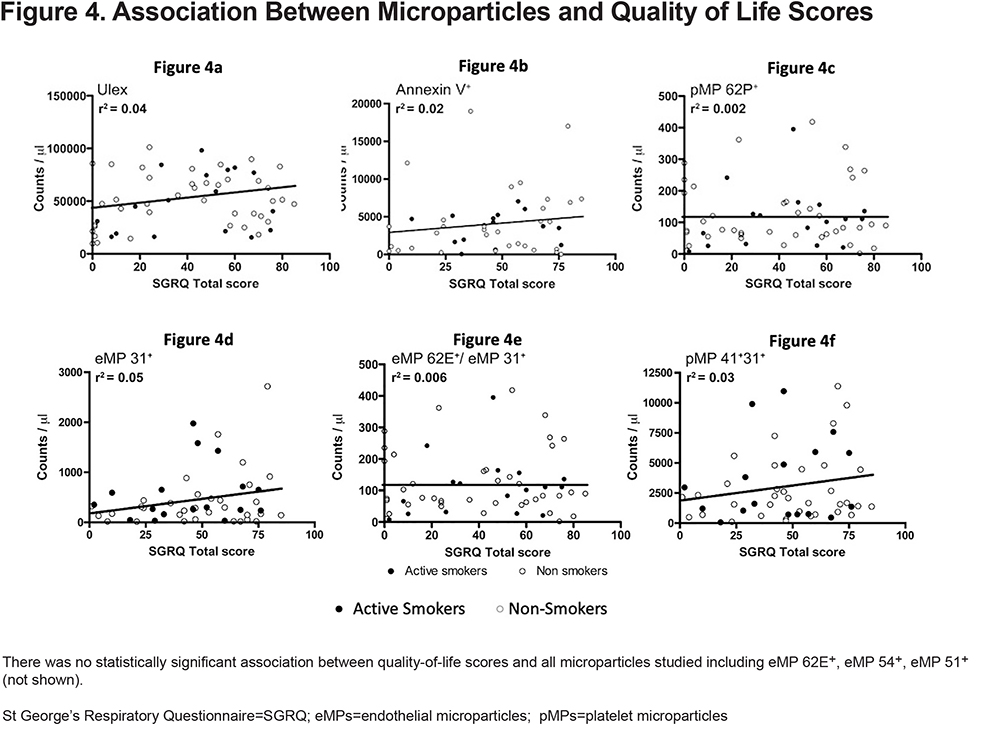
Microparticle Level Correlation with BODE, DLCO and Quality of Life Scores
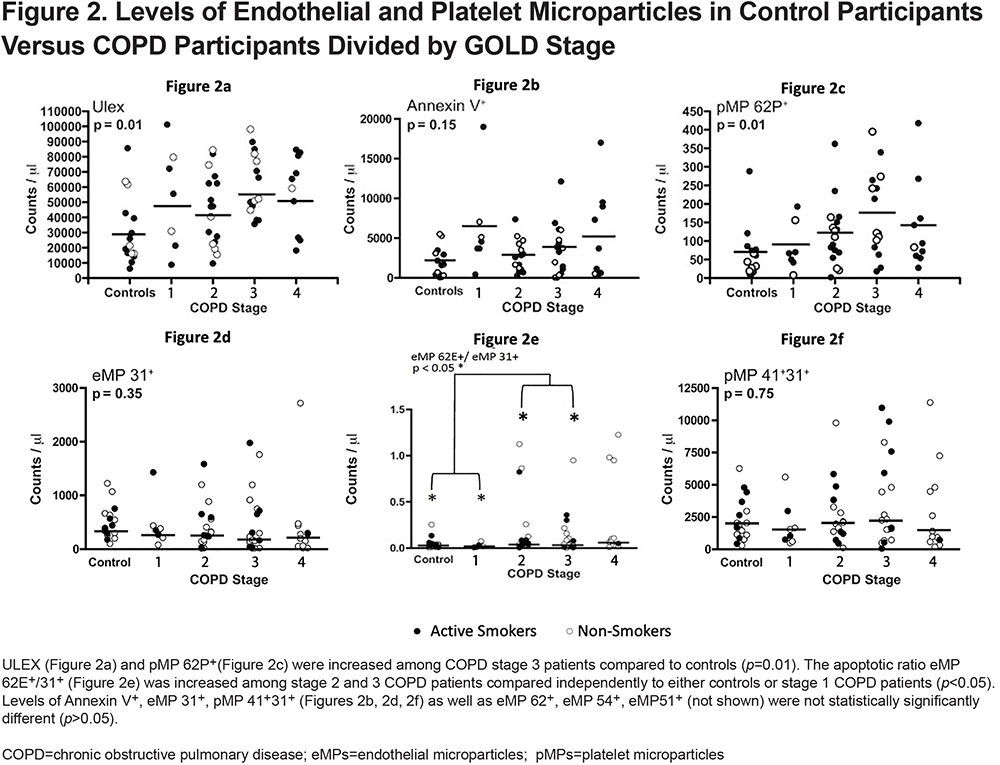
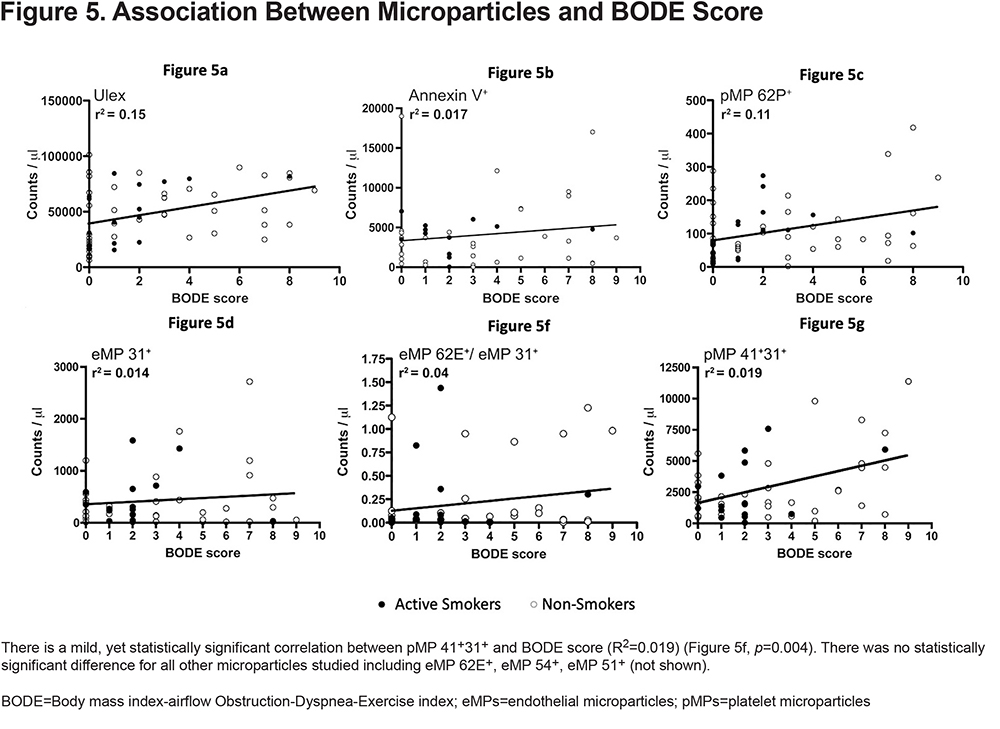
The association between MPs and BODE score, DLCO, and quality of life scores was evaluated by linear regression adjusting for important variables known to alter MP levels. We found no correlation between MPs and the percent predicted DLCO (Figure 3). We were also unable to find a significant correlation between MPs and quality of life scores (Figure 4). Finally, when we looked at that association between BODE score and MPs (Figure 5), the coefficient of determination (R2) for the association of pMP 41+31+ and the BODE score showed a mild, yet statistically significant correlation R2=0.019 (p=0.004) (Figure 5f).
Microparticle Levels in Acute Exacerbation Versus Stable COPD Participants
In 17 patients we obtained MP levels in both acute exacerbations and in the stable state (Figure 6). There was no difference in the apoptotic ratio as well as most MPs levels when paired comparisons were made. The only MP that had a statistically significant difference in concentration (decreased during acute exacerbation) was ULEX lectin (P=0.04). (Figure 6a)

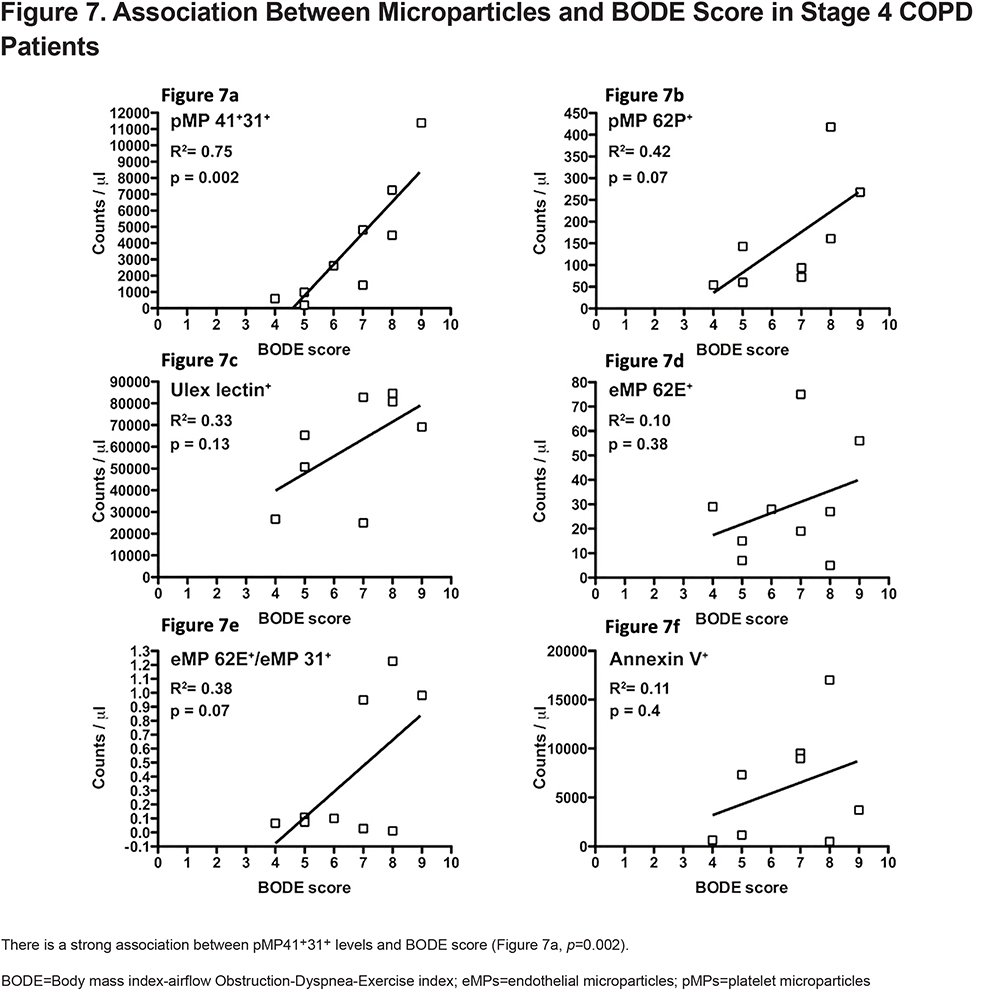
Microparticle Levels in Stage 4 Participants
In 9 patients with COPD GOLD Stage 4, eMPs and pMPs were correlated with severity by BODE score (Figure 7). There is a strong association between BODE score and the pMP levels. BODE score explains 75% of the variability seen in pMP 41+31+ levels (R2=0.75). (Figure 7a).
Discussion
Our study centered around measuring various endothelial and platelet-derived microparticles in relation to relevant COPD features and exacerbation status. This hypothesis was based on the literature that shows COPD is associated with increased endothelial apoptosis and dysfunction as well as platelet dysfunction/hypercoagulability. We did not find any difference in the level of MPs between control participants and COPD participants. These results were still apparent even after adjusting for confounding factors like smoking history, age, coronary artery disease, etc.
Previous studies have reported that COPD individuals have increased CD31+ (PECAM) and MPs compared to controls as well as to less severe COPD individuals.27,28 Takahashi et al (N=127) reported this same association with CD62E+ (E selectin), however, the larger Multi-Ethnic Study of Atherosclerosis COPD study (MESA) (N=180) was unable to show a statistically significant difference between CD62E+ or CD51+ MPs between COPD individuals and controls.27 Lastly, Takahashi et al reported that both CD31+ and CD62E+ MP were elevated during acute exacerbations, but we were unable to show this same conclusion. These discrepancies may be explained by population differences and variations in sample recollection and processing. Our study used a validated methodology processed in a laboratory with ample experience in these types of measures.4,12,29
ULEX is an MP of particular interest although few studies have incorporated its use in characterizing eMPs in COPD, possibly due to reports that the lectin ULEX is an unsuitable biomarker for endothelium for in vivo studies due to its lack of specificity.6 Nevertheless, previous publications support its tropism for human endothelium.30,31 Furthermore, mechanistic studies into the pathophysiology of COPD have used ULEX lectin-coated magnetic beads to isolate human pulmonary endothelium32 suggesting that it may in fact be useful for examining changes in COPD-related lung endothelium. Interestingly, ULEX+ lectin+ was the only MP elevated in COPD stage 3 patients compared to controls (Figure 2, p=0.01). We also clearly detected that ULEX lectin+ MP levels drop during acute COPD exacerbations with later clinical stabilization (Figure 6a, p=0.04). Given that certain microRNAs upregulated in COPD patients can inhibit proper endothelial tube formation and sprouting,32 it is possible that more advanced COPD-induced endothelial dysregulation results in less ULEX+ microparticles/epitopes being expressed in pulmonary vasculature and thus, an increase in COPD individuals’ plasma. During COPD exacerbations, acute-onset repair mechanisms may explain decreased ULEX+ lectin measured in patient plasma at this stage. Further research in this area could help recognize ULEX+ as a potential marker of COPD exacerbations.
We also did not find a clear association between MPs and either DLCO or HRQoL which matches previous studies. For example, the MESA group showed that while CD31+ eMP levels were inversely associated with DLCO, in their fully adjusted model, there was no association between either CD51+ or CD62E+ with diffusing capacity.27 This highlights 2 things: the inherent heterogeneity when it comes to measuring MPs as well as the fact that certain ones may be better indicators than others of structural lung dysfunction.
One unique analysis of our study was to assess the eMP 62E+/eMP 31+ apoptotic ratio in this population. A lower ratio means higher levels of endothelial apoptosis as mentioned before. We found that early-stage smokers (controls and stage 1 COPD) independently had increased apoptosis compared with more advanced COPD stages. These findings are in line with a previous report showing that smokers with normal spirometry and low DLCO have a lower eMP 62E+/eMP 31+ ratio in comparison with participants with normal spirometry, normal DLCO, nonsmokers.33 This is likely a reflection of ongoing endothelium damage at early stages of the disease when most participants do not have clinical evidence of disease. Better understanding of the apoptotic ratio might help early recognition of COPD as well as early interventions to prevent progression of the disease.
Furthermore, the MESA COPD Study27 found that CD31+ eMPs were elevated in both mild COPD and participants with emphysema compared to controls while CD62E+ eMPs, indicative of endothelial activation, were elevated only in severe COPD participants and participants with hyperinflation. While our group was unable to show this isolated increase in CD 62E+ eMPs among severe COPD participants, likely due to our smaller sample size, our results highlight that the use of the apoptotic ratio is a marker sensitive enough to reflect the apoptosis and activation process that occurs in COPD that should be further explored.
MP assessment in COPD may be of increased value in assessing the pathophysiologic processes that occur in late COPD. We found a minor, yet statistically significant association between pMP 41+/31+ and the BODE score (Figure 6, p=0.004, R2=0.019) overall, but this association became stronger and clearer when we look at our COPD stage 4 participants (Figure 7, p=0.002, R2=0.75). This may suggest increased platelet activation as the disease progresses. Several publications have demonstrated the association between thrombocytosis and platelets activation with increased 1-year mortality after COPD exacerbation and a protective role of antiplatelet therapy in patients with acute exacerbations.20,34 Our findings are intriguing because to our knowledge, the association between increased pMPs with BODE score within a single cohort of stage 4 COPD patients has never been described before, making a new potential connection between worsening outcomes and platelets activation.
Limitations
Some limitations of our study may explain some of our discrepant results compared to similar studies. The most important is probably sample collection and processing techniques. MP levels are not normally distributed and show great variability between patients. Furthermore, the inherent process of collecting serum and plasma specimens, preparing them for flow cytometry, and measuring MP levels offer several opportunities for variability.35,36 For example, it has been reported that simple agitation may further increase MP levels in serum samples35 and MP measurement techniques lack standardization, thus contributing to differences in recorded values.36,37 We collected our samples following a strict protocol and samples were processed in a laboratory with years of expertise in performing these techniques.5 The other important limitation is sample size, as our study involved a fewer number of participants compared to others,27,28 thus a lack of power may explain the negative results. Nevertheless, our study has a size comparable to other studies,32,38,39 and has highlighted how analysis of some MPs (such as the apoptotic ratio) can be readily assessed in smaller populations. Lastly, a significant portion of our study population was on statin therapy which, due to its anti-inflammatory role, may impact the formation, release, and number of MPs. Further studies are needed to quantify this impact.
Conclusion
We found that most MPs measured do not correlate significantly with COPD status, COPD severity, or exacerbations in our cohort. The apoptotic eMP 62E+/eMP 31+ ratio may be a useful marker of early endothelium apoptosis and early recognition of the disease process. Lastly, platelet activation assessed by pMP 41+31+ increases with disease severity and may be an important feature for stage 4 COPD patients.
Acknowledgments
Author contributions: JK was responsible for organizing, writing, and drafting the first draft of the manuscript as well as formatting figures, tables and statistical analyses. JL was responsible for coordinating the participant recruitment, performing clinical examinations, data collection/organization, and final editing of the manuscript. MC was responsible for study design, clinical examinations, participant recruitment, coordinating specimen laboratory measurement, and final editing of the manuscript. MC collected patient samples/data as well as statistical analyses, figure creation, and initial drafting of the manuscript. All authors have contributed to the intellectual content of the article and gave approval for publication.
Declaration of Interest
The authors report no conflicts of interest in this work.