Running Head: The Heterogeneity of COPD Patients
Funding Support: None.
Date of acceptance: June 28, 2021 │ Published online: July 2, 2021
Abbreviations: chronic obstructive pulmonary disease, COPD; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; Global initiative for chronic Obstructive Lung Disease, GOLD; Global Lung Function Initiative, GLI; lower limit of normal, LLN; COPD Genetic Epidemiology study, COPDGene; pulmonary function test, PFT; idiopathic pulmonary fibrosis, IPF; interstitial lung disease, ILD; alpha-1-antitrypsin, A1AT; computed tomography, CT; standard deviation, SD; preserved ratio-impaired spirometry, PRISm; Austrian Burden of Obstructive Lung Disease, BOLD; alpha-1-antitrypsin deficiency, AATD
Citation: Alabi FO, Alkhateeb HA, DeBarros KM, et al. The heterogeneity of COPD patients in a community-based practice and the inadequacy of the Global Initiative for Chronic Obstructive Lung Disease criteria: a real-world experience. Chronic Obstr Pulm Dis. 2021; 8(3): 396-407. doi: http://doi.org/10.15326/jcopdf.2021.0229
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous, chronic, inflammatory disease of the airways that is often associated with limited airflow and deterioration of the alveoli and local vasculature.1 It is estimated that COPD affects 384 million people worldwide, which corresponds to 11.6% of the global population, and there are about 16 million cases within the United States.2 It is currently the third leading cause of death globally with over 3 million reported deaths in 2019, which accounts for approximately 6% of total deaths worldwide.
The current standard for diagnosing COPD requires an airflow limitation that is not fully reversible. The Global initiative for chronic Obstructive Lung Disease (GOLD)3 defines this as a post-bronchodilator forced expiratory volume in 1 second (FEV1) to a forced vital capacity (FVC) ratio of < 0.70. This is consistent with the American Thoracic Society/ European Respiratory Society classifications.4 GOLD further provides guidelines for classifying COPD severity based on the degree of post-bronchodilator FEV1 impairment, with stages ranging from GOLD 1–4.
The predictive value of FEV1 is questionable. Several studies have demonstrated that FEV1 impairment is a good tool for predicting disease progression and correlates with symptoms, exacerbations, and mortality.5 Others have found little correlation between FEV1 impairment and exacerbations. Han et al analyzed data from 4484 patients with COPD using the GOLD A-D classification system. Over the span of 3 years, there was no difference in the number or severity of exacerbations when comparing COPD patients in group C (composed of patients with a severe reduction of FEV1<50, low symptoms, and a history of >2 exacerbation episodes) to group B (composed of patients with a mild to moderate reduction of FEV1>50, high symptoms, and a history of 1 or fewer exacerbation episodes).6 In group D, which had the highest exacerbation number and severity, the history of exacerbation episodes was a greater predictor for future exacerbations than FEV1.6
When evaluating the burden of COPD, estimates are often based on spirometry-defined COPD data. As such, the COPD global prevalence of 11.7% is likely a gross under-representation of the true burden of COPD.1 Even GOLD guidelines admit that defining COPD by spirometry alone leads to under-recognition and under-diagnosis of the disease.3
Despite the many guidelines that are available for diagnosing COPD, there are still patients who fall through the cracks and cannot be classified using any strict guidelines. These patients are not included in most COPD studies and are not equally recruited for almost all phase 3 drug trials that are performed on COPD patients. Although some of our COPD patients do not meet the strict criteria for diagnosing COPD, the guidelines are unclear regarding how to approach this population in clinical practice. We were particularly curious to learn and describe the percentage and characteristics of the patients who did not meet the guidelines’ definition of COPD compared to those who met the GOLD definition of COPD in a real-world practice.
Objectives
This study aims to describe and better understand the heterogeneity of real-world patients diagnosed with COPD in a community-based practice and evaluate the diagnostic sensitivity of the GOLD criteria for diagnosing COPD.
Methods
We performed a retrospective analysis of all established patients with a diagnosis of COPD at Florida Lung, Asthma & Sleep Specialists between January 2015 and December 2018. As a minimum of 12 months of follow-ups were required for inclusion in the study, patients who had been newly diagnosed with COPD after January 2018 were excluded from the analysis.
Study Participants
The eligible patients were men and women aged ≥40 years with physician-diagnosed COPD who required therapy for ≥12 months. The inclusion criteria were as follows:
- must have had a diagnosis of COPD for at least a year,
- must have a COPD Assessment Test (CAT) score ≥10,
- must be on treatment for COPD,
- smoking history must be documented in their charts,
- never smokers must have had significant secondhand exposure to tobacco or exposure to an environmental noxious substance or biomass fuels,
- must have a valid pulmonary function test (PFT) at the time of diagnosis.
The demographic variables that were used for analysis included age, sex, and race. In addition, comorbidity history related to the respiratory system was abstracted from their charts.
Data Collection
The following data was collected from their charts:
Smoking history:
- Current, former, or never smoker
Comorbidities:
- History of asthma
- History of idiopathic pulmonary fibrosis (IPF)
- History of interstitial lung disease (ILD)/pulmonary fibrosis
Laboratory data:
- Alpha-1 antitrypsin (A1AT) level
- A1AT genotype (MM, ZZ, MS, MZ)
Chest computed tomography (CT) scan data:
- Presence of emphysema
- Presence of bronchiectasis
- Findings of pulmonary fibrosis/ILD/IPF on CT scan of the chest
PFT data:
- Percentage of predicted FEV1
- FEV1/FVC ratio lower limit of normal (LLN)
- Pre-bronchodilator FEV1/FVC ratio
- Post-bronchodilator FEV1/FVC ratio
- Post-bronchodilator FEV1≥12%
- Post-bronchodilator FVC≥12%
- GOLD criteria for COPD based on the fixed ratio of FEV1/FVC<70%
- Global Lung Function Initiative (GLI) criteria for COPD based on the FEV1/FVC ratio <LLN.
Statistical Analysis
The data was analyzed using SPSS version 24 software (IBM SPSS) and formulated into figures and tables. The data were given as the mean (standard deviation [SD]) if it were normally distributed or the median (interquartile range) if it was nonparametric.
Ethical Considerations
This study was approved by the Western Institutional Review Board-Copernicus Group, and the need for consent was waived due to the retrospective nature of the study. There is no more than minimal risk to the participants of this study.
Results
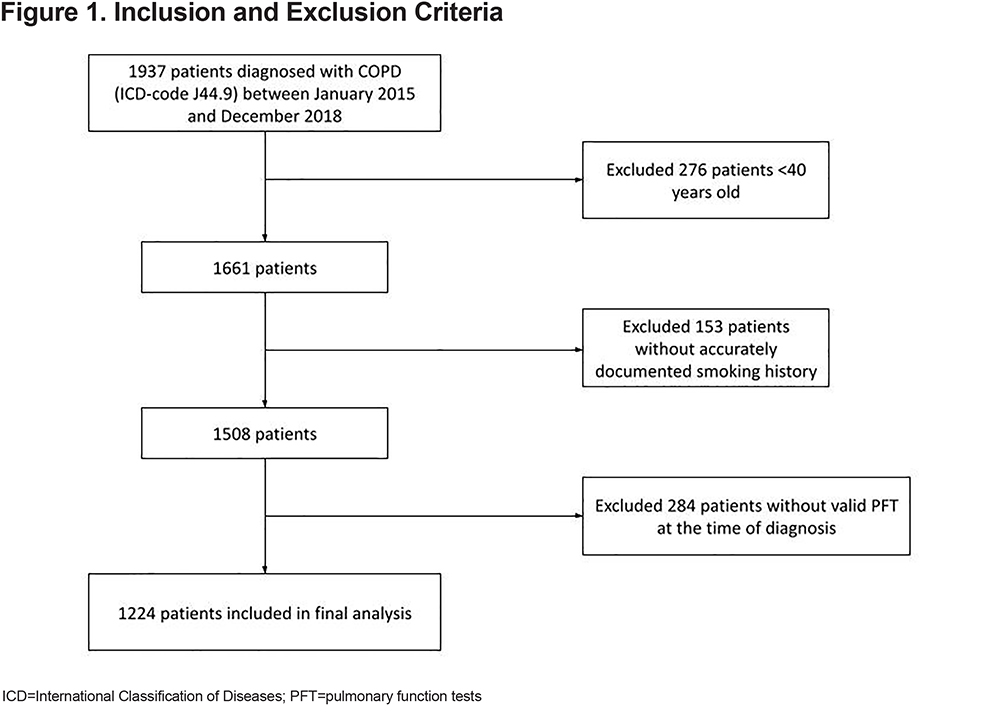
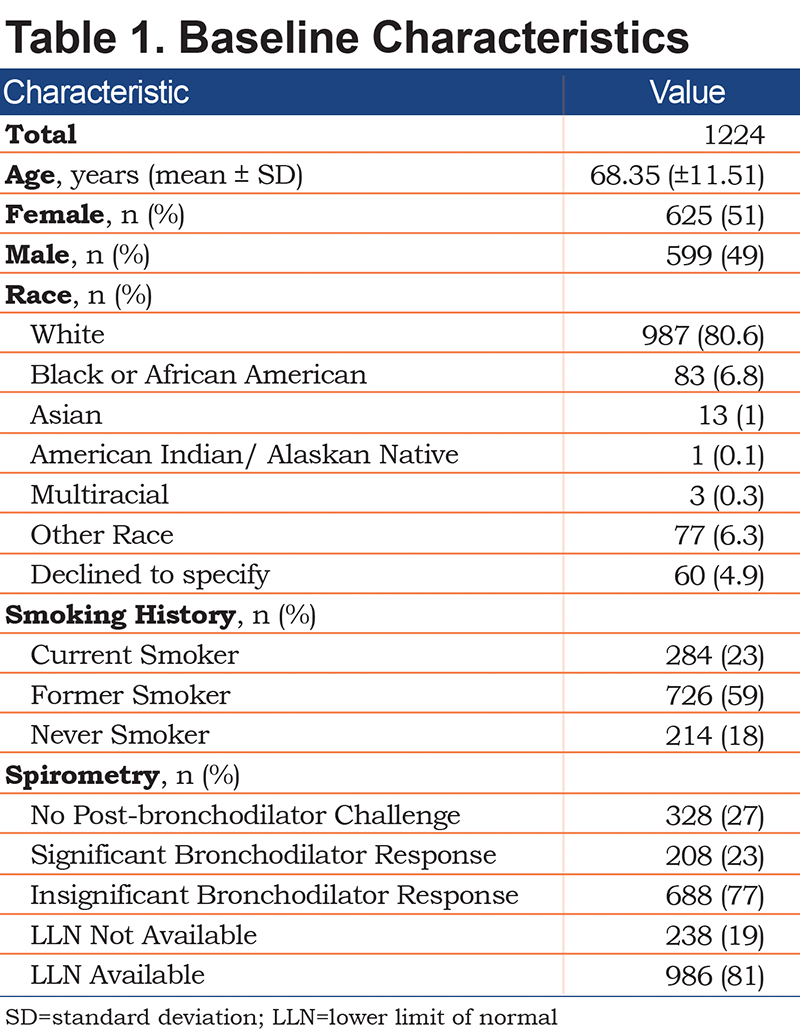
Between January 1, 2015 and December 31, 2018, 1224 of the 1937 patients screened met the inclusion criteria (Figure 1). The demographic data of the 1224 patients with COPD are presented in Table 1. Of the patients, 51% were female, the average age was 68.3±11.5 years, and 81% of the population were White. Additionally, 18% were never smokers, and 23% were still actively smoking. Table 1 shows that the spirometric measurements did not include LLN and post-bronchodilator testing in 27% and 19% patients, respectively. Approximately 23% of the patients had COPD in combination with asthma, and about 1% of the patients had a history of IPF (Table 2).
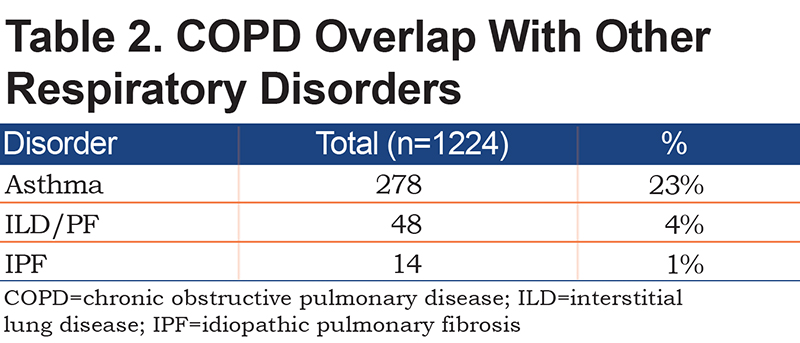
Chest CT scans were available in 841 patients (Table 3). Radiographic evidence of bronchiectasis was present in 15% of the cases, and 8% had evidence of interstitial lung disease (IPF/nonspecific interstitial pneumonia). Among the patients who had the chest CT scans, 51% had radiographic evidence of emphysema (Table 4). Additionally, 21% of the patients with emphysema on the CT scan of the chest had normal spirometry, among which 12% had a history of asthma. Among the 49% of patients without emphysema on the CT chest scan, 21% had normal spirometry.
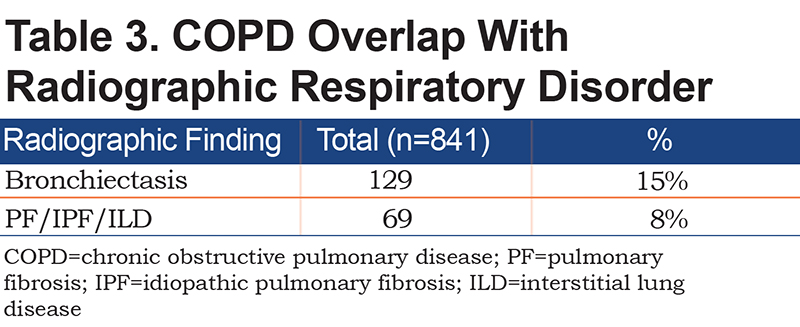
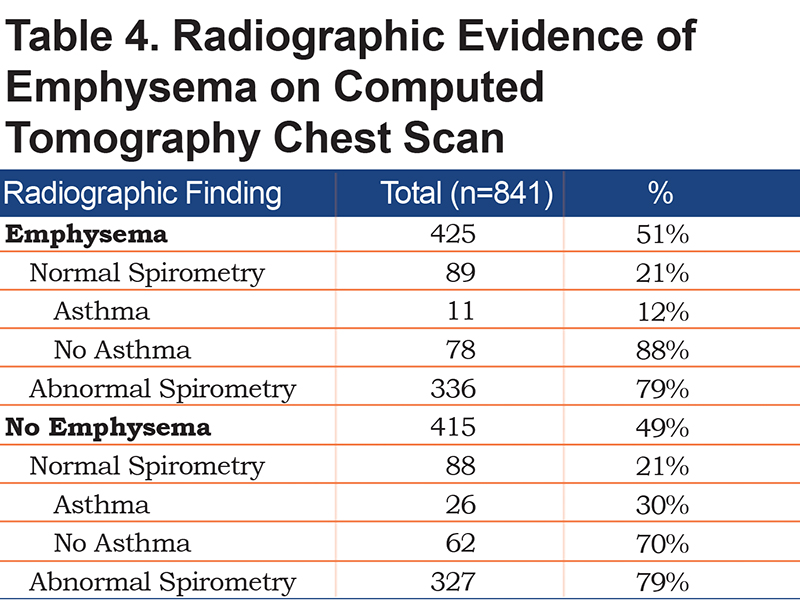
Figure 2 shows the distribution of COPD based on various criteria. A COPD diagnosis based on the GOLD criteria with a fixed FEV1/FVC ratio of <70% was seen in 43% of the patients. Moreover, 26% of the patients met the LLN criteria by the GLI, 21% had normal spirometry, and 36% qualified as preserved ratio-impaired spirometry (PRISm). A1AT genotype testing was available for 153 patients, and the antigen levels were present in 31 patients (Table 5). Of the patients, 88% had the MM genotype, 8% had the MS genotype, and 3% had the MZ genotype. Additionally, 30 of the 31 patients had an A1AT level above 57mg/dL by nephelometry.
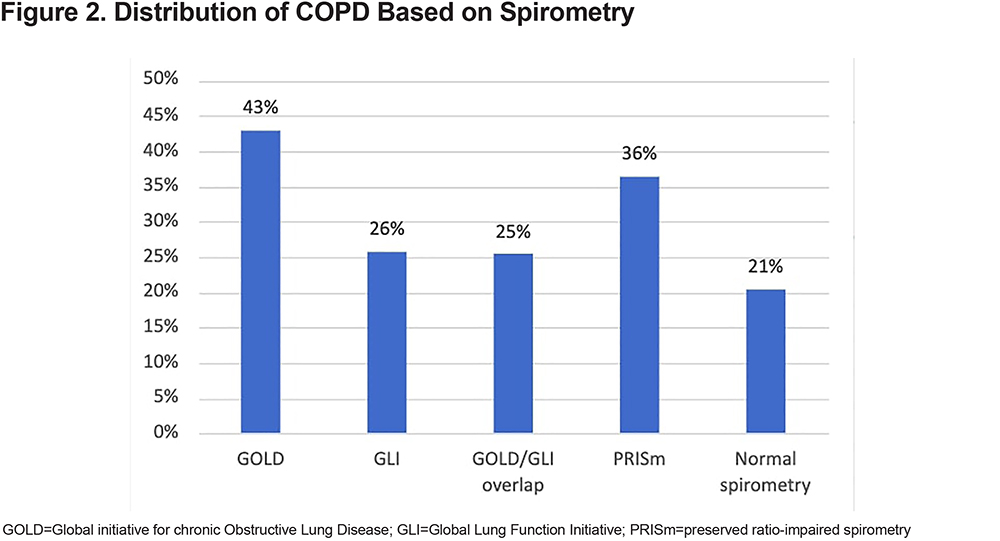
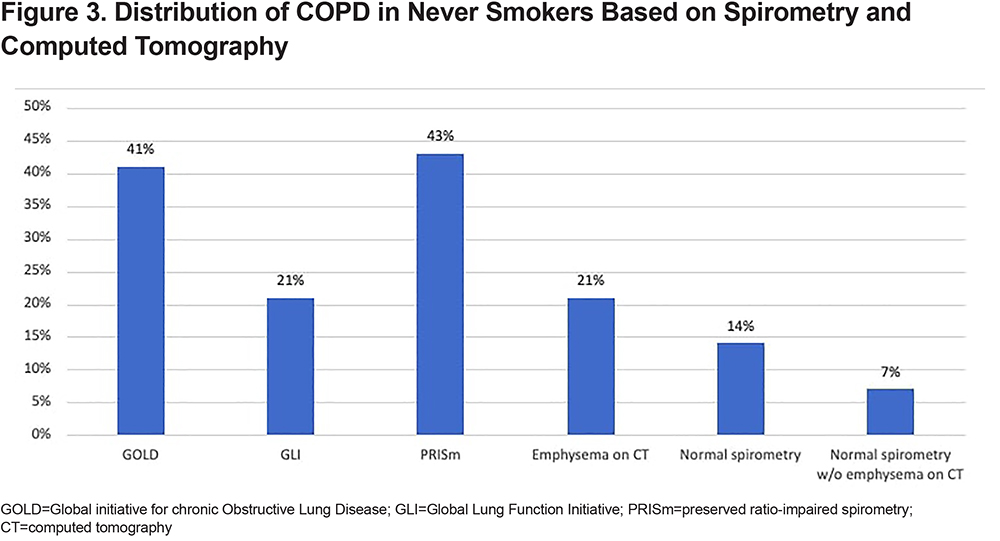
Among the never smokers with COPD, 41% met the GOLD criteria for COPD diagnosis, and 21% and 43% met the GLI and PRISm criteria, respectively. Figure 3 shows the distribution of COPD in never smokers based on spirometry and chest CT scan. In the never smoker COPD group, 14% had normal spirometry and 21% had emphysema on their chest CT scans. Additionally, 7% of the never smoker COPD group showed normal spirometry and an absence of emphysema on their chest CT scans.
The percentage of patients with COPD based on GOLD was the same, irrespective of their smoking status, as 43% of the current smokers met the GOLD criteria, whereas 41% of the never smokers met the same criteria. Spirometry showing PRISm was mostly seen in never smokers compared to former or current smokers. Emphysematous changes on the chest CT scans were mostly seen in current smokers (Figure 4).
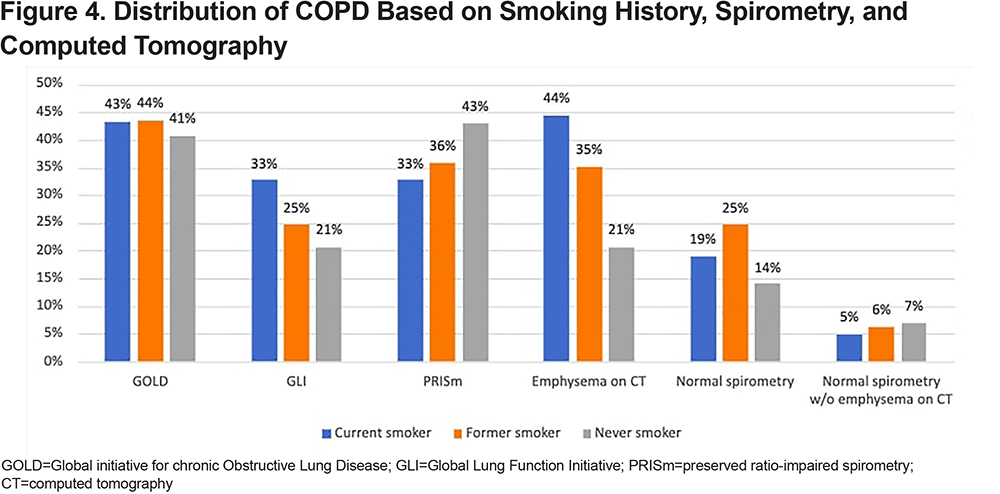
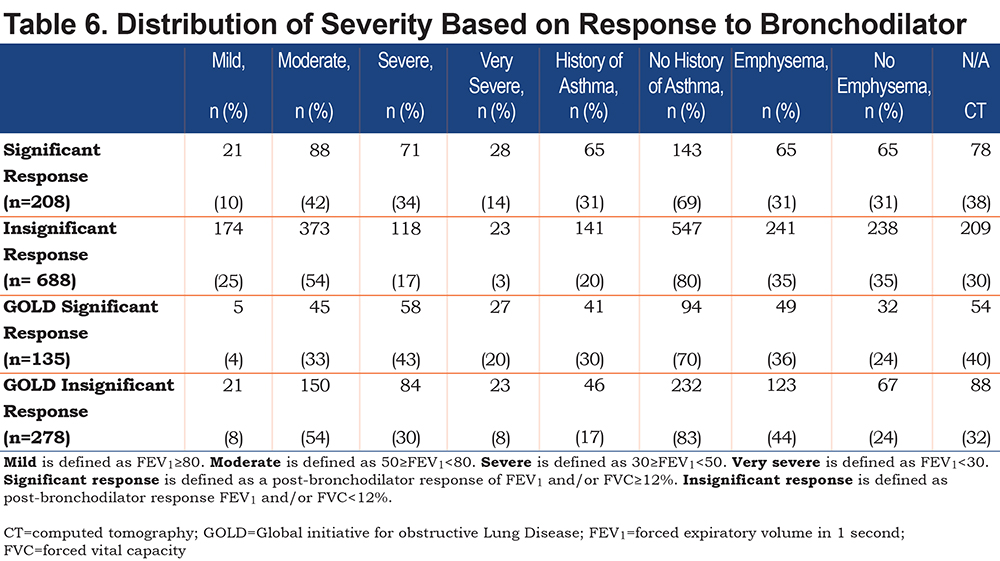
Table 6 shows the distribution of the severity of COPD based on the response to bronchodilator testing. Approximately 73% of the 1224 patients with COPD in this study had spirometry with bronchodilator testing, 23% had a positive response to the bronchodilator testing with a change of ≥12% and >200ml in FVC and/or FEV1, and 77% of the 896 patients with bronchodilator testing did not have a significant response (Table 1). Moderate COPD, defined as 50%≥FEV1<80%, was the most common severity classification in both patients who responded to bronchodilators (42%) and those who did not (54%). Mild COPD, defined as FEV1>80%, was more common in non-responders (25%) than in responders (10%), while very severe COPD, defined as FEV1<30%, was more common in responders (14%) than in non-responders (3%). Among the 413 patients who met the GOLD criteria for COPD diagnosis, 33% had a positive response to bronchodilator testing, and 67% did not have a significant response. Moderate severity was the most common among the patients with COPD based on GOLD who did not respond to bronchodilator testing. Among the responders, the severe severity stage was the most common at 43%. The percentage of patients who showed asthma and emphysema on the chest CT scans was the same for the bronchodilator responders and non-responders.
Discussion
COPD is a heterogenous, chronic, progressive, and debilitating disease, and the prevalence has continued to steadily increase over the last 50 years. It is now the third-ranked cause of death globally after cardiovascular disease and strokes.7 Despite this alarming statistic, it remains poorly understood by the general public and grossly underdiagnosed by health care providers. The difficulty in diagnosing COPD stems from many factors, including restrictive diagnostic criteria, reliance on smoking history for the diagnosis, failure to report/ask about symptoms, a scientific society recommendation to only perform spirometry on symptomatic smokers, the lack of reliable COPD screening tests, and the unpredictable natural course of this disease.
GOLD has been instrumental in the advancement of COPD pathogenesis and treatment.3 Since the initial report in 2001,8 the approach to COPD management has been constantly updated and refined. The paradigm shift in the management of COPD with more emphasis on the ABCD classification scheme (symptoms and exacerbation risk) and less emphasis on the spirometric data was the focus of the GOLD 2019 report. In 2019, GOLD’s board of directors stated that the “world should take COPD very seriously,” which was meant to stress the seriousness of this disease, the attributed mortality, and the burgeoning economic implications with a paucity of research funding compared to cardiovascular diseases and cancer.9 Despite the increasing prevalence and the laudable GOLD contributions in the areas of pathogenesis and management, research on enabling a timely and accurate diagnosis is lagging. The GOLD reports clearly stated and acknowledged the inadequacy of the GOLD criteria, which is based on a fixed ratio of FEV1/FVC<70%, for the diagnosis of COPD in some patients. Despite the acknowledgement of this limitation and the fact that it is well known to most practicing providers that many patients with COPD do not fit into the strict GOLD criteria, there is currently no recommendation by any scientific society on how to handle these cases in clinical practice.
Emerging data suggest that airway limitation should not be a strict requirement for COPD diagnosis. In a study of 2736 patients, respiratory symptoms were present in 50% of current or former smokers with a preserved lung function (post-bronchodilator FEV1/FVC≥0.70 and FVC>LLN). Symptomatic current and former smokers with a preserved lung function had a higher exacerbation rate than asymptomatic current and former smokers with mild-to-moderate COPD. Additionally, they had increased radiographic evidence of airway-wall thickening without emphysema.10
The renewed interest to redefine the diagnosis of COPD has been charged by the COPD Genetic Epidemiology (COPDGene®) group, who are leading the way in this area. They have proposed using the following 4 disease characteristics to diagnose COPD: exposure history (defined as tobacco smoking); symptoms (defined as dyspnea and/or chronic bronchitis); CT abnormality (defined as emphysema, gas trapping, and/or airway wall thickening); and abnormal spirometry.11Their definition of abnormal spirometry includes traditional GOLD patients with an impaired ratio <0.70, as well as patients with PRISm (low FEV1<80% with normal FEV1/FVC≥0.70). 12 Using these 4 disease characteristics, they grouped patients into 3 COPD categories: Possible COPD for patients with presence of 2 characteristics, Probable COPD for patients with presence of 3 characteristics and Definite COPD for patients with presence of 4 characteristics. The GOLD criteria would have diagnosed 46% of the 8784 participants of the COPDGene study with COPD, whereas under the new proposed criteria, 82% of the 8784 participants would be diagnosed with either possible, probable, or definite COPD.11
Our study found that among the 1224 symptomatic patients with physician-diagnosed COPD, 43% met the GOLD criteria for COPD diagnosis. Additionally, 57% of the COPD patients in this study did not meet the GOLD criteria of FEV1/FVC ratio <70%, and they experienced symptoms with a CAT score ≥10 that warranted being placed on medications for COPD. Our finding was similar to that of Woodruff et al10 who found that respiratory symptoms were present in 50% of current or former smokers with preserved spirometry. In another COPDGene study that was published in 2015, the GOLD 0 group had a reduced quality of life, as evidenced by their St George’s Respiratory Questionnaire scores and reduced 6-minute walk tests compared to never smokers. Moreover, 54% of the patients in the GOLD 0 group also experienced respiratory-related impairments compared to never smokers, and 42.3% had CT evidence of airway-wall thickening or emphysema.13 Our study population differed slightly from the COPDGene study because current or previous smoking history was not required for inclusion in our study. Using the proposed COPDGene definition of COPD,11 about 40% of individuals who would have been missed by the GOLD criteria progressed over 5 years with similar rates of mortality as those with an FEV1/FVC < 70%.11
The COPDGene 2019 definition for COPD is an improvement over the GOLD criteria. It will improve and enhance our ability to diagnose COPD in a timely fashion, thereby providing the unique opportunity for early intervention, which may result in halting or slowing down disease progression and possibly reducing mortality.14 The COPDGene 2019 definition was derived primarily from a database of heavy smokers with a >45-pack-year history, which may reduce the overall sensitivity of the definition in real-life practice or in areas where most COPD patients are never smokers. COPDGene did not include a never smoker group; all never smokers were excluded from the study. The only exposure history that was accounted for in the new proposed definition was smoking, which reduces the generalization of the diagnostic criteria in areas where smoking is not as relevant in the pathogenesis of COPD.
There is some controversy regarding the use of a fixed ratio for diagnosis, with some arguing that the LLN of FEV1/FVC is a better diagnostic criterion. A fixed ratio can over-diagnose older patients over the age of 65 and under-diagnose younger patients under the age of 45 compared to using the LLN.15 However, despite this discrepancy, the LLN is not superior to the fixed ratio when evaluating COPD-related hospitalizations and mortality.16 In this study, the GLI criteria with the LLN was less sensitive when identifying COPD compared to the GOLD criteria. GLI criteria identified 26% of COPD compared to 43% by the GOLD criteria.
About 50% of patients who show PRISm on spirometry are predicted to develop frank airflow limitation.17 Additionally, 36% of our patients with symptomatic COPD had PRISm documented on their spirometry. Studies have shown that the patients with predominant airway disease progress through the PRISm (low FEV1 with normal FEV1/FVC) phase into GOLD stages 2–4, whereas patients with an emphysematous-predominant pathway primarily progress through the GOLD stage 1 into GOLD stages 2–4.12 The prevalence of PRISm in COPDGene was 12.3%. These patients had increased dyspnea and a reduced 6-minute walk distance compared to the controls.18,19 The Rotterdam study showed that PRISm is associated with increased mortality and a heightened cardiovascular burden. About 15% of the patients in this study transitioned to normal spirometry, whereby 35% remained in PRISm.17 In the Rotterdam study, all patients with PRISm were current smokers, whereas in our study, 18% of the patients had no smoking history.
Although tobacco smoking is considered the leading cause of COPD, it is not the only way to develop it. While the prevalence of COPD is higher in smokers and former smokers compared to never smokers, an estimated one-fourth of adults with COPD have never smoked.20 They can exhibit similar symptoms and impairment to their quality of life compared to smokers.21 Factors associated with an increased risk of COPD in nonsmokers include poverty, a low education level, older age, body mass index changes, occupational exposure to dust, exposure to biomass for cooking or heating, history of tuberculosis, childhood respiratory illness, or a family history of COPD.21-24
The GOLD report has exposure history as one of the requirements for the diagnosis COPD, in addition to symptoms and spirometric criteria. Although smoking remains the most important risk factor for COPD in the western world, this leaves many COPD patients undiagnosed because their risk factors are unrelated to smoking.25 The Austrian Burden of Obstructive Lung Disease (BOLD) study found that 18% of airway diseases among never smokers are irreversible.21 Additionally, 18% of the patients in our study were never smokers, although half of them had experienced second-hand exposure to tobacco. The findings in the BOLD study were similar to ours since the prevalence of COPD in nonsmokers22 was 23%. The risk factors for COPD in never smokers include an advancing age, asthma, and female gender with lower education levels.21 Additional risk factors for COPD in never smokers include early life disadvantages, such as childhood asthma, childhood respiratory infections, and maternal smoking, which have all been associated with reduced lung function in adults. The impact of childhood disadvantages could be as significant as the disadvantages of heavy smoking.26 A childhood history of asthma is a strong risk factor for COPD; among children who developed severe asthma by the age of ten, 44% will meet the diagnosis of COPD according to the GOLD criteria by the age of 50, independent of their smoking status. Moreover, 17% of children with mild to moderate asthma will reach GOLD criteria stage 1 COPD.27
Some of our never smoking patients were exposed to biomass fuels, and others to known occupational or environmental fumes. Among the never smokers, 21% had evidence of emphysema on the chest CT scans, and some demonstrated either a fixed obstructive airway disease or abnormal diffusion capacity. As only the presence of emphysema on the chest CT scans was abstracted in our study, the percentage of patients with evidence of COPD on chest CT scans was likely to have been underestimated. The COPDGene study used the quantitative chest CT, which is not routinely performed when assessing COPD patients, and evaluated for the presence of small airway involvement.28
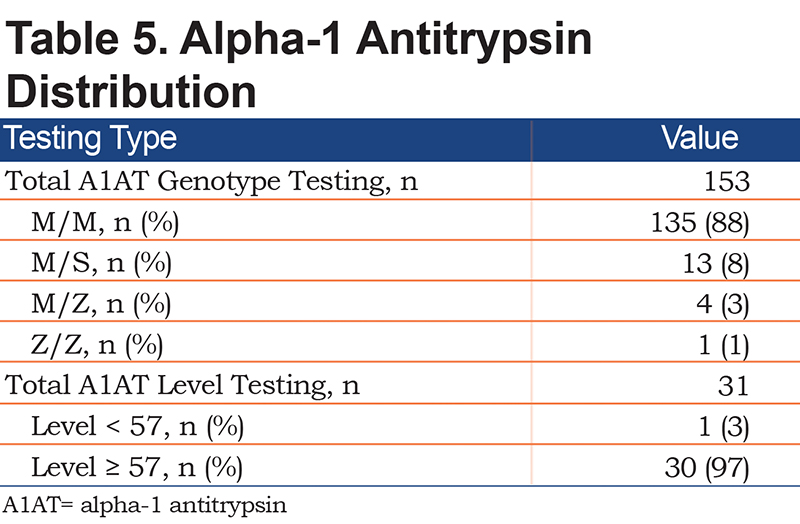
Another risk factor for COPD is alpha-1-antitrypsin deficiency (AATD), which is a genetic disorder that results in varying levels of serum A1AT. There is an established inverse relationship between serum concentrations of A1AT and the risk of developing COPD. The ZZ genotype presents the lowest serum concentration, followed by SZ, SS, MZ, MS, and MM, respectively. About 5% of people with COPD are believed to have AATD; however, this prevalence is likely an overestimate given that most of the data used to evaluate AATD prevalence in COPD patients is provided by referral centers and specialty clinics, which increases the likelihood of referral bias.29 The true prevalence of severe AATD is much lower, and it has been reported as being as low as 0.15% to 1.9% in several studies.30 Moreover, 88% of the 135 COPD patients in our study had the A1AT genotype MM, 11% had heterozygous genotype MZ, with homozygous ZZ found in 1% of COPD patients. Heterozygosity of the Z variant has been reported to be the major cause of intermediate AATD (mostly MZ), which accounted for 8%, and ZZ homozygosity was detected in 1.9% of the patients.31
This study has many limitations due to it being retrospective in nature. The patients were included in the study based on physician-diagnosed COPD, which may cause the results to be erroneous. However, we reduced the probability of including non-COPD patients in the study by requiring that never smokers have at least 1 significant environmental or occupational exposure to noxious stimuli. The findings of emphysema in our study may be underestimated due to a lack of independent review of chest CT scans. Moreover, we were only able to abstract the presence of emphysema on the chest CT scans, which underestimated the percentage of COPD patients with other abnormal radiographic findings such as airway wall thickening or air trapping. The smoking history was not quantitated by the patients’ pack-year history, which could have led to falsely attributing the cause of some of the patients’ COPD to tobacco smoking. Exacerbation history was not collected and reported in this study since this information was not uniformly obtained by the providers in the practice The COPD diagnostic criteria based on the GOLD criteria is too restrictive, and too many patients are left undiagnosed or receive a delayed diagnosis. The COPDGene group has proposed redefining diagnostic criteria through an integrated approach using environmental exposure, clinical symptoms, CT imaging, and spirometric criteria.11 This renewed definition is welcome and overdue. Our study, which was performed at a community-based practice, shed some light on the inadequacy of the GOLD criteria and augmented the COPDGene group’s conclusion that it is time to redefine the diagnostic criteria for COPD. We also hope to reemphasize the importance of considering other exposure history besides smoking as a risk factor for developing COPD especially in regions with a diverse and heterogeneous population.
Acknowledgements
Author contributions: FOA and HAA were responsible for conception and design of the study. HAA, KMD, PSBB, RLS, MKZ, and RAI were responsible for data acquisition. FOA and HAA provided analysis of the data, interpretation of the results, and drafted the initial manuscript. FOA, FU, and NM were involved in the editing and revisions of the manuscript. All authors significantly contributed content of the article and gave final approval of the version to be published.
Declaration of Interest
The authors have no known conflicts of interest to disclose.