Running Head: Losartan Trial for Emphysema
Funding Support: Supported by grants from the National Heart, Lung, and Blood Institute U01HL128951 and the American Lung Association.
Date of acceptance: July 6, 2021. Published online: August 2, 2021
Abbreviations: Losartan Effects on Emphysema, LEEP; chronic obstructive pulmonary disease, COPD; high-resolution computed tomography, HRCT; Hounsfield units, HU; angiotensin converting enzyme, ACE; angiotensin receptor blockers, ARBs; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; total lung capacity, TLC; residual volume, RV; transforming growth factor beta, TBFb; computed tomography, CT; National Institutes of Health, NIH; National Heart, Lung, and Blood Institute, NHLBI; American Lung Association, ALA; Pulmonary Trials Cooperative, PTC; protocol leadership groups, PLGs; network management core, NEMO; Airways Clinical Research Centers, ARC; data and safety monitoring board, DSMB; post-bronchodilator, BD; percentage of lung voxels with a density less than -950 HU, pct950; modified Medical Research Council, mMRC; Patient-Reported Outcomes Measurement Information System Questionnaire: Physical Function Short Form 20a, PROMIS-20a; pulse oximetry oxygen saturation, SpO2; Food and Drug Administration, FDA; coronavirus disease 2019, COVID-19
Citation: American Lung Association Airways Clinical Research Centers. Wise R, Holbrook JT, Brown RH, et al. Losartan effects on emphysema progression randomized clinical trial: rationale, design, recruitment, and retention. Chronic Obstr Pulm Dis. 2021; 8(4): 414-426. doi: http://doi.org/10.15326/jcopdf.2021.0210
Online Supplemental Material: Read Online Supplemental Material (186KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease that is associated with increasing morbidity and mortality in the United States.1 Current treatments for COPD consist mainly of inhaled bronchodilators and anti-inflammatory agents that are used for treatment of respiratory symptoms and prevention of exacerbations. Although smoking cessation slows the progression of the disease,2,3 there are no pharmacologic agents that clearly modify disease progression.
Angiotensin receptor blockers (ARBs) are potential agents to modify the progression of COPD. In a retrospective database study, Mancini found that ARB-treated COPD patients had reduced mortality and COPD hospitalization regardless of cardiovascular risk factors.4,5 Andreas and colleagues6 tested whether 4 months of irbesartan treatment would increase skeletal muscle strength in 30 patients with COPD. Although there was no effect on peripheral or respiratory muscle strength, the ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) increased (p=0.07), and total lung capacity (TLC) (p< 0.001) and residual volume (RV) (p<0.08) were decreased in the irbesartan group compared to placebo.6
There is evidence that these changes reflect direct biological effects of ARBs on the lung beyond the anti-hypertensive effect for which they are clinically indicated. Angiotensin 1 receptors are expressed in lung tissue and are involved in apoptosis of alveolar epithelial cells7,8 which is theorized to be an essential component of emphysema progression.9 There is compelling evidence that the lung remodeling effects of angiotensin 1 receptor activation is mediated by transforming growth factor beta (TGFb) signaling. Fibrillin-deficient mice exhibit emphysema, which is caused by excessive signaling by TGFb, which can be prevented or reversed by the angiotensin receptor blocker losartan.10,11 Murine models have shown that losartan is effective in preventing the progression of emphysema. Podowski and colleagues found that cigarette-smoke exposed mice develop TGFb-mediated emphysema that can be inhibited or reversed by losartan.12 A pilot placebo-controlled proof of concept study in COPD patients showed that losartan was well-tolerated and was associated with lack of progression of emphysema in the subgroup of patients who had computed tomography (CT) evidence of emphysema at baseline (Clinical Trials.gov, NCT00720226).13
Therefore, the Losartan Effects on Emphysema Progression (LEEP) trial was initiated to definitively test the hypothesis that losartan would slow progression of emphysema in COPD patients who exhibited radiographic evidence of emphysema. The purpose of this manuscript is to describe the LEEP trial protocol and initial experience with recruitment of participants.
Methods
Study Organization
The LEEP trial was jointly sponsored by the National Institutes of Health’s National Heart, Lung, and Blood Institute (NIH-NHLBI) and the American Lung Association (ALA) through the Pulmonary Trials Cooperative (PTC) clinical trial structure. The PTC is a consortium organized by the NIH-NHLBI that comprises protocol leadership groups (PLGs) and a network management core (NEMO).14 The PLGs were responsible for the design and data coordination for individual participating trials and the NEMO was responsible for oversight and coordination of trial sites. Specifically, the NEMO performed certification of study sites and personnel, distributed drug and capitation payments, and managed the electronic submission of HRCT scans.
The LEEP trial (NCT02696564) was conducted by the PLG at Johns Hopkins Bloomberg School of Public Health in coordination with the NEMO at the University of Pittsburgh. LEEP study sites were members of the American Lung Association Airways Clinical Research Centers (ALA-ACRC) network; the PLG is also the data coordinating center for the ACRC network. LEEP was monitored by an independent data and safety monitoring board (DSMB) that reports to the NIH-NHLBI.
In addition to LEEP, the PTC also conducted 2 randomized trials evaluating interventions for COPD: “Intervention Study In Overweight Patients With COPD” (NCT02634268)15 and “Redefining Therapy In Early COPD for the Pulmonary Trials Cooperative” (NCT02867761),16 and one that evaluated antimicrobials for interstitial pulmonary fibrosis, “Clinical Efficacy of Antimicrobial Therapy Strategy Using Pragmatic Design in Idiopathic Pulmonary Fibrosis” (NCT02759120).17
Trial Design
LEEP was a blinded, parallel, placebo-controlled trial comparing losartan 100 mg/day to placebo (p=0.08) (Figure 1 ). The primary outcome was the rate of emphysema progression as measured by the difference in quantitative high-resolution computed tomography (HRCT) measurements of emphysema.
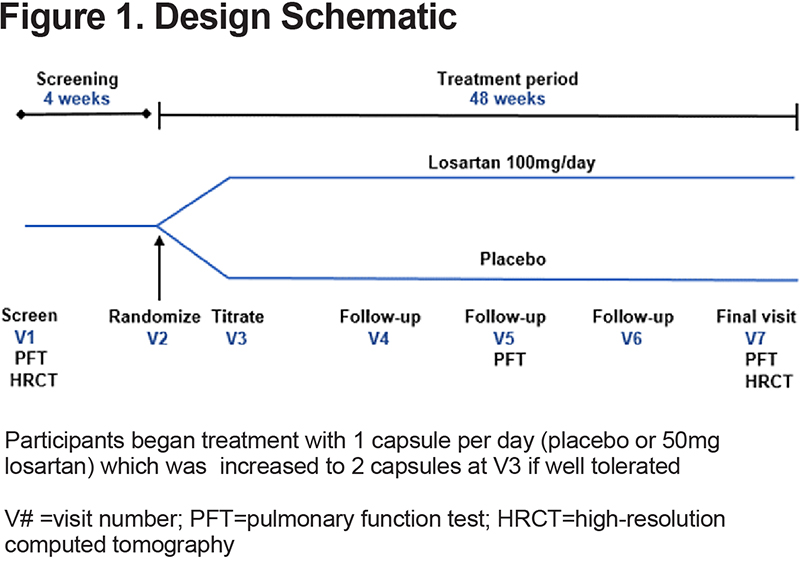
Participants
Men and women diagnosed with mild to very severe airway obstruction (post-bronchodilator [BD] FEV1/FVC ratio ≤ 0.70 and FEV1 20%–80% predicted),18 age 40 years or older, with a history of 10 pack years or greater of cigarette smoking and HRCT scan with emphysema score of 5%-35% of voxels with density <-950 Hounsfield units (HU) were eligible.
Exclusion criteria (eTable 1 in the online supplement) included current use of angiotensin converting enzyme (ACE) or angiotensin receptor blocker medication or indications for their use (heart failure, diabetic nephropathy, or recent myocardial infarction), hypotension or untreated hypertension, and treatment with potassium sparing diuretics, potassium supplementation, or elevated serum potassium levels. Participants were also excluded if adequate HRCT scans could not be obtained (e.g., prior lung resection surgery, evidence of other lung disease, and presence of pacemakers or other metallic objects in the chest). There were 7 study visits over a 52-week period (eTable 2 in the online supplement).
Study Treatment
After eligibility was established, individuals were randomly assigned by computer to receive either losartan or placebo on a 1:1 allocation basis. The PLG prepared randomization assignments in permuted blocks, stratified by study site. Uniquely numbered bottles of masked study drug were distributed to participants. Verification of correct dispensing was ascertained by retention of a tear-off label and data entry of the dispensed bottle number.
Treatment was titrated with participants beginning on 50 milligrams (mg)/day (1 capsule of over-encapsulated losartan) or one capsule of placebo for the first two weeks. If 1 capsule was well tolerated and systolic blood pressure was >90 mm Hg and the diastolic blood pressure was >60 mm Hg, the dose was increased to 100 mg/day (2 capsules) or 2 capsules of placebo for 48 weeks, which is the maximum recommended dose for treatment of hypertension.
Primary Outcome
The extent of emphysema was quantified as the percentage of lung voxels with a density less than -950 HU (pct950) at baseline and after 48 weeks of follow-up. We elected to use pct950 rather than the log15th percentile of lung density as an indicator of the extent of emphysema. Pct950 is equally sensitive in our studies and is a naturally interpretable number that has relevance to the visual interpretation of lung imaging.
HRCT scans were evaluated at a reading center, masked to treatment assignment. VIDA software was initially used to evaluate scans, however, we switched to using an open-source software (Slicer)19 after the VIDA software was no longer available. Slicer output will also be used for evaluation of secondary HRCT outcomes, e.g., percentage of emphysema for each lobe.
We based the standardization of the scanners between centers on the previous work of 2 large multicenter CT emphysema studies, the COPD Genetic Epidemiology study and SPIROMICS, that used experts in the field and company technical expertise to determine optimal settings within platforms and comparable scanner settings across platforms to maximize emphysema detection and minimize variation between scanners.20,21 The acquisition and reconstruction kernels were specified for each scanner manufacturer and model, and participants were scanned with the same HRCT machine using the same settings at baseline and follow-up. Certification and calibration of each scanner is verified by scanning of a phantom at baseline and every month. To ensure that scans are done at TLC, participants are coached and trained in the proper breathing maneuver by experienced pulmonary research coordinators prior to HRCT scans.
Secondary Outcomes
Secondary outcome measures encompass patient-reported outcomes including general and disease-specific quality of life: St George’s Respiratory Questionnaire,22 the modified Medical Research Council (mMRC) dyspnea scale,23 the COPD Assessment Test24 and the Patient-Reported Outcomes Measurement Information System Questionnaire: Physical Function-Short Form 20a (PROMIS-20a).25 FEV1 and FVC are measured before and after 2 inhalations of albuterol in accordance with American Thoracic Society/European Respiratory Society (ATS/ERS) standards and using National Health and Nutrition Examination Survey (NHANES) reference values,26,27and HRCT measures of emphysema are measured in each of the lobes of the lung separately, including TLC, mean lung density, 15th percentile density, emphysema progression for each lobe and measures of airway lumen and wall thickness.28,29 Adherence to the drug was measured by self-report and pill counts from returned study drug bottles.
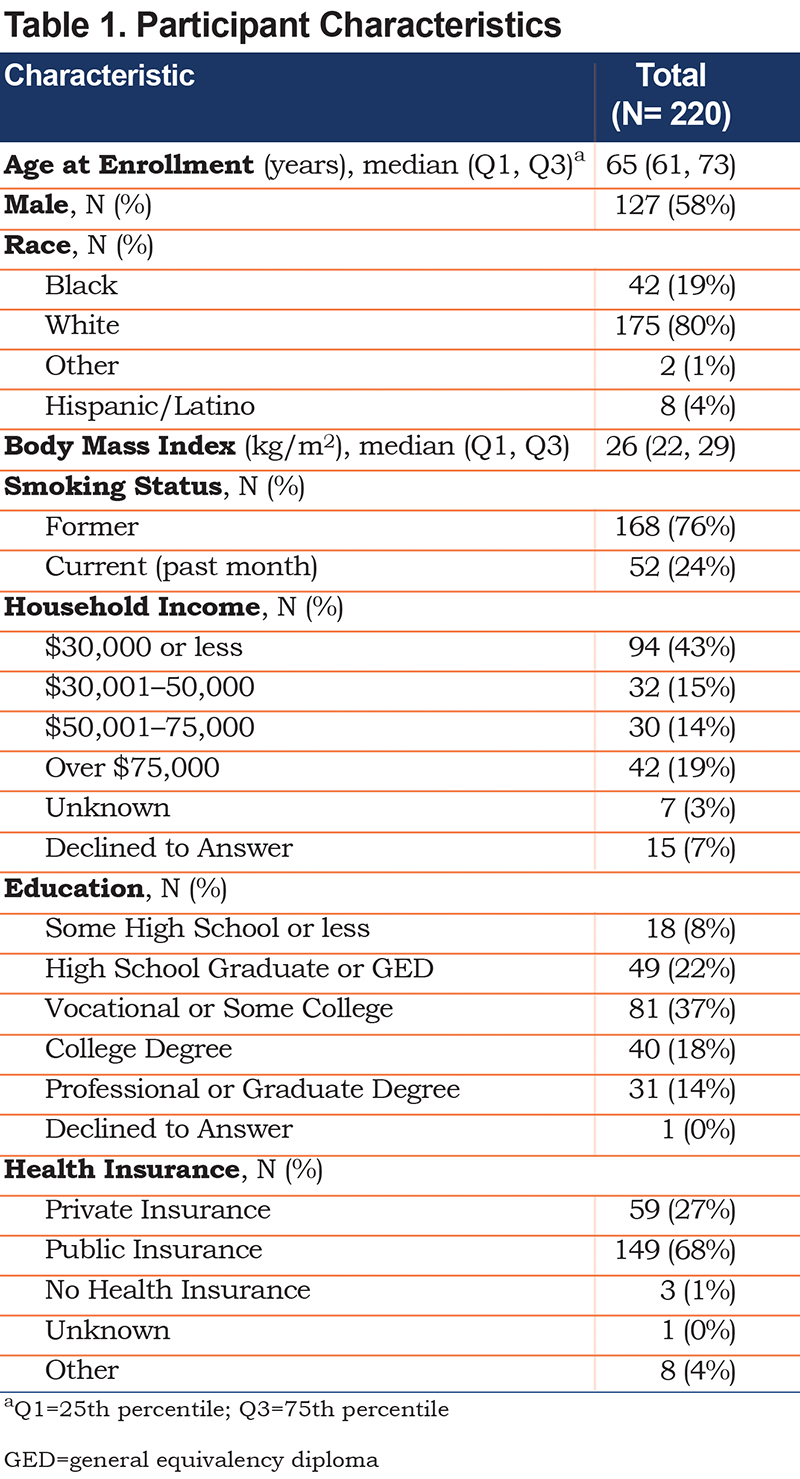
Sample Size and Power
The trial was designed for 90% power with a 2-sided type 1 error of 5% for a minimum detectable difference in the change in emphysema progression of 2% per year assuming a standard deviation of the difference of 4%. The sample size for the trial (220 participants; 110 per group) includes 20% additional participants to account for poor adherence and missing data.
Previous studies in emphysema patients show an annual increase in disease progression of 2%-3% with a standard deviation (SD) of 4% for emphysema patients and greater than the disease progression found in patients with COPD without emphysema.30,31 There has been no minimally important difference (MID) established for rate of change of pct950, therefore, a reasonable estimate of the MID to use for research is about 2%/year based on ½ SD.32 Using a cross-sectional, anchor-based approach in patients with radiographic emphysema, a 2% difference in pct950 would translate into 4.1 units of SGRQ or 3.6% predicted FEV1 which are close to the MID for those variables.33-36
Validation of Slicer Software
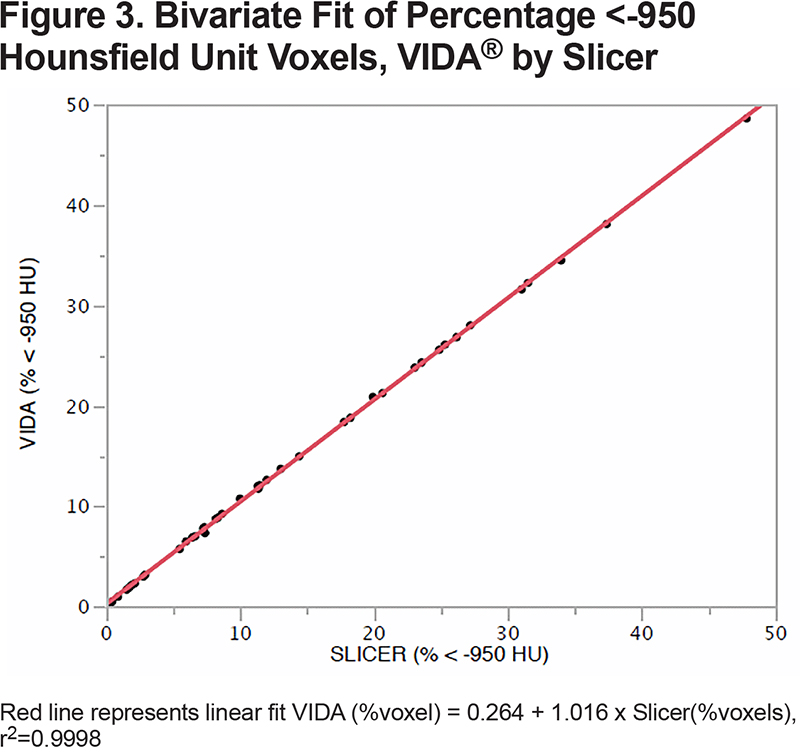
Agreement between the VIDA37 and Slicer software quantitative values for measurement of %voxels less than -950 HU was evaluated with linear regression using the first in 44 HRCTs obtained from participants of the LEEP trial.
Statistical Analysis
Baseline characteristics of participant and screening data were summarized with medians and proportions. The primary analysis will estimate the difference in response between the losartan and placebo groups by comparing between-group means of changes from baseline percentage of emphysema over 48 weeks. We will employ generalized linear mixed models using covariates that include site, baseline values for percentage of emphysema, an indicator variable for treatment group, and if needed, interaction terms between time covariates and treatment group.
The primary analysis will focus on least squares mean change in percentage of emphysema at 48 weeks for losartan versus placebo. We will reject the null hypothesis that losartan is equivalent to placebo if the 95% confidence interval of the estimated least squares mean change excludes 0.0. A robust variance estimate will be used to guard against incorrect inferences due to misspecification of the assumed serial correlation structure among repeated observations on each participant. The primary analysis will be based on the random treatment assignment regardless of actual treatment use (intention to treat).
Similar analytic methods will be applied to secondary outcome measures. For quantitative analysis of each of the 5 lobes of the lungs, we will use general linear mixed models to simultaneously estimate the treatment effects for the 5 lobes and the covariance matrix to evaluate the null hypothesis that effects are the same across the lobes. All analysis performed for this report was done using SAS V9 software.
Results
Initially 22 clinical centers of the ALA-ACRC network started recruitment for the trial. Four new consortium sites were added during the trial and 2 of the original sites dropped out after completing follow-up on a total of 3 participants. The trial was approved by local institutional review boards (IRBs) at each site. All participants signed 1 consent form that described the study and potential risks, including exposure to radiation during the HRCT scans. Participants were screened for pregnancy and recent COPD exacerbations prior to each HRCT scan.
We anticipated a 6-month period from the release of the protocol to opening recruitment at clinical sites, and, once recruitment was open, a nominal rate of recruitment of 10 participants per month for about 22 months. We projected that clinical sites would need to screen medical records of 12-16 individuals to establish preliminary eligibility for HRCT scanning and that about 50% of individuals with HRCT scans would demonstrate sufficient emphysema to be randomized. In reality, it took 22 months after the start of funding for the first participant to be randomized in June 2017 because of the time required to harmonize procedures and outcome measures among the PTC trials and the time required to recruit and certify sites. The last participant was randomized 32 months later in February of 2020, for a mean accrual of 6.7 participants per month.
Recruitment was slower than expected from the start. We took several steps in 2018 to enhance recruitment. These included recruiting 5 additional sites, increasing capitation payments, adding direct payments for study scans that disqualified otherwise eligible patients, and revising the eligibility criteria.
Five new sites were recruited, 4 in 2018 and 1 in 2019. Three of the sites enrolled 7 participants. Two sites that were recruited in 2018 were not successful. One of the sites was certified but never enrolled a participant and the other site was never certified because of onerous requirements for specification of data management system settings.
In 2018, supplemental capitation payments of $1200, provided by the ALA, were added to encourage recruitment; sites were paid retrospectively for enrolled participants so that the best recruiting sites were not penalized. Specific payments for HRCT scans of individuals whose scan did not meet the HRCT criteria for the trial were also added. Initially, we estimated a cost of $250 per scan and increased that cost by 50% to cover scans on ineligible patients. Actual costs for scans at sites varied from $115 to $760 per scan which hampered screening at some sites. We modified the payment system to pay $250 directly for scans that established ineligibility for potential participants who were otherwise eligible. The HRCT reading center also screened clinical HRCT scans of potentially eligible patients to identify those likely to qualify based on a study scan to reduce the number of study scans on patients unlikely to qualify.
We monitored exclusion criteria to identify recruitment barriers. For participants who signed consent, % predicted FEV1 was the most common reason for ineligibility, 53% as of March 2018. Many investigators believed that the requirement for a % predicted FEV1 of 30% to 70% excluded otherwise good candidates for the trial. After discussion of safety implications, and review and approval by the PTC DSMB and IRBs, the range of acceptable % predicted FEV1 was expanded to 20% to 80% starting in June 2018. The percentage of screened participants excluded based on FEV1 decreased to 44% by March of 2019 and was 31% overall at the end of the trial. The definition of chronic oxygen use was changed from “> 2 L/min nasal cannula at rest or clinical evidence of cor pulmonale” to “resting pulse oximetry oxygen saturation (SpO2) <89% on 2L nasal cannula continuous flow, unless at altitude > 4000 feet, then resting SpO2 <89% on 4L NC continuous flow” to account for higher flow rates typically used in Denver, Colorado.
A total of 2779 participants were screened for preliminary eligibility resulting in 560 participants who gave written consent for eligibility evaluations and 220 who were randomly assigned to receive losartan or placebo (Figure 2). The most common reasons for ineligibility at screening were use of a contraindicated medication, e.g., angiotensin receptor blockers or ACE inhibitors (25%), lack of interest (19%), and/or another medical exclusion, e.g., kidney disease (13%).
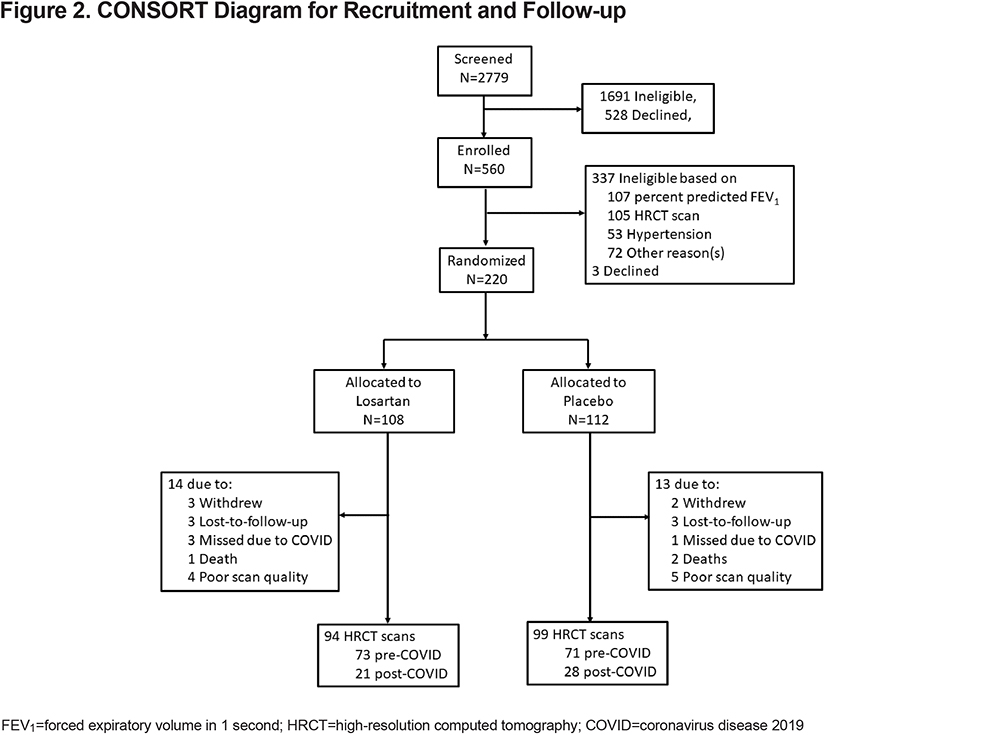
Sixty-one percent of the 560 individuals who signed consent were not eligible (e-Table 3 in the online supplement). The most common reasons for ineligibility were: % predicted FEV1 not in range (31%), ineligibility based on HRCT (31%) or hypertension (16%). Of the 340 HRCT scans performed for eligibility assessment, 105 did not meet the requirement for emphysema of 5%-35% voxels with density less than -950 HU and 15 who were eligible based on HRCT did not meet all of the eligibility criteria or declined to participant. Characteristics of the 220 randomized participants are shown on Table 1 .
In December 2017, six months after opening the trial, the software that was licensed for measurement of the primary outcome, quantitative HRCT, became unavailable for use by external investigators because of a change in the company’s business model (VIDA, Coralville, Iowa). Accordingly, the HRCT reading center conducted a validation study with open-source Slicer software and re-measured all acquired scans up to that date. The agreement between the HRCT quantitative agreement was close, the slope (standard error) was 1.05 (0.002) % < -950 HU for the 44 scans analyzed by both programs (Figure 3)
The Food and Drug Administration (FDA) recalls of valsartan in July 2018 and losartan in November 2018 and in January 2019 adversely affected recruitment. The FDA announced that some commercial supplies of these drugs were contaminated with a probable human carcinogen, N-nitrosodiethylamine, and recalled several batches from the market.38 The study drug used in LEEP was not subject to recall, however, the recalls were widely publicized and led many patients to decline participation in a clinical trial of losartan.
The negative publicity generated by the recalls led to a steep drop in accrual starting in the summer of 2018. About the same time, we implemented national and local strategies to promote the trial. The ALA developed a social media strategy to increase visibility of the trial including geographically and demographically targeted advertising on Google in June 2018 and on Facebook in December 2018. The COPD Foundation launched a geographically targeted email and newsletter campaign to promote the trial to members of its social network (COPD360 social) in October 2018. In July of 2019, sites were offered individual grants of up to $5000 to develop local strategies. Six sites took advantage of the grants to create advertisements for buses and subways, local cable television stations, and newspapers. One site used the funds to conduct intensified electronic medical record queries.
Shortly after the last patient was randomized in February 2020 the coronavirus disease 2019 (COVID-19) pandemic emerged around the world. On March 12, 2020, sites were notified that participant follow-up could be conducted by telephone and spirometry was not required. In-person visits were permitted for blood collections for safety laboratory draws if deemed essential for patient safety and in line with local research restrictions.
Fifty-eight participants were under active follow-up at that time. The visit window for obtaining HRCT scans for assessment of the primary outcome was extended by 12 weeks in order to allow time for re-opening of research visits. Of the 58 participants under follow-up, 26 had scans in the visit window, 23 had scans in the extended window, and 5 had scans after the extended window closed, the latest was at 75 weeks. Four participants did not have a scan. During the pre-pandemic period, 14 of 148 participants had scans after the final visit window closed, the latest was at 62 weeks.
Where possible, participants were asked to measure their own blood pressure with home devices at the time of study visits. Follow-up spirometry, which is considered an aerosol- generating procedure, was deemed non-essential for the remaining participants. Data collected on spirometry will be analyzed with those spirometry data considered to be data missing completely at random. We also began to survey participants who provided consent to monitor the effects of COVID-19 pandemics on a cohort of well-characterized COPD patients.
Discussion
In this manuscript, we outline the rationale, protocol, and recruitment experience for the LEEP trial. The trial was confronted with several unforeseen challenges: delayed start of recruitment, variable research cost of HRCT scans, high rates of patients screening-out, the negative effects of recall of lots of losartan for contamination by carcinogens, the withdrawal of the license for the HRCT quantitative analysis software, and the closure of research sites due to the COVID-19 pandemic.
Although harmonization of procedures, reporting, and data across the PTC trials delayed opening the trial, it allowed production of uniform reports for monitoring all 4 PTC trials and will allow data to be easily combined across trials. Furthermore, in the process of developing the procedures, the 4 PLGs and the NEMO were able to discuss best practices on data collection and quality control.
We had to conduct a large number of screening HRCT examinations to enroll our target population and our initial underestimate of the variability in HRCT scan costs may have impacted enrollment. Switching to a system of paying for each scan regardless of eligibility, as long as a participant met all other criteria, allowed more patients to be screened at sites with high HRCT costs. We also emphasized that the HRCT reading center was willing to review clinical HRCT scans to identify patients likely to be eligible, thus reducing the number of participants who were ineligible based on HRCT. It is difficult to know whether either measure was effective since paying for scans likely increased the number of scans for screening while review of clinical scans likely reduced the number.
Monitoring eligibility criteria is a widely utilized and useful tool for identifying barriers to recruitment. Although it is difficult to isolate the effects of individual actions, modifying the criterion for % predicted FEV1 was associated with a decrease in consented patients excluded based on FEV1 from 52% before the protocol revision to 44% several months after the revision and an overall rate of 31%. Revising the criterion regarding chronic oxygen use to account for oxygen saturation and altitude had no effect.
Losartan is a generic drug that is widely prescribed for common COPD comorbidities including hypertension, heart failure, renal disease, and coronary artery disease. Thus, it was not unexpected that a major reason for screening out many patients who would otherwise be eligible was current use or a firm indication for this class of drug. Further, because losartan is widely available, it is likely that some COPD patients decided to take this drug without enrolling in a trial based on hope that it might be effective.
The FDA recalls of specific lots of losartan and valsartan due to contamination with carcinogens led to a period of almost no enrollment. Fortunately, the lots of drugs that we packaged and distributed for LEEP were not subject to recall and we were able to acquire additional drug supplies free of contamination to complete the study. The recalls were widely publicized so it was essential for us to prepare materials to explain that the study drug had not been recalled for prospective participants and enrolled participants who expressed concern. We also independently tested newly purchased study drugs for contamination before packaging new supplies.
We instituted a number of strategies to address lagging recruitment including adding sites, modifying an eligibility criterion, providing small grants for local advertising, frequent meetings with site personnel to discuss recruitment activities, ALA-sponsored social media campaigns, enlistment of publicity from the COPD Foundation, and increased site capitation. Despite the negative effects of the losartan recalls we were able to increase the recruitment rate from about 5 per month in the first 16 months to about 8 per month in the second 16 months of recruitment. No one strategy stood out as being the most successful which emphasizes the importance of employing multiple strategies for recruitment.
The primary outcome for the trial was quantitative HRCT analysis, so it was critical to have stable analytic tools throughout the course of the trial. However, the vendor of our initial HRCT analysis platform withdrew external licensing agreements for business purposes which required us to validate and institute a different open-source software and conduct reanalysis of our initial baseline data. Fortunately, there was a nearly perfect agreement between the 2 platforms. Extending the window for the HRCT scans during the pandemic allowed us to complete follow-up in all but 4 of the 58 participants under follow-up in March 2020.
The structure of LEEP, a public-private partnership between the NIH and the ALA, permitted enough financial and administrative flexibility to respond to these unanticipated events and successfully complete study enrollment. Without this cooperative structure and access to additional funds, we would not have been able to complete LEEP accrual and follow-up. The trial completed follow-up in February 2021 and data analysis is currently underway.
Acknowledgements
We are most grateful for all the research coordinators who helped us with data collection and the COPD patients who participated at 16 centers of the ALA-ACRC in the United States.
Per NHLBI policies, de-identified participant data and related documents, including study protocol and statistical analysis plan, will be shared through the Biological Specimen and Data Repository Information Coordinating Center. Data sharing will follow NHLBI access policies. Data will be made available within 60 days of publication of the primary manuscript and will be available indefinitely.
Author contributions: All authors have contributed to manuscript drafting and/or critical revision, approved the final manuscript and agree to be held accountable for all aspects of the work.
Clinical Trial Registration: NCT00720226
American Lung Association Airways Clinical Research Centers
Baylor College of Medicine, Houston: Nicola Hanania, MD (Principal Investigator), Mustafa Atik, MD (Co-investigator), Marianna Sockrider, MD (Co-Investigator) Laura Bertrand (Lead Coordinator)
Columbia University Medical Center, New York: Emily DiMango, MD (Principal Investigator), Tarnjot Saroya (Lead Coordinator)
Cornell University, New York: Robert J. Kaner, MD (Principal Investigator), Bradley Pua, MD (Co-investigator), Xiaoping Wu, MD (Co-investigator), Lianne De La Cruz (Lead Coordinator)
Duke University Medical Center, Durham: Loretta G. Que, MD (Principal Investigator), Catherine Foss, RRT (Lead Coordinator), Anne Mathews, MD (Co-investigator), Isaretta Riley, MD (Co-investigator), Antoinette Santoro, MS (Coordinator)
Louisiana State University, New Orleans: Kyle Happel, MD (Principal Investigator), Connie Romaine, MSN (Lead Coordinator), Sarah Jolley (Co-Investigator), Matthew Lammi, MD (Co-investigator), Richard Tejedor, MD (Co-investigator), Paula Lauto, RN (Coordinator), Marie Sandi (Coordinator)
Mount Sinai-National Jewish Health Respiratory Institute, New York: Linda Rogers, MD (Principal Investigator), Sonali Bose, MD (Co-investigator), Chelsea Chung (Coordinator)
Mount Sinai-National Jewish Health Respiratory Institute, Denver: Barry Make, MD (Principal Investigator), Jami Henriksen (Lead Coordinator), Flavia Hoyte, MD (Co-investigator), Hayley Posoff (Lead Coordinator)
New York University, New York: Joan Reibman, MD (Principal Investigator), Karen Carapetyan (Lead Coordinator)
Northwestern University Feinberg School of Medicine, Chicago: Ravi Kalhan, MD (Principal Investigator), Jenny Hixon (Lead Coordinator), Sharon Rosenberg, MD (Co-investigator), Lewis Smith, MD
Pacific Northwest VA Puget Sound Health Care System, Seattle: Laura Feemster (Principal Investigator), David Au (Co-investigator), Brenda Patterson
Rush University Medical Center, Chicago: James Moy, MD (Principal Investigator), Ben Hu (Lead Coordinator)
St. Vincent Health System, Indianapolis: Michael Busk, MD, MPH (Principal Investigator), Ellen Looney (Lead Coordinator)
Temple University Health System Lung Center, Philadelphia: Gerard Criner, MD (Principal Investigator), Francine McGonagle (Coordinator), Antoinette Santoro (Coordinator)
University of Alabama at Birmingham Lung Health Center, Birmingham: Mark Dransfield (Principal Investigator), Mike Wells (Co-investigator), Surya Bhatt (Co-investigator), Trisha Parekh (Co-investigator), Necole Seabron (Coordinator), Renita Holmes (Coordinator), Elizabeth Westfall (Center Director)
University of Arizona, Tucson: Lynn B. Gerald, PhD, MSPH (Principal Investigator), Monica Kraft, MD (Principal Investigator), Michele Simon, CRC (Lead Coordinator), Sam Afshin, MD (Study Physician), Natalie Provencio (Coordinator)
University of California, San Diego: Stephen C. Lazarus, MD (Principal Investigator), Julian Silva, MA (Coordinator)
University of California, San Francisco: Stephen C. Lazarus, MD (Principal Investigator), Julian Silva, MA (Coordinator)
University of Chicago Asthma and COPD Center, Chicago: Edward T. Naureckas, MD (Principal Investigator), Virginia Zagaja (Coordinator)
University of Florida, Jacksonville: James Cury, MD (Principal Investigator), Fallon Wainwright (Lead Coordinator), Katrina Maloney (Coordinator), Vandana Seeram (Co-investigator),
University of Illinois Breathe Chicago Center, Chicago: Jerry A. Krishnan, MD, PhD (Principal Investigator), Lauren Castro (Coordinator), Julie DeLisa (Coordinator), Wendy Hasse (Coordinator), Sai Illendua (Coordinator), Ellen Stein (Coordinator), Prieto-Centurion Valentin, MD (Co-investigator), Julie DeLise (Coordinator)
University of Michigan, Ann Arbor: MeiLan Han, MD, MS (Principal Investigator), Alan Baptist, MD (Co-investigator), Wassim Labaki, MD (Co-investigator), Catherine Meldrum, MD (Co-investigator), Mary Kay Hamby (Coordinator), Gretchen Bautista (Project Manager), Rysso Kelly (Coordinator)
University of Pittsburgh Emphysema/COPD Research Center, Pittsburgh: Divay Chandra, MD (Principal Investigator), Elizabeth Stempkowski, BA, CCRC (Lead Coordinator), Jessica Bon Field, MD (Co-investigator), Craig Riley, MD (Co-Investigator)
University of South Florida, Tampa: Thomas Casale, MD (Principal Investigator), Catherine Smith (Lead Coordinator), Amber Pepper, MD (Co-investigator), Claudia Gaefke, MD (Co-Investigator), Dennis Ledford (Co-investigator), Juan Car Cardet, MD (Co-investigator), Richard Lockey, MD (Co-investigator), Raul Villarreal, MD (Co-investigator),
University of Vermont Lung Center, Colchester: David Kaminsky, MD (Principal Investigator), Erika Gonyaw (Lead Coordinator), Charles Irvin, PhD (Principal Investigator), Anne Dixon, MA, BM, BCh (Co-investigator)
Washington University, St. Louis: Mario Castro, MD (former Principal Investigator), Brenda Patterson, RN (Coordinator), Kaharu (Cajal) Sumino, MD (Co-investigator), Jaime Tarsi, RN (Coordinator)
Western Connecticut, Danbury: Douglas Kahn, MD (Principal Investigator), Margaret Mukway (Coordinator)
Chairman’s Office, University of Alabama, Birmingham: William C. Bailey, MD (chairman)
Data Coordinating Center, Johns Hopkins University Center for Clinical Trials, Baltimore: Janet Holbrook, PhD (Co-principal Investigator), Robert Wise, MD (Co-principal Investigator), Anne Casper (Coordinator), Cathy Ewing (Coordinator), Heather Hazucha, MPH (Coordinator), Alexis Rea, MPH (Lead Coordinator), Gem Roy, MD (Coordinator), Emily Szilagyi (Coordinator), Dave Shade, JD (Information System), Andrea Lears (Analyst), Jill Meinert (Analyst), Joy Saams, MSRN (former Lead Coordinator), Robert Henderson, MS (Biostatistician), Ashley McCook, MS (Biostatistician)
HRCT Reading Center, Johns Hopkins University, Baltimore: Robert Brown, MD (Principal Investigator), Allison Brooker (Coordinator)
ALA-ACRC Biorepository, Nemours Children's Health, Jacksonville: Kathryn Blake, PharmD (Principal Investigator), Ed Mougey (Co-investigator)
Project Office, American Lung Association, New York: Alexandra Sierra, MA, Susan Rappaport, MPH, Albert Rizzo, MD.
Project Office, National Heart, Lung, and Blood Institute, Division of Lung Diseases, Washington, DC: Tony Punturieri, MD, PhD (Program Officer), Lisa Viviano. BSN (Clinical Trials Specialist, Office of the Director)
Network Management Core, University of Pittsburgh, Pittsburgh: Stephen Wisniewski, PhD (Principal Investigator), Frank C. Sciurba, MD, FCCP (Principal Investigator), Amanda Baucom, MSCP (Coordinator), Maria Brooks, PhD (Biostatistician), Heather Eng (Data Manager), Yulia Kushner (Administrative Assistant), Jeffery Martin (IT Support), Vicky Palombizio (Coordinator), Jennifer Stevenson, MLS (Data Manager), Mary Tranchine (Financial Administrator), Alexander Washy (Systems Analyst)